Abstract
Nuclear factor Y (NF-Y) gene family is an important transcription factor composed of three subfamilies of NF-YA, NF-YB and NF-YC, which is involved in plant growth, development and stress response. In this study, 63 tobacco NF-Y genes (NtNF-Ys) were identified in Nicotiana tabacum L., including 17 NtNF-YAs, 30 NtNF-YBs and 16 NtNF-YCs. Phylogenetic analysis revealed ten pairs of orthologues from tomato and tobacco and 25 pairs of paralogues from tobacco. The gene structure of NtNF-YAs exhibited similarities, whereas the gene structure of NtNF-YBs and NtNF-YCs displayed significant differences. The NtNF-Ys of the same subfamily exhibited a consistent distribution of motifs and protein 3D structure. The protein interaction network revealed that NtNF-YC12 and NtNF-YC5 exhibited the highest connectivity. Many cis-acting elements related to light, stress and hormone response were found in the promoter of NtNF-Ys. Transcriptome analysis showed that more than half of the NtNF-Y genes were expressed in all tissues, and NtNF-YB9/B14/B15/B16/B17/B29 were specifically expressed in roots. A total of 15, 12, 5, and 6 NtNF-Y genes were found to respond to cold, drought, salt, and alkali stresses, respectively. The results of this study will lay a foundation for further study of NF-Y genes in tobacco and other Solanaceae plants.
Similar content being viewed by others
Introduction
Transcription factors (TF), also known as trans-acting factors, can specifically bind to specific sequences upstream of the 5 'end of eukaryotic genes, so as to activate or inhibit transcription expression of downstream genes in specific growth and development stages or specific tissues1. Nuclear factor Y(NF-Y) is an important transcription factor widely existing in eukaryotes, also known as CCAAT Binding Factor (CBF) or Heme Activator Protein (HAP)2. NF-Y consists of three conserved subunits, NF-YA (HAP2/CBF-B), NF-YB (HAP3/CBF-A), and NF-YC (HAP5/CBF-C), and is a heterotrimer transcription factor complex3,4. Among them, the NF-YA subunit is usually localized within the nucleus, with a core conserved domain consisting of two conserved alpha-helical domains (A1, A2). A1 is composed of 20 amino acids, which is located in the N terminal of the core region and can interact with the NF-YB subunit and NF-YC subunit. A2 is composed of 21 amino acids, which is located in the C terminal of the core region and specifically recognizes and binds to CCAAT cis-acting elements5. Both NF-YB and NF-YC subunits contain conserved Histone Fold domains (HFD), also known as Histone Fold motifs (HFM), with three or four α-helices5,6. HFD on the NF-YB subunit is similar to core histone H2B, except that HFD on the NF-YC subunit is more similar to core histone H2A7,8. Previous studies reported that NF-YB subunits can be divided into LECL and non-LECL, LEC1 was composed of LEC1 and LEC1-like (L1L), and the 55th aspartic acid (D) in its domain was considered to be a specific amino acid of LEC19.
The NF-Y trimer complex is formed by the polymerization of three subunits within the cell. Firstly, NF-YB and NF-YC in the cytoplasm recombine to form heterodimers due to the presence of HFD, and then transfer from the cytoplasm to the nucleus. NF-YA is then recruited by the heterodimer just formed in the cytoplasm to form the NF-Y complex. Finally, NF-YA in the mature complex specifically binds to the cis-element CCAAT to inhibit or activate downstream gene expression10. A single NF-YA subunit cannot function and must be combined with the NF-YB/NF-YC heterodimer to form a triplet to bind to the CCAAT cis-element10. In addition, studies have shown that NF-YB/NF-YC heterodimers can also bind other transcription factors other than NF-YA subunits to regulate downstream gene expression7.
A large number of studies had shown that NF-Y genes play an important role in plant growth and development, abiotic and biological stress response10,11,12,13. In Arabidopsis thaliana, NF-YC3/C4/C9 and GA-repressing DELLA protein RGA-Like 2 (RGL2) were involved in regulating the expression of ABI5 gene, affecting the synthesis of abscisic acid, which were related to seed dormancy and germination, and NF-YC3/C4/C9 also promoted photomorphogenesis11. In Triticum aestivum L., TaMADS29 and TaNF-YB1 regulated wheat kernel development through direct interaction14. During the ripening process of tomato fruits, the NF-Y complex composed of NF-YB8a/8b/8c, NF-YC1a/1b/1d/9 and NF-YA11/9 can regulate the transcription of CHS1 by regulating the H3K27me3 level at the CHS1 site, affecting flavonoid biosynthesis and thus tomato fruit color15. In alfalfa, MtNF-YC6 and MtNF-YC11 interacted with MtNF-YB12 and MtNF-YB17 and participated in the regulation of arbuscular development16. PdbNF-YA11 in poplar was involved in the resistance of poplar to Alternaria infection by regulating jasmonic acid (JA) synthesis and signaling pathways17. The expression of AhNF-YA4/A8/A11, NF-YB4 and NF-YC2/C8 genes in peanut and PgNF-YB09, PgNF-YC02 and PgNF-YC07-04 genes in ginseng had been shown to be induced by salt stress18,19. In peach, 9 NF-Ys genes were identified to be up-regulated under drought stress, indicating that they could be used as candidate functional genes to further study drought resistance of peach20. Although the NF-Y gene family has been extensively studied in plants, it has been little studied in tobacco.
Tobacco is an important cash crop and one of the important model plants used in scientific research. Drought, cold, salt and other abiotic stresses have been affecting the growth and development of tobacco plants21,22. Therefore, the identification of tobacco stress-related genes will be of great significance for the improvement of tobacco varieties, the enhancement of tobacco resistance, and the promotion of tobacco growth and development. In this study, NF-Y genes in tobacco were identified and analyzed for physicochemical properties, subcellular localization prediction, phylogeny, gene structure and conserved motifs, promoter cis-acting elements, protein 3D structure, protein interaction network, and expression in plant tissues and abiotic stresses. This study provided a comprehensive understanding of the NF-Y gene family in tobacco, and the results laid a foundation for further study on the function of NF-Y genes and the improvement of tobacco varieties.
Results
Identification and sequence analysis of NF-Y gene family members in tobacco
A total of 63 NF-Y genes (17 NF-YAs, 30 NF-YBs, 16 NF-YCs) were identified in tobacco by BLAST and HMMER (Table 1), and these genes were named according to their subfamilies (NtNF-YA1 to NtNF-YA17, NtNF-YB1 to NtNF-YB30, NtNF-YC1 to NtNF-YC16). The physicochemical properties and subcellular localization of the 63 NtNF-Y proteins were shown in Table 1. The length of the amino acid sequence encoded by the NtNF-Y genes ranged from 104 to 353 aa. The theoretical isoelectric point (pI) ranged from 4.58 to 9.69. The molecular weight (MW) of NtNF-Y proteins ranged from 11.54 to 40.55 KDa. Ten of the 63 NtNF-Y proteins were considered stable proteins (Instability index < 40). The sequences and properties of 63 NtNF-Y proteins were significantly different. All members of NtNF-Y were located in the nucleus, while four members of NtNF-YCs, NtNF-YC3/C8/C15/16, were also located in the cytoplasm.
Multiple alignments and phylogenetic tree of NtNF-Y protein
Multiple alignment revealed that NtNF-Y family proteins had conserved domains, as shown in Fig. 1. The conserved domain of NtNF-YAs comprises of two core subdomains (Fig. 1A). One subdomain is responsible for NF-YB/C interactions, while the other subdomain is involved in DNA binding. The conserved domain of NtNF-YBs consisted of one domain that bound to DNA and another domain that interacted with NF-YA and NF-YC proteins (Fig. 1B). NtNF-YCs contained NF-YA interaction domains separated by NF-YB interaction domains, and DNA-binding domains were embedded in the first NF-YA interaction domain (Fig. 1C). In addition, NtNF-YB3/B5/B12/B28 were found to have an aspartic acid (Asp)-55 residue (Fig. 1B), indicating that NtNF-YB3/B5/B12/B28 may be LEC1 type genes.
Multiple alignments of the conserved domain of tobacco NF-Y proteins. The DNA binding, NF-YA and NFYB/YC subunit interaction domains were marked in black lines. (A) Multiple alignments of the NtNF-YA conserved domains. (B) Multiple alignments of the NtNF-YB conserved domains. (C) Multiple alignments of the NtNF-YC conserved domains. The amino acids in the red box represented the key amino acids that distinguish LEC1 from non-LEC1.
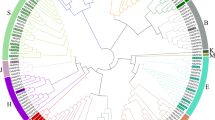
To investigate and elucidate the phylogenetic relationships among tobacco, Arabidopsis, rice and tomato NF-Y proteins, a phylogenetic tree was constructed (Fig. 2, Supplementary Table S1). Phylogenetic tree showed that all NF-Y proteins can be clustered into three branches, the same subfamily members clustered on the same branch, except Solyc06g016750. Ten pairs of orthologous genes from tomato and tobacco and 25 pairs of paralologous genes from tobacco (seven pairs of NtNF-YAs, ten pairs of NtNF-YBs, eight pairs of NtNF-YCs) were observed. In addition, in the NF-YB subfamily, NtNF-YB3/B5/B12/B28 clustered together with AtNF-YB6 (LEC1)/B9 (L1L), OsNF-YB7 (L1L)/B9 (LEC1) and 15 tomato NF-YB members clustered together to form the LEC1 branch, while the remaining NF-YB subfamily members form the non-LEC1 branch (Fig. 2).
Phylogenetic analysis of NF-Y proteins identified in Nicotiana tabacum (Nt), Arabidopsis thaliana (At), Oryza sativa (Os), and tomato. Based on the full-length amino acid sequence of NF-Y, the phylogenetic tree was constructed by neighbor-joining (NJ) method. The three subfamilies were color-coded: green for NF-YA, red for NF-YB, and blue for NF-YC. The NF-Ys of tobacco, Arabidopsis, rice and tomato were marked with circular, five-pointed star triangular and rectangle patterns respectively. The bootstrap values were shown on the branches.
Gene structures, conserved domains and motifs
To better understand the evolution and diversity of NtNF-Y members, gene structure and conserved motifs were investigated (Fig. 3). The NtNF-YAs subfamily contained the conserved CBFB_NFYA domain, and both the NtNF-YBs and NtNF-YCs subfamilies had the conserved CBFD_NFYB_HMF domain (Fig. 3B). In addition, the NtNF-YC subfamily had a unique HAP5 domain (Fig. 3B). The three subfamilies had different domains, suggesting that each of them had a unique function, whereas the same domains were also present in NtNF-YBs and NtNF-YCs, suggesting that these two subfamilies had similar functions. The composition of motifs was different among the three subfamilies (Supplementary Figs. S1, S2). The three subfamilies of NtNF-YAs, NtNF-YBs and NtNF-YCs each contain four to five conserved motifs, and members of the same subfamily had similar motif distribution.
Phylogenetic relationships, gene structures and conserved domains composition of NtNF-Y genes. (A) Neighbor-joining phylogenetic tree of NtNF-Ys. The NF-YA, NF-YB, and NF-YC subfamilies were represented in green, red, and blue, respectively. (B) Conserved domains of NtNF-Ys. Colored boxes indicate different conserved domains. (C) Exon/intron structures of NtNF-Ys. The yellow boxes represented exons, the green boxes represented UTRs and the black lines represented introns.
The introns of NtNF-Ys were diverse (Fig. 3C). The gene structures of NtNF-YAs members were similar, most of them contain five introns, only three contain six introns, and one contains four introns, which were relatively stable. The gene structures of NtNF-YBs and NtNF-YCs were significantly different. 16 NtNF-YBs with no introns, two NtNF-YBs with one and two introns, respectively, and the remaining members with four to seven introns. Eight members of the NtNF-YCs subfamily had no or only one intron, two members had 11 and 12 introns, respectively, and the rest had between three and six introns.
Promoter Cis-acting elements of NtNF-Y genes
In addition to the common core elements TATA-box and CAAT-box, 59 cis-acting elements were identified in the promoter region of NtNF-Y genes (Fig. 4). In general, it can be divided into five categories: the first category was related to growth and development, the second category was related to hormone response, the third category was related to light response, the fourth category was related to stress response, and the fifth category was other cis-type components. Among them, the types of optical response related components were the largest. Most of NtNF-Y genes had cis-acting elements related to hormone and stress response, and a small number of photoresponsive elements, such as Box 4, TCT-motif, GATA-motif, G-box, etc. NtNF-YC16 and NtNF-YB11 had the most ABRE (abscisic acid responsiveness) elements, both with 9. G-box elements were the most numerous in NtNF-YB11 with 10, followed by NtNF-YC16 with 9. NtNF-YB5 had the most light-responsive elements Box 4 with a total of 8. The RE (anaerobic induction) element was present in almost all NtNF-Y genes. The number of ARE elements in NtNF-YB22 was the highest, with 10, while the number of ARE elements in other genes was no more than 5 (Fig. 4). These results suggested that the NtNF-Y genes family may play an important role in multiple stress and hormone responses, especially in anaerobic and abscisic acid (ABA) responses.
NtNF-Y genes promoter cis-acting regulatory elements. The numbers in the box represented the number of cis-acting elements. Detailed information of cis-acting elements was provided in Supplementary Table S2.
Protein 3D structure of NtNF-Y gene family
The 3D structure of the NtNF-Y protein consisted of α-helices and random curl, and the same subfamily had similar 3D structure (Fig. 5). The NtNF-YA conserved domain consisted of two α-helices located in two core subdomains, while the NtNF-YB and NtNF-YC conserved domains are both composed of four α-helices located in the core subdomains of DNA binding and protein interactions (Fig. 5).
Protein–protein interaction (PPI) network of NtNF-Y gene family
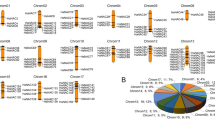
The protein interaction network of NtNF-Ys contained a total of 40 NtNF-Y proteins (10 NtNF-YAs, 18 NtNF-YBs, and 12 NtNF-YCs), with complex interactions among the three subfamilies of NF-YA, NF-YB, and NF-YC (Fig. 6). NtNF-YC12 and NtNF-YC5 had the highest connectivity, followed by NtNF-YC16, NtNF-YC9, NtNF-YC15, NtNF-YC13, NtNF-YC8, NtNF-YC3 and NtNF-YB11. NtNF-YC3, NtNF-YC8, NtNF-YC13 and NtNF-YC15 had strong interaction with NtNF-YA8 and NtNF-YA12. NtNF-YC5 and NtNF-YC12 had strong interactions with NtNF-YB16, NtNF-YB13, NtNF-YA4 and NtNF-YA16 (Fig. 6).
Expression patterns of NtNF-Y genes in different tissues of tobacco
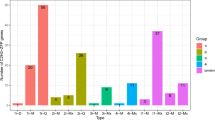
In order to investigate the expression patterns of these NtNF-Y genes in different tobacco tissues, the RNA-seq data of NtNF-Y genes in three different tissues (roots, stems and stem apexes) were obtained and analyzed. The results showed that among the 63 NtNF-Y genes, 54 genes were expressed in at least one tissue, nine genes were not expressed in all three tissues, and eight genes were highly expressed in all three tissues (Fig. 7). The expression levels of most NtNF-YA subfamily members in roots were higher than those in stems and shoot apexes. NtNF-YB9/B14/B15/B16/B17 and B29 were specifically expressed in roots (Fig. 7).
Expression analysis of NtNF-Y genes under different abiotic stress
To clarify the role of NtNF-Y genes in response to diverse abiotic stresses, transcriptome data encompassing low temperature, drought, salt, and alkali stress conditions were collected, and the differentially expressed genes across these distinct stress types were analyzed. The results showed that multiple NtNF-Y genes were involved in different abiotic stress processes (Fig. 8). Under cold stress, the expression levels of 15 NtNF-Y genes (six NtNF-YA genes, four NtNF-YB genes, and five NtNF-YC genes) significantly changed. Among them, the expression levels of only two genes (NtNF-YC1 and NtNF-YB10) were significantly up-regulated, while the expression levels of the remaining 13 genes were significantly down-regulated. Under drought stress, the expression levels of seven NtNF-Y genes (six NtNF-YA genes, one NtNF-YB gene) were significantly up-regulated. The expression levels of five NtNF-Y genes (two NtNF-YA genes, two NtNF-YB genes, and one NtNF-YC gene) were significantly down-regulated. The expression levels of three NtNF-Y genes (NtNF-YA2, NtNF-YA6, NtNF-YC16) were significantly up-regulated under salt stress, while the expression levels of two NtNF-Y genes (NtNF-YB10, NtNF-YB21) were significantly down-regulated. Under alkali stress, the expression of only one NtNF-Y gene (NtNF-YA2) was significantly up-regulated, while the expression of the other five NtNF-Y genes (two NtNF-YA genes and three NtNF-YB genes) was significantly down-regulated. In addition, multiple NtNF-Y genes were observed to function under two or more stresses. For example, NtNF-YA3/A4/A7 and NtNF-YC6 simultaneously responded to low temperature and drought stress, NtNF-YA2 simultaneously responded to low temperature, salt and alkali stress, and NtNF-YB11 simultaneously responded to low temperature, drought and alkali stress (Fig. 8).
Differentially expressed genes (DEGs) of NtNF-Y under different abiotic stresses (cold, drought, NaCl and NaHCO3). The color scale represented the size of the log2 fold change. The red boxes, blue boxes, and gray boxes indicate significant up-regulation, significant down-regulation, and no significant change in NtNF-Y genes under the corresponding conditions, respectively.
Discussion
Nuclear factor Y (NF-Y) is a heterotrimeric transcription factor complex composed of three subunits: NF-YA, NF-YB and NF-YC. It is widely found in eukaryotic organism and is an important transcription factor. It plays an important role in plant growth and development, abiotic and biological stress response. NF-Y gene family members have been identified in a variety of plants, such as Arabidopsis thaliana (30 NF-Ys)7,23,24, rice (34 NF-Ys)25, and potato (37 NF-Ys)8. A total of 63 NtNF-Y genes were identified in the genome of tobacco, including 17 NtNF-YAs, 30 NtNF-YBs and 16 NtNF-YCs, which was about twice as many as Arabidopsis, rice and potato. Arabidopsis thaliana, rice and potato are diploid, and Nicotiana tabacum is allotetraploid. This may be related to whole-genome duplication during tobacco formation. In addition, the number of other gene families, such as POD226, HSP9027, MADS-box28, NAC29, etc., identified in tobacco also showed a similar situation.
NF-YAs contained CBFB_NFYA domain, motif 3, motif 4, motif 7 and motif 9. NF-YBs contained CBFD_NFYB_HMF domain, motif 1, motif 2, motif 5 and motif 6. NF-YCs contained CBFD_NFYB_HMF and HAP5 domains, motif 1, motif 2, motif 8 and motif 10 (Fig. 3). Although some motifs were missing in individual members, each subfamily showed its unique domain and motif composition on the whole, suggesting the conservation of its function and the reliability of classification. The tertiary structure of NtNF-Ys proteins predicted by homology modeling showed that the tertiary structure of NtNF-Y proteins consisted of α-helices and random coiled-coils, with similar tertiary structures in the same subfamily (Fig. 5). Among them, the tertiary structures were more consistent among NtNF-YAs compared to NtNF-YBs and NtNF-YCs, suggesting a more conserved function of NtNF-YAs. These tertiary structure models of NtNF-Y proteins laid the foundation for the study of their biological functions. Protein interactions predictions indicated complex interactions among the three subfamilies of NtNF-Y, with NtNF-YC12 and NtNF-YC5 showing the highest connectivity, suggesting that they may have more important functional roles (Fig. 6). These results provide a rich genetic resource for future research. The structure of important NtNF-Y proteins and the interaction mechanism of important NtNF-Y proteins need to be further investigated in the future, which is of great significance for analyzing the mechanism of NtNF-Y function in tobacco and the improvement of tobacco varieties.
Analysis of the cis-acting promoter elements of tobacco NtNF-Ys showed that NtNF-Ys contained many cis-acting elements related to light response, plant growth and development, hormone response and stress response, similar to the results of NF-Y promoter cis-acting elements in other plants, such as Z. jujuba30, watermelon31 and banana32. These results suggested that NF-Y may play an important role in plant growth and development and stress tolerance. In this study, the phylogenetic tree analysis of NF-Ys in tobacco, Arabidopsis, rice and tomato was performed. According to the genes with known functions in the phylogenetic tree, the functions of other genes can be inferred. Among members of Arabidopsis NF-Y gene family, the regulation of AtNF-YA2/A3/A5/A7/ A10 and AtNF-YB1 was related to drought stress7,11. In addition, OsNF-YA7 and A10 in rice are also involved in the regulation of drought stress33,34. In the NF-Y phylogenetic trees of Arabidopsis thaliana, rice, tomato and tobacco as shown in Fig. 2, NtNF-YA15/A13 clustered together with AtNF-YA2/A10 and OsNF-YA10, NtNF-YA1/A5/A6/A7/A9/A11/A14 clustered together with AtNF-YA3/A5, NtNF-YA8/A12 were clustered with AtNF-YA7 and OsNF-YA7, and NtNF-YB2/B4/B7/B8/B20/B30 were clustered with AtNF-YB1, suggesting that these tobacco NF-Y gene family members may have similar functions. Arabidopsis AtNF-YB2/B3 can promote flowering by activating the key flowering regulator FLOWERING LOCUS T (FT)35,36. AtNF-YC3/C4/C9 is also necessary for flowering37. Overexpression of AtNF-YC1/C2 can activate flowering24,38. In rice, overexpression of OsNF-YB8/B9/B10/C2/C4 affects flowering36,39,40. The NtNF-YB10/B11/B21/C3/C8/C9/C13/C15/C16 in tobacco had high homology with the NF-Y genes related to flowering in Arabidopsis thaliana and rice (Fig. 2). They may be involved in the flowering process of tobacco and need further study. In addition, ZmNF-YC2 and PtNF-YB1 have also been identified to play a role in flowering regulation in maize and poplar41,42. Studies have shown that OsNF-YB2/B3/B4 affects chloroplast biosynthesis43, and the orthologous gene NtNF-YB30 of OsNF-YB3 may be a candidate gene involved in chloroplast synthesis (Fig. 2). The study of Arabidopsis thaliana showed that AtNF-YC1/C3/C4/C6/C9 regulated photomorphogenesis and hypocotyl elongation7,44,45. It can be seen from the phylogenetic tree that tobacco NtNF-YC3/C8/C9/C13/C15/C16 had high homology with the five NF-YC members that regulate photomorphogenesis in Arabidopsis thaliana (Fig. 2). In addition, promoter cis-acting element analysis showed that NtNF-YC3 had more light-responsive element Box4 and NtNF-YC16 had higher light-responsive element G-box (Fig. 4). Therefore, NtNF-YC3 and NtNF-YC16 may be potential candidate genes for regulating photomorphogenesis. In addition, studies have shown that CsNF-YC2 and CsNF-YC9 were involved in chloroplast photomorphogenesis in cucumber, and a CsNF-YC2/-YC9-CsTIC21 model was proposed46.
Many studies have found that NF-Y transcription factors play an important role in plant growth and development and abiotic stress13. The analysis of the promoter cis-acting elements of the tobacco NF-Y gene family also found many cis-acting elements related to plant growth and development and stress response (Fig. 4). In this study, the expression patterns of NtNF-Y genes in different tissues (roots, stems and stem apexes) of tobacco were analyzed. The results showed that 54 genes were expressed in at least one tissue, among which NtNF-YA4/A7/A11/A13/A14/A15/A16 and NtNF-YC16 were highly expressed in three tissues, indicating that these eight genes may play an important role in the whole growth and development of tobacco, especially in the process of root growth (Fig. 7). Similarly, Tartary buckwheat also found that most of the FtNF-Y genes (63.15 %) were expressed in all tissues, and nearly half of the FtNF-Y genes (44.74 %) were highly expressed in roots47. AtNF-YA2 and AtNF-YA10 are related to root development in Arabidopsis13,48. In the phylogenetic tree, NtNF-YA13 and NtNF-YA15 were clustered with AtNF-YA2 and AtNF-YA10 in the same branch (Fig. 2), and NtNF-YA13 and NtNF-YA15 had higher expression levels in roots (Fig. 7), indicating that NtNF-YA13 and NtNF-YA15 may be potential candidate genes involved in root development. In Brassica napus, the expression of most BnNF-Y genes was up-regulated under drought treatment49,50. In peach, nine PpNF-YA genes were identified to be up-regulated for expression under drought stress, among which PpNF-YB2 and PpNF-YA5 were drought-resistant candidates20. In addition, studies in maize, tea and Z. jujuba also showed that NF-Y genes play an important role in drought stress response30,51,52. In this study, transcriptome analysis under drought stress showed that the expression of seven NtNF-Y genes was up-regulated and the expression of five genes was down-regulated (Fig. 8). Among them, NtNF-YA6/A7/A8/A12/A13 had higher homology with drought-related NF-Y genes in Arabidopsis and rice (Fig. 2), indicating that NtNF-YA6/A7/A8/A12/A13 may play an important role in tobacco resistance to drought stress7,11,33,34. Transcriptome analysis under different abiotic stresses showed that multiple NtNF-Y genes responded to two or more abiotic stresses at the same time. Similarly, multiple BnNF-Y and MsNF-Y genes were found to respond to a variety of abiotic stresses in Brassica napus and alfalfa49,50,53. This indicated that the functions of NtNF-Y genes may be diverse. These NF-Y genes were widely involved in the growth and development and stress resistance of tobacco, which were worthy of further study. In the future, it is expected to cultivate tobacco multi-resistant high-quality germplasm through new technologies such as CRISPR gene editing technology to improve the quality of tobacco leaves54.
Conclusions
In this study, based on the whole genome of Nicotiana tabacum, a total of 63 tobacco NF-Y genes were identified, including 17 NF-YAs, 30 NF-YBs, and 16 NF-YCs. Their gene structure and protein characteristics were analyzed, and their phylogeny, promoter cis-acting elements, protein 3D structure and protein interaction network, as well as expression analysis in plant tissues and under abiotic stresses were investigated. The NtNF-Y genes contained numerous cis-acting elements associated with hormone, stress, and light responses. NtNF-YB9/B14/B15/B16/B17/B29 were tissue-specific and specifically expressed in roots. 15, 12, 5, and 6 NtNF-Y genes responded to cold stress, drought stress, salt stress, and alkali stress, respectively, and several NtNF-Y genes functioned under two or more stresses. In conclusion, this study laid a foundation for further study on the structure and function of NF-Y gene family in tobacco, and provided rich genetic resources for tobacco variety improvement.
Methods
Identification of NF-Y gene family members in tobacco
Genome data of Nicotiana tabacum L. cv. TN90 were available from the NCBI (https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_000715135.1/)55. The amino acid sequences of 30 NF-Y genes (10 NF-YAs, 10 NF-YBs, 10 NF-YCs) in Arabidopsis thaliana were obtained from the Arabidopsis Information Resource (TAIR, https://www.arabidopsis.org/)56. The amino acid sequences of 34 NF-Y genes (11 NF-YAs, 11 NF-YBs, 12 NF-YCs) in rice (Oryza sativa L.) and 59 NF-Y genes (10 NF-YAs, 29 NF-YBs, 20 NF-YCs) in tomato (Solanum lycopersicum L.) were downloaded from Plant Transcription Factor Database (PlantTFDB, http://planttfdb.gao-lab.org/)57.
The Hidden Markov Model (HMM) for NF-YA(PF02045) and NFY-B/C(PF00808) were downloaded from the pfam database in InterPro (https://www.ebi.ac.uk/interpro/entry/pfam/)58, respectively. The genome-wide protein sequences of tobacco were searched for genes containing NF-Y conserved domains using HMMER v3.159, and these genes were screened based on a certain E-value (< 1× 10-10). The specific NF-YA HMM and NF-YB/C HMM in tobacco were constructed by using hmmbuild in HMMER v3.1. Using the new tobacco-specific HMM, the whole genome protein sequence of tobacco was searched again by using HMMER v3.1, and all genes with E-value less than 0.01 were selected. The amino acid sequences of 30 NF-Y genes in Arabidopsis thaliana were used for Blast (E-value= 1e−5) in the tobacco genome protein sequence to search for potential NF-Y gene family members in tobacco. The candidate NF-Y members obtained by the above two methods were combined. The conserved domains of these genes were identified using the online tool NCBI Batch CD-search (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi)60. The genes without related domains were removed. When multiple transcripts existed for the same gene, the longest transcript was selected as the NF-Y gene. Finally, the candidate tobacco NF-Y genes were obtained and named.
Analysis of physicochemical property and prediction of subcellular localization
Using the protein sequence of tobacco NF-Y, various properties of the protein, such as theoretical isoelectric point, amino acid number, instability coefficient, molecular weight, etc., were analyzed through the ExPASy-ProtParam website (https://web.expasy.org/protparam/)61. The subcellular localization of tobacco NF-Y proteins was predicted by Cell-PLoc 2.0 (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/)62,63.
Multiple alignment and construction of phylogenetic tree
Multiple alignments of NF-Y protein sequences in tobacco, Arabidopsis, rice and tomato were performed using ClustalX v2.164. The phylogenetic tree was constructed by the Neighbor-Joining (NJ) method through MEGA765, the P-distance model was selected, the Bootstrap value was set to 1000, and Pairwise Deletion was selected for gap processing. The phylogenetic tree was beautified using iTOL (https://itol.embl.de/)66. The results of multiple comparisons were embellished using GeneDoc.
Analysis of gene structure, domains, and conserved motifs
Gene structures of NtNF-Y gene family members were analyzed from tobacco genome annotation files by TBtools67. The conserved domains of NF-Ys were identified using NCBI Batch CD-search (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi). Conserved motifs of the NtNF-Y protein were identified via the MEME website (https://meme-suite.org/)68 with a maximum Motifs number of 10 and other parameters by default. The results were visualized using TBtools.
Cis-acting element analysis of promoters
The upstream 2000 bp sequences of the NtNF-Y genes were extracted from the tobacco genome and its annotation file using TBtools. The cis-elements of the NtNF-Y genes promoter were predicted using the PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)69 and the heat map was drawn by R package.
Homologous modeling of 3D protein structure and protein–protein interaction (PPI) network analysis
The tertiary structure of the NtNF-Y protein was predicted from the protein sequence of the NF-Y gene by homology modeling at the online website SWISS-MODEL (https://swissmodel.expasy.org/interactive/)70.
The NtNF-Y protein interaction network was constructed using NtNF-Y protein sequences by STRING (Search Tool for the Retrieval of Interacting Genes / Proteins, Version 11.5, https://string-db.org/)71. The disconnected nodes in the network were hidden. Medium confidence (0.400) was chosen as the minimum required interaction score. The protein interaction network was visualized by Cytoscape v3.7.272.
Transcription data analysis
The raw transcriptome sequencing data of Nicotiana tabacum under low temperature stress (SRP097876), alkali stress (NaHCO3 treatment, SRP193166), salt stress (NaCl treatment, SRP193166), drought stress (SRP399263) and different plant tissues (SRP101432) were downloaded from the Sequence Read Archive database (SRA, https://www.ncbi.nlm.nih.gov/sra)73 through the prefetch command in the SRA Toolkit.
Transcriptome sequencing data in sra format were converted to fastq format by the fastq-dump command in SRA Toolkit. The raw data were quality-checked with FastQC and then removed the adapter and cut off the first 12 bases of reads using Trimmomatic74 to get clean reads. The genome annotation file of tobacco was converted from gff format to gtf format by GffRead75 as an input file for the StringTie software76. Tobacco genome index was constructed and clean reads were aligned to the tobacco reference genome by using HISAT277 to generate the corresponding sam files. Convert sam files to the reordered bam files using Samtools78. Through the StringTie software, the reordered bam file was used as the input file, and the gtf file of the tobacco genome was used to assist the assembly to obtain the gene abundance file and the assembled transcript GTF file. Then, the count values were obtained via the prepDE.py script provided by StringTie based on the assembled transcript GTF file obtained in the previous step. According to the gene count matrix obtained in the previous step, differentially expressed genes under different stresses were analyzed using R package DESeq279. The differently expressed genes screening standard was padj < 0.05 and | log2FoldChange | > 1. The heat map based on the value of log2 fold change was made by using the Heatmap program in TBtools.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Strader, L., Weijers, D. & Wagner, D. Plant transcription factors-being in the right place with the right company. Curr. Opin. Plant Biol. 65, 8. https://doi.org/10.1016/j.pbi.2021.102136 (2022).
Laloum, T., De Mita, S., Games, P., Baudin, M. & Niebel, A. CCAAT-box binding transcription factors in plants: Y so many?. Trends Plant Sci. 18, 157–166. https://doi.org/10.1016/j.tplants.2012.07.004 (2013).
Myers, Z. A. & Holt, B. F. NUCLEAR FACTOR-Y: Still complex after all these years?. Curr. Opin. Plant Biol. 45, 96–102. https://doi.org/10.1016/j.pbi.2018.05.015 (2018).
Nardone, V., Chaves-Sanjuan, A. & Nardini, M. Structural determinants for NF-Y/DNA interaction at the CCAAT box. Biochim. et Biophys. Acta (BBA) Gene Regul. Mech. 1860, 571–580. https://doi.org/10.1016/j.bbagrm.2016.09.006 (2017).
Chaves-Sanjuan, A. et al. Structural determinants for NF-Y subunit organization and NF-Y/DNA association in plants. Plant J. 105, 49–61. https://doi.org/10.1111/tpj.15038 (2021).
Nardini, M. et al. Sequence-specific transcription factor NF-Y displays histone-like DNA binding and H2B-like ubiquitination. Cell 152, 132–143. https://doi.org/10.1016/j.cell.2012.11.047 (2013).
Zhao, H. et al. The Arabidopsis thaliana nuclear factor Y transcription factors. Front. Plant Sci. 7, 11. https://doi.org/10.3389/fpls.2016.02045 (2017).
Xuanyuan, G. C. et al. Genome-wide screening and identification of nuclear Factor-Y family genes and exploration their function on regulating abiotic and biotic stress in potato (Solanum tuberosum L.). Gene 812, 16. https://doi.org/10.1016/j.gene.2021.146089 (2022).
Li, J. et al. Genome-wide analysis of the poplar NF-Y gene family and its expression in floral bud development of Populus tomentosa. Trees-Struct. Funct. 34, 285–296. https://doi.org/10.1007/s00468-019-01917-3 (2020).
Zhang, H. et al. Crucial abiotic stress regulatory network of NF-Y transcription factor in plants. Int. J. Mol. Sci. 24, 4426. https://doi.org/10.3390/ijms24054426 (2023).
Swain, S., Myers, Z. A., Siriwardana, C. L. & Holt, B. F. III. The multifaceted roles of NUCLEAR FACTOR-Y in Arabidopsis thaliana development and stress responses. Biochim. Biophys. Acta Gene Regul. Mech. 1860, 636–644. https://doi.org/10.1016/j.bbagrm.2016.10.012 (2017).
Zanetti, M. E., Ripodas, C. & Niebel, A. Plant NF-Y transcription factors: Key players in plant-microbe interactions, root development and adaptation to stress. Biochim. Biophys. Acta-Gene Regul. Mech. 1860 645–654. https://doi.org/10.1016/j.bbagrm.2016.11.007 (2017).
Kishor, P. B. K. et al. Nuclear factor-Y (NF-Y): Developmental and stress-responsive roles in the plant lineage. J. Plant Growth Regul. 42, 2711–2735. https://doi.org/10.1007/s00344-022-10739-6 (2023).
Liu, G. et al. TaMADS29 interacts with TaNF-YB1 to synergistically regulate early grain development in bread wheat. Sci. China Life Sci. 66, 1647–1664. https://doi.org/10.1007/s11427-022-2286-0 (2023).
Wang, J. et al. NF-Y plays essential roles in flavonoid biosynthesis by modulating histone modifications in tomato. New Phytol. 229, 3237–3252. https://doi.org/10.1111/nph.17112 (2021).
Deng, C. et al. MtNF-YC6 and MtNF-YC11 are involved in regulating the transcriptional program of arbuscular mycorrhizal symbiosis. Front. Plant Sci. 13, 16. https://doi.org/10.3389/fpls.2022.976280 (2022).
Huang, Y. et al. Expression patterns of the poplar NF-Y gene family in response to Alternaria alternata and hormone treatment and the role of PdbNF-YA11 in disease resistance. Front. Bioeng. Biotechnol. 10, 16. https://doi.org/10.3389/fbioe.2022.956271 (2022).
Wan, Q. et al. Genome-wide identification and abiotic stress response pattern analysis of NF-Y gene family in peanut (Arachis Hypogaea L.). Trop. Plant Biol. 14, 329–344. https://doi.org/10.1007/s12042-021-09295-2 (2021).
Liu, M. M. et al. Transcriptome-wide characterization, evolutionary analysis, and expression pattern analysis of the NF-Y transcription factor gene family and salt stress response in Panax ginseng. BMC Plant Biol. 22, 11. https://doi.org/10.1186/s12870-022-03687-6 (2022).
Li, M., Li, G., Liu, W., Dong, X. & Zhang, A. Genome-wide analysis of the NF-Y gene family in peach (Prunus persica L.). BMC Genom. 20, 612. https://doi.org/10.1186/s12864-019-5968-7 (2019).
Dana, M. M., Pintor-Toro, J. A. & Cubero, B. Transgenic tobacco plants overexpressing chitinases of fungal origin show enhanced resistance to biotic and abiotic stress agents. Plant Physiol. 142, 722–730. https://doi.org/10.1104/pp.106.086140 (2006).
Gao, Y. et al. NtRAV4 negatively regulates drought tolerance in Nicotiana tabacum by enhancing antioxidant capacity and defence system. Plant Cell Rep. 41, 1775–1788. https://doi.org/10.1007/s00299-022-02896-5 (2022).
Siefers, N. et al. Tissue-specific expression patterns of Arabidopsis NF-Y transcription factors suggest potential for extensive combinatorial complexity. Plant Physiol. 149, 625–641. https://doi.org/10.1104/pp.108.130591 (2009).
Petroni, K. et al. The promiscuous life of plant NUCLEAR FACTOR Y transcription factors. Plant Cell 24, 4777–4792. https://doi.org/10.1105/tpc.112.105734 (2012).
Yang, W. J., Lu, Z. H., Xiong, Y. F. & Yao, J. L. Genome-wide identification and co-expression network analysis of the OsNF-Y gene family in rice. Crop J. 5, 21–31. https://doi.org/10.1016/j.cj.2016.06.014 (2017).
Cheng, L. et al. Genome-wide identification and analysis of the class III peroxidase gene family in tobacco (Nicotiana tabacum). Front. Genet. 13, 916867. https://doi.org/10.3389/fgene.2022.916867 (2022).
Song, Z. et al. Genome-wide identification and expression analysis of HSP90 gene family in Nicotiana tabacum. BMC Genet. 20, 1–12. https://doi.org/10.1186/s12863-019-0738-8 (2019).
Bai, et al. Genome-wide identification, gene structure and expression analysis of the MADS-Box gene family indicate their function in the development of tobacco (Nicotiana tabacum L.). Int. J. Mol. Sci. 20, 5043. https://doi.org/10.3390/ijms20205043 (2019).
Li, W. et al. NAC family transcription factors in tobacco and their potential role in regulating leaf senescence. Front. Plant Sci. 9, 1900. https://doi.org/10.3389/fpls.2018.01900 (2018).
Panzade, K. P., Kale, S. S., Manoj, M. L., Kothawale, S. P. & Damse, D. N. Genome-wide analysis and expression profile of nuclear factor Y (NF-Y) gene family in Z. jujuba. Appl. Biochem. Biotechnol. 194, 1373–1389. https://doi.org/10.1007/s12010-021-03730-6 (2022).
Yang, J., Zhu, J. H. & Yang, Y. X. Genome-wide identification and expression analysis of NF-Y transcription factor families in watermelon (Citrullus lanatus). J. Plant Growth Regul. 36, 590–607. https://doi.org/10.1007/s00344-017-9670-1 (2017).
Yan, H. L. et al. Genome-wide identification, characterization and expression analysis of NF-Y gene family in relation to fruit ripening in banana. Postharvest Biol. Technol. 151, 98–110. https://doi.org/10.1016/j.postharvbio.2019.02.002 (2019).
Lee, D. K. et al. The NF-YA transcription factor OsNF-YA7 confers drought stress tolerance of rice in an abscisic acid independent manner. Plant Sci. 241, 199–210. https://doi.org/10.1016/j.plantsci.2015.10.006 (2015).
Najafabadi, M. S. Improving rice (Oryza sativa L.) drought tolerance by suppressing a NF-YA transcription factor. Iran. J. Biotechnol. 10, 40–48 (2012).
Kumimoto, R. W. et al. The nuclear factor Y subunits NF-YB2 and NF-YB3 play additive roles in the promotion of flowering by inductive long-day photoperiods in Arabidopsis. Planta 228, 709–723. https://doi.org/10.1007/s00425-008-0773-6 (2008).
Hwang, Y. H. et al. Functional conservation of rice OsNF-YB/YC and Arabidopsis AtNF-YB/YC proteins in the regulation of flowering time. Plant Cell Rep. 35, 857–865. https://doi.org/10.1007/s00299-015-1927-1 (2016).
Kumimoto, R. W., Zhang, Y., Siefers, N. & Holt, B. F. NF-YC3, NF-YC4 and NF-YC9 are required for CONSTANS-mediated, photoperiod-dependent flowering in Arabidopsis thaliana. Plant J. 63, 379–391. https://doi.org/10.1111/j.1365-313X.2010.04247.x (2010).
Hackenberg, D., Keetman, U. & Grimm, B. Homologous NF-YC2 subunit from Arabidopsis and tobacco is activated by photooxidative stress and induces flowering. Int. J. Mol. Sci. 13, 3458–3477. https://doi.org/10.3390/ijms13033458 (2012).
Kim, S. K. et al. OsNF-YC2 and OsNF-YC4 proteins inhibit flowering under long-day conditions in rice. Planta 243, 563–576. https://doi.org/10.1007/s00425-015-2426-x (2016).
Das, S., Parida, S. K., Agarwal, P. & Tyagi, A. K. Transcription factor OsNF-YB9 regulates reproductive growth and development in rice. Planta 250, 1849–1865. https://doi.org/10.1007/s00425-019-03268-2 (2019).
Su, H. et al. Identification of ZmNF-YC2 and its regulatory network for maize flowering time. J. Exp. Bot. 72, 7792–7807. https://doi.org/10.1093/jxb/erab364 (2021).
Wang, R. K., Zhu, L., Zhang, Y., Fan, J. F. & Li, L. L. Genome-wide analysis of poplar NF-YB gene family and identified PtNF-YB1 important in regulate flowering timing in transgenic plants. BMC Plant Biol. 19, 9. https://doi.org/10.1186/s12870-019-1863-2 (2019).
Miyoshi, K., Ito, Y., Serizawa, A. & Kurata, N. OsHAP3genes regulate chloroplast biogenesis in rice. Plant J. 36, 532–540. https://doi.org/10.1046/j.1365-313X.2003.01897.x (2003).
Myers, Z. A. et al. NUCLEAR FACTOR Y, subunit C (NF-YC) transcription factors are positive regulators of photomorphogenesisin Arabidopsis thaliana. Plos Genet. 12, 30. https://doi.org/10.1371/journal.pgen.1006333 (2016).
Tang, Y. et al. Arabidopsis NF-YCs mediate the light-controlled hypocotyl elongation via modulating histone acetylation. Mol. Plant. 10, 260–273. https://doi.org/10.1016/j.molp.2016.11.007 (2017).
Ke, X. et al. Cucumber NUCLEAR FACTOR-YC2/-YC9 target translocon component CsTIC21 in chloroplast photomorphogenesis. Plant Physiol. 192, 2822–2837. https://doi.org/10.1093/plphys/kiad296 (2023).
Yan, H. L. et al. Genome-wide analysis of the NF-Y gene family and their roles in relation to fruit development in Tartary buckwheat (Fagopyrum tataricum). Int. J. Biol. Macromol. 190, 487–498. https://doi.org/10.1016/j.ijbiomac.2021.09.001 (2021).
Sorin, C. et al. A miR169 isoform regulates specific NF-YA targets and root architecture in Arabidopsis. New Phytol. 202, 1197–1211. https://doi.org/10.1111/nph.12735 (2014).
Wang, J., Jin, Z. Y., Zhou, M. J., Yu, Y. J. & Liang, M. X. Characterization of NF-Y transcription factor families in industrial rapeseed (Brassica napus L.) and identification of BnNF-YA3, which functions in the abiotic stress response. Ind. Crop. Prod. 148, 15. https://doi.org/10.1016/j.indcrop.2020.112253 (2020).
Xu, L. et al. Multiple NUCLEAR FACTOR Y transcription factors respond to abiotic stress in Brassica napus L. PLoS One 9, 11. https://doi.org/10.1371/journal.pone.0111354 (2014).
Cao, L. et al. Genome-wide identification of NF-Y gene family in maize (Zea mays L.) and the positive role of ZmNF-YC12 in drought resistance and recovery ability. Front. Plant Sci. 14, 1159955. https://doi.org/10.3389/fpls.2023.1159955 (2023).
Wang, P. J. et al. Identification, expression, and putative target gene analysis of nuclear factor-Y (NF-Y) transcription factors in tea plant (Camellia sinensis). Planta 250, 1671–1686. https://doi.org/10.1007/s00425-019-03256-6 (2019).
An, Y. X., Suo, X., Niu, Q. C., Yin, S. X. & Chen, L. Genome-wide identification and analysis of the NF-Y transcription factor family reveal its potential roles in salt stress in Alfalfa (Medicago sativa L.). Int. J. Mol. Sci. 23, 20. https://doi.org/10.3390/ijms23126426 (2022).
Chen, K. L., Wang, Y. P., Zhang, R., Zhang, H. W. & Gao, C. X. CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu. Rev. Plant Biol. 70, 667–697. https://doi.org/10.1146/annurev-arplant-050718-100049 (2019).
Sayers, E. W. et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 50, D20–D26. https://doi.org/10.1093/nar/gkab1112 (2022).
Lamesch, P. et al. The Arabidopsis Information Resource (TAIR): Improved gene annotation and new tools. Nucleic Acids Res. 40, D1202–D1210. https://doi.org/10.1093/nar/gkr1090 (2012).
Tian, F., Yang, D. C., Meng, Y. Q., Jin, J. P. & Gao, G. PlantRegMap: Charting functional regulatory maps in plants. Nucleic Acids Res. 48, D1104–D1113. https://doi.org/10.1093/nar/gkz1020 (2020).
Paysan-Lafosse, T. et al. InterPro in 2022. Nucleic Acids Res. 51, D418–D427. https://doi.org/10.1093/nar/gkac993 (2023).
Finn, R. D., Clements, J. & Eddy, S. R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 39, W29-37. https://doi.org/10.1093/nar/gkr367 (2011).
Lu, S. et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 48, D265–D268. https://doi.org/10.1093/nar/gkz991 (2020).
Gasteiger, E. et al. Protein Identification and Analysis Tools on the Expasy Server 571–607 (Humana Press, 2005).
Chou, K. C. & Shen, H. B. Plant-mPLoc: A top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS One 5, e11335. https://doi.org/10.1371/journal.pone.0011335 (2010).
Chou, K. C. & Shen, H. B. Cell-PLoc: A package of Web servers for predicting subcellular localization of proteins in various organisms. Nat. Protoc. 3, 153–162. https://doi.org/10.1038/nprot.2007.494 (2008).
Larkin, M. A. et al. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948. https://doi.org/10.1093/bioinformatics/btm404 (2007).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. https://doi.org/10.1093/molbev/msw054 (2016).
Letunic, I. & Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293–W296. https://doi.org/10.1093/nar/gkab301 (2021).
Chen, C. et al. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 13, 1194–1202. https://doi.org/10.1016/j.molp.2020.06.009 (2020).
Bailey, T. L. & Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 2, 28–36 (1994).
Lescot, M. et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 30, 325–327. https://doi.org/10.1093/nar/30.1.325 (2002).
Waterhouse, A. et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303. https://doi.org/10.1093/nar/gky427 (2018).
Szklarczyk, D. et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 51, D638–D646. https://doi.org/10.1093/nar/gkac1000 (2023).
Shannon, P. et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504. https://doi.org/10.1101/gr.1239303 (2003).
Katz, K. et al. The sequence read archive: A decade more of explosive growth. Nucleic Acids Res. 50, D387–D390. https://doi.org/10.1093/nar/gkab1053 (2022).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. https://doi.org/10.1093/bioinformatics/btu170 (2014).
Pertea, G. & Pertea, M. GFF utilities: GffRead and GffCompare. F1000Research https://doi.org/10.12688/f1000research.23297.1 (2020).
Pertea, M. et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 33, 290–295. https://doi.org/10.1038/nbt.3122 (2015).
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37, 907–915. https://doi.org/10.1038/s41587-019-0201-4 (2019).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079. https://doi.org/10.1093/bioinformatics/btp352 (2009).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 1–21. https://doi.org/10.1186/s13059-014-0550-8 (2014).
Acknowledgements
This research was funded by the Foundation of Innovation Team Project for Modern Agricultural Industrious Technology System of Shandong Province (SDAIT-25-01).
Author information
Authors and Affiliations
Contributions
L.Y. and K.S. conceived and designed the study and revised the manuscript. Y.T., X.Z. and H.L. collected important background information. K.S. and B.L. conducted transcriptome data processing. Y.T. and Y.S. conducted data analysis and Y.T. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tian, Y., Song, K., Li, B. et al. Genome-wide identification and expression analysis of NF-Y gene family in tobacco (Nicotiana tabacum L.). Sci Rep 14, 5257 (2024). https://doi.org/10.1038/s41598-024-55799-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55799-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.