Abstract
With the rapid spread of wireless technologies and increasing electromagnetic energy, electromagnetic waves (EMW) have become a severe threat to human health. Therefore, minimizing the harmful effects of electromagnetic wave radiation is possible through the development of high-efficiency EMW absorption coatings. The aim of this work was to generate microwave absorbance coatings containing synthesized nano-CuFe2O4 and nano-CaFe2O4. Firstly, nano-CuFe2O4 and nano-CaFe2O4 were synthesized using the sol–gel method. Then, their structure, electrical, dielectric, and magnetic properties were investigated to find out the possibility of using these materials in high-frequency applications (e.g., microwave absorbance coatings). After that, two dosages (2.5 wt% and 5 wt%) of nano-CuFe2O4 and nano-CaFe2O4 were incorporated into epoxy resin to prepare modified epoxy resin as microwave coatings. The dielectric studies show that the AC conductivity of the prepared samples is high at high frequencies. Additionally, the magnetic properties reveal a low coercivity value, making these samples suitable for high-frequency devices. The microwave results illustrate that adding nano-ferrites with high content enhances the absorption characteristics of the tested films. The results showed that the two films have two absorption bands with RL < –10 dB ranging from 10.61 to 10.97 GHz and from 10.25 to 11.2 GHz. The minimum return loss achieved for the two cases is −13 and −16 dB, respectively. Indicating that the film coated with CuFe has a better absorption value than the one coated with CaFe.
Similar content being viewed by others
Introduction
Recently, electromagnetic radiation has become widespread due to the rapid progress of wireless communication technology, particularly the explosive expansion of 5G and human over-reliance on many smart devices. As a result, the problem of electromagnetic pollution is getting more severe1,2,3,4,5. As a result, the problem of electromagnetic pollution is getting more severe. An excess of electromagnetic waves can cause electromagnetic disturbances, which can impede signal transmission and potentially disrupt the regular operation of electronic and intelligent infrastructure6,7,8,9. Even more concerning, studies show that prolonged exposure to high electromagnetic wave density is harmful to health and increases the threat of disease1. Most researchers agree that developing materials that absorb electromagnetic waves is a simple and effective way to minimise electromagnetic pollution.
Microwave absorption materials (MAMs) are useful materials capable of absorbing electromagnetic radiation and microwaves because of their low reflection and dispersion coefficients. The fundamental method of operation for microwave absorbers is to first transform microwave energy via a specific physical mechanism into a different kind of energy, and then to use a dissipation motion to transform that new type of energy back into heat energy. A high-performance microwave absorber must have a broad bandwidth and excellent absorption10,11. These materials are essential for a wide range of military and civil applications, such as healthcare, warfighter anti-radar monitoring, national defense security, and electronic reliability. The rapid development of information technology has led to a boom in the research of nanomaterials for microwave absorption applications in recent years. As a result, there has been a lot of interest in the development of high-performance MAMs at the nanoscale that are low-cost, have a broad bandwidth, and exhibit robust absorption10.
Ferrites are one of the most essential nanomertrais types that have attracted a lot of attention recently. Spinel ferrites are composite metal oxides with ferric ions that are crucial for industries. Their usual structural formula is MFe2O4, where M is a divalent metal ion like Zn2+, Co2+, Ni2+, Mg2+, Fe2+, Mn2+ etc., whose ionic radius varies from 0.6 to 1 Å12,13. Spinel ferrites have superior electric and magnetic properties, surface active sites, a large specific surface area, outstanding chemical stability, variable size and form, and varied applicability in every field of science and technology, among other distinctive physicochemical characteristics14,15. Moreover, high magnetic permeability, resistivity, excellent eddy current environmental friendliness, favorable mechanical properties, and magnetic loss are among the advantages of ferrite-based microwave absorbing materials, which are primarily employed to disperse electromagnetic radiation16.
On the other hand, radar-absorbing coatings with the benefits of easy production, high bonding force, and easy control of electromagnetic parameters are one of the most effective approaches to radar stealth technology to minimize the pollution of electromagnetic radiation17,18,19,20. It is well known that absorbing coatings are usually composed of MAM and an insulator matrix. MAMs are responsible for absorbing the EM wave, while the insulator matrix was to shield MAM and keep the shape of the radar-absorbing coatings21,22. Recently, epoxy coatings containing ferrites have attracted a lot of interest as potential microwave-absorption candidates with strong microwave-absorbing capabilities16. However, to the best of our knowledge, few works were pertaining to study the epoxy coatings containing ferrites as microwave absorbing coatings.
The aim of this work was to generate microwave absorbance coatings containing synthesized nano-CuFe2O4 and nano-CaFe2O4. After synthesis step, their structure, electrical, dielectric, and magnetic properties are investigated to find out the possibility of using these materials in high-frequency applications (e.g. microwave absorbance coatings). Then, two dosages (2.5 wt%, and 5 wt%) of nano-CuFe2O4 and nano-CaFe2O4 were incorporated into epoxy resin using ball-mill to prepare modified epoxy resin as microwave coatings. Thereafter, the return loss of four films coated with different contents of nano-ferrites is measured using VNA to check the microwave absorption of the proposed material. Also, the electromagnetic features of these samples are measured in terms of complex permittivity using Keysight Dielectric probe kit N1501A.
Experimental part
Materials
Calcium, copper, and ferric nitrates were obtained from WinLab, UK. Cetyltrimethylammonium bromide (CTAB) and sodium hydroxide were obtained from Adwic Co., Egypt. Epoxy resin consists of bisphenol A and polyamide as the curing agents. This ratio was obtained from Kemapoxy, Egypt. All the used extenders and solvents were supplied from local and international companies with normal chemical grades.
Preparation of the prepared nano-ferrites
As presented in Fig. 1, Fe(NO3)3⋅9H2O (0.2 M) and Ca(NO3)3⋅4H2O (0.1 M) were stirred at ambient temperature to prepare nano-CaFe2O4. Also, Fe(NO3)3⋅9H2O (0.2 M) and Cu(NO3)3⋅3H2O (0.1 M) were mixed to prepare nano-CaFe2O4. Then, 5% (w/v) CTAB was added to the two mixtures as a dispersing agent with continuous stirring at high speed. After that, 2 N NaOH was gradually added while being stirred frequently until the pH reached 8. Following thorough precipitation, the precipitates were cleaned with ethanol and deionized water before being set aside to dry at 80 °C. The dried dark red powders were then annealed at 550 °C in a muffle.
Characterization of the prepared nano-ferrites
The morphology and elemental composition of the synthesized nano-ferrites were investigated using scanning electron microscope connected to energy dispersive X-ray spectroscopy (SEM/EDX-JEOL JED 2300). Transmission electron microscope (TEM-JEOL JX 1230) was used to determine the particle shapes and sizes of the nano-ferrites. X-ray powder diffraction patterns (XRD) were investigated at ambient temperature using a Philip’s diffractometer (Model PW1390), employing Ni-filtered Cu Kα radiation (λ = 1.5404 Å). The diffraction angle, 2θ, was scanned at a rate of 2°/min. Particle Sizing Systems\ZPW388 obtained from Santa Barbara, Calif., USA was used. X-ray photoelectron microscopy (XPS) was performed by (KRATOS-AXIS) Ultra spectrometer. The magnetic properties were performed at room temperature using a vibrating sample magnetometer (VSM; Lake Shore, USA), and the maximum magnetic field is 20 KG.
The dielectric and electrical properties of the nano-ferrites were carried out over a frequency range (10–1 to 107) and at ambient temperature. A high-resolution alpha analyzer (Novocontrol Technologies, GmbH & Co. KG) is employed to achieve these measurements. For measurements, a disc of the sample is sandwiched between two gold-plated brass electrodes of 10 mm in diameter in parallel plate geometry. The complex dielectric function was obtained by:
where ε' is a real part and ε'' is an imaginary part or dielectric loss, i = √−1, and ν is the frequency. The complex conductivity σ* = σ' + iσ" and electric modulus M* = M' + iM" are determined and interpreted according to their relationships, as detailed in23,24. The frequency dependence of the three parameters (ε′, M″, and σ′) at room temperature will be considered here. These three parameters are interrelated to each other according to:
which,
Production of coatings formulations containing nano-ferrites
In this work, a ball mill was used to prepare epoxy resin composite coating according to the proportion of nano-ferrites to epoxy resin mass fractions of 2.5% and 5%. Firstly, nano-CaFe2O4 or nano-CuFe2O4 was dispersed in a mixed solution of xylene and N-butanol (weight ratio of 7:3) using ultrasonic for 0.5 h. After that, the dispersed solution was mixed with the epoxy in a ball mill for 1 h. Finally, four paint formulations based on epoxy containing nano-CaFe2O4 or nano-CuFe2O4 at 2.5 and 5%, which were denoted as CaFe (2.5%), CaFe (5%), CuFe (2.5%), and CuFe (5%), were obtained.
Methods of testing and evaluation of coating
Several ASTM standards, including hardness (ASTM D 6577), ductility (ASTM D 5638), impact resistance (ASTM D 2794), and pull-off strength (ASTM D 4541), were used to evaluate the coated films' elasticity, strength, and flexibility.
Microwave setup
\(\tan \delta = {{\varepsilon ^{\prime}} \mathord{\left/ {\vphantom {{\varepsilon ^{\prime}} {\varepsilon ^{\prime\prime}}}} \right. \kern-0pt} {\varepsilon ^{\prime\prime}}}\) The ability of materials to absorb microwaves is directly linked to their electromagnetic characteristics. To investigate the absorption properties of microwaves, the electromagnetic features of coatings containing nano-CaFe2O4 and nano-CuFe2O4 were measured using SPEAG-DAK 3.5 (200 MHz to 20 GHz). In this work, one thickness (1.5 mm) was used, according to the literature25, to examine only the effect of both concentrations on the absorption properties of microwaves. These measurements included the samples' relative complex permittivity, Eq. (1), which is made up of real and imaginary parts. The real part represents the storage capacity of dielectric energy, while the imaginary part indicates the dissipation of dielectric. The dielectric loss tangent is used to describe the electromagnetic wave absorption properties of the absorbers. The reflection coefficients of rubber absorber sheets were measured using a transmission line technique in the 8–12 GHz frequency range at room temperature. A rectangular sample of rubber was placed in an aluminium specimen holder that connected the two waveguide sections, each 60 mm in length. The two waveguide sections were then connected to two ports of a Rhode and Schwartz model ZVA67 VNA vector network analyzer through two waveguide adaptors, as shown in Fig. 2. A full two-port transmission-reflection-line (TRL) calibration was performed to eliminate any loss due to the sample holder9. From the measured S11, the reflection loss (RL) was calculated as:
Results and discussion
X-ray diffraction (XRD)
Figure 3 depicts the XRD patterns for nano-ferrites. According to the standard XRD data of nano-CaFe2O4 (JCPDS Card No. 78-4321), the distinctive diffraction peaks of nano-CaFe2O4 were observed at 2θ = 20°–70°26. The common nano-CuFe2O4 peaks are situated at 2θ = 30.1°, 35.6°, 38.7°, 42.8°, 47.3°, 57.1°, 62.95°, and 71.2°, and they correspond perfectly with the standard CuFe2O4 XRD data cart (JCPDS Card No. 034-0425)27. Furthermore, the Scherrer equation was used to examine the average crystal size of both nano-ferrites as follows:
where K = 0.89 is the Scherer constant, β is the width of the peak, θ is the Braggs diffraction angle, and λ is the wavelength of the X-ray28. The average crystal size of nano-CaFe2O4 was calculated to be 42.4 nm, while nano-CuFe2O4 was found to be 46.2 nm, clearly indicating that the synthesized nano-ferrites are of nano-scale.
To examine the morphology and particle size of both nano-CaFe2O4 and nano-CuFe2O4, TEM and SEM were employed. TEM in Fig. 4a, b demonstrates that nano-CaFe2O4 has a particle size range of 8.18 to 25.7 nm and nano-CuFe2O4 has a particle size range of 13.37 to 33.70 nm. Additionally, the aggregation of nano-CaFe2O4 and nano-CuFe2O4 is made clear by TEM images, which may be triggered by the high surface energy and magnetic forces that occur between nanoparticles. On the other hand, SEM micrographs of synthesized nano-ferrites show their spinel structure, as seen in Fig. 4c, d.
Energy dispersive X-ray analysis (EDX)
EDX analysis was employed to figure out the constituent elements of nano-CaFe2O4 and nano-CuFe2O4. According to an EDX investigation of the spectrum shown in Fig. 4e, f, the Fe, Ca, and O elements have been identified, which confirms the generation of nano-CaFe2O4. Additionally, EDX for nano-CuFe2O4 precisely determines the presence of Cu, Fe, and O.
Zeta potential
Figure 5 demonstrates the zeta potential of nano-CaFe2O4 and nano-CuFe2O4. Zeta potential is widely recognized for providing insight on the stability of nanoparticles in the media in which they are dispersed. The particle is considered stable if the potential is within the range of + 30 to −30 mV. The figure illustrates that both nano-ferrites fall within the indicated range, demonstrating that they are stable and do not aggregate under the intended usage conditions29.
X-ray photoelectron spectroscopic (XPS) analysis
The possible valence states of each cation in nano-CuFe2O4 and nano-CaFe2O4 have been determined by performing XPS analysis. Figure 5b is the full XPS spectra of nano-CuFe2O4 and nano-CaFe2O4. The XPS spectra exhibit characteristic peaks at binding energies representing C 1s, O 1s, Fe 2p, Ca 2p, and Cu 2p. The Fe 2p3/2 and Cu 2p3/2 spectra can be well-fitted by two synthetic curves, indicating two possible valences for each ion, as shown in Fig. 5c, d. In Fig. 5c, two peaks are shown at binding energies of 710.02 and 709.9 eV for the Fe 2p3/2 line in nano-CuFe2O4 and nano-CaFe2O4, respectively. These peaks are attributed to Fe2+. On the other hand, the peaks at binding energies of 712.63 and 711.78 eV in both nano-CuFe2O4 and nano-CaFe2O4, respectively, are attributed to Fe3+30,31. Figure 5d displays two peaks at binding energies of 933.2 and 936.06 eV for the Cu 2p3/2 line, which are attributed to Cu1+ and Cu2+, respectively30,32. As shown in Fig. 5e, there are two peaks at 350.14 and 346.62 eV, corresponding to Ca 2p1/2 and Ca 2p3/2, respectively, demonstrating the presence of Ca2+33,34,35.
Dielectric and electrical investigations
The real part of complex permittivity, ε', is illustrated graphically as a function of frequency for CaFe2O4 and CuFe2O4 at room temperature in Fig. 6a. Three different trends are clearly shown in the figure. The lower range of frequencies (0.1–10 Hz) shows a clearly linear increase with decreasing frequency. This originated from the contribution of charge carriers’ transport, which causes ac conductivity. Further increases in frequency show shoulder-like behavior in the intermediate range of frequencies. This behavior may be attributed to the well-known interfacial polarization due to the accumulation of some ions at the interfaces at the borderers between different components and their heterogeneous structures36.
Similar behavior was also shown on the frequency dependence of dielectric loss ε′′(f), as illustrated in Fig. 6b. This confirms that both parameters are not independent. At the higher limit of the frequency window (≥ 10 MHz), the permittivity values of both compositions collapse together and become independent of frequency. This can be explained according to the fact that the alteration of all kinds of polarizations lags behind the frequency of the externally applied electric field. This phenomenon became well known in many composites recently37.
The representation of the imaginary part of the electric modulus M''(f) is usually used in dielectric characterization because it suppresses undesirable capacitance effects due to electrode contacts and provides a clear view of the DC conduction and dipole relaxation36,37,38. Figure 7 illustrates graphically the imaginary part of the electric modulus as a function of frequency. The two investigated processes are clear peaks in this representation. Both processes seem to be faster in the case of the CuFe2O4 composite. This may indicate that the degree of freedom of the Cu ions is higher than that of the calcium ions.
Generally, this investigated behavior can be explained on the basis of the Maxwell–Wagner bi-layered model, which is in agreement with Koop's dispersion phenomenological theory39,40. In this model, the dielectric material is composed of two layers. One of them is the grain boundary, which is a conductive layer, and the other is the grain boundary, which is a poorly conductive layer. The grain boundary is more active at lower frequencies, while its activity becomes less and the grain's activity dominates at higher frequencies. This is due to the movement of charge carriers (electrons) in the material that can move in the direction of the electric field by hopping mechanisms to the grain boundary at lower frequencies. The charge carriers accumulate at the grain boundary (a poorly conductive layer), forming space-charge polarization and causing the high value of the dielectric constant. As the frequency increases, the probability of reaching the grain boundary by the charge carriers is weak because they cannot follow the direction of the applied electric field, which thus causes the dielectric constant to decrease until it reaches a constant value40,41,42.
In the presented study, ε' has a high value at lower frequencies because the electron hopping between cations (Fe3+ ↔ Fe2+ in both the CaFe2O4 and CuFe2O4 samples besides Cu2+ ↔ Cu1+ in the CuFe2O4 sample as confirmed from XPS) takes place in the direction of the applied field, whereas it has a low value at higher frequencies due to the electron hopping between cations not following the alternating field41,43. Additionally, CuFe2O4 displayed improved dielectric properties compared to CaFe2O4 over the frequency range. This refers to the increase in charge carrier number that decreases the resistance of the sample, and consequently, the polarization increases, resulting in a high value of ε'40,44,45.
The dependence of AC electrical conductivity on frequency for CaFe2O4 and CuFe2O4 at room temperature is shown in Fig. 8. It is observed that the conductivity increases as the frequency increases for the investigated samples due to the increased field applied to the charge carriers, which causes an increase in their hopping or tunnelling46. In ferrites, the electrical conductivity is due to the exchange of electrons between ions of the same element that have different valence states41,47. At low frequencies, the conductivity is low because of the grain boundary effect that reduces hopping electrons between Fe3+ and Fe2+. In contrast, the effect of the grain boundary becomes less at high frequencies, and the grain effect is dominant, and leading to hopping electrons41. Also, the AC conductivity of CuFe2O4 is higher than that of CaFe2O4 owing to the cationic distribution in the samples and the flow of charge carriers because of the grain effect, which is more active than the grain boundary effect44,47.
From the obtained results, it can be concluded that the real part of the complex permittivity and dielectric loss of the prepared nano-ferrites showed the dielectric behavior of these samples. Therefore, they can be used as dielectric materials in low-frequency devices. In addition, the AC conductivity of these samples has high values at high frequencies, making them also suitable for high-frequency devices48.
Magnetic properties
Figure 9 shows the magnetic hysteresis plots of nano-CaFe2O4 and nano-CuFe2O4 at room temperature. The inset figure illustrates the magnified vision of the low-field magnetization region. The S shape in the hysteresis loop for the examined samples indicates the ferromagnetic behavior of the samples, which is somewhat strong for nano-CuFe2O4 while weak for nano-CaFe2O4. The values of magnetic parameters as coercivity, Hc, remanent magnetization, retentivity, Mr, saturation magnetization, Ms, and squarence ratio, Mr/Ms, are calculated from hysteresis plots and tabulated in Table 1.
It is noticed that the value of Ms for nano-CuFe2O4 is higher than that of nano-CaFe2O4. The coercivity, Hc, of the samples is very low, which means that these samples can be considered soft magnets49,50. Moreover, the low value of coercivity indicates the negligible hysteresis loss of microwave energy which makes these samples candidates for high-frequency devices2,51.
Coatings evaluation
Mechanical properties
The mechanical features of CaFe (2.5%), CaFe (5%), CuFe (2.5%) and CuFe (5%) are shown in Fig. 10a. The figure shows that the hardness of CaFe (5%) and CuFe (5%) is higher than those containing 2.5%, which are approximately 240.56 and 243, respectively. Coatings containing 5% provided the best hardness values due to their high content in the film, resulting in the formation of a uniform film that had acceptable hardness. Meanwhile, the coatings with 5% had low impact resistance and ductility due to the presence of a significant quantity of epoxy rings that formed an inflexible and dense macromolecular framework. These characteristics, which result in a stiff and brittle structure, are a direct consequence of the hardener's depletion of all reactive sites.
Pull-off strength results
The pull-off test was used to determine the level of adhesion for coated panels that were both dry and wet. An equation has been used to estimate the adhesion loss (ψ) values as follows:
where αD denotes the dry adhesion and αw represents the wet adhesion.
Figure 10b demonstrates that the films coated with CaFe (5%) and CuFe (5%) delivered the best dry and wet adhesion strengths with low adhesion loss, which are approximately 4 and 4.8%, respectively, while the adhesion loss of CaFe (2.5%) and CuFe (2.5%) is in the range of 31.57 and 29.59%, respectively. The presence of nano-ferrites with high content has improved both adhesion strength and reduced adhesion loss. This is attributed to the good arrangement of the nano-particles, which can block the whole voids in the matrix and form a tight film that could restrict the diffusion of aqueous solution through the film, so the adhesion is not affected52.
Microwave measurements
The variation of the return loss of the four films coated with CaFe (5% and 2.5%) and CuFe (5% and 2.5%) is shown in Fig. 11. From this result, it can be observed that adding nano-ferrites with high content enhances the absorption characteristics of the tested films, as the two films coated with 2.5% of nano-ferrites have a return loss of around −5 dB. However, the two films coated with (5% wt) of nano-ferrites have two absorption bands with RL < –10 dB ranged from 10.61 to 10.97 GHz for CuFe and from 10.25 to 11.2 GHz for CaFe, with centre frequencies of 10.75 GHz and 10.8 GHz for CuFe and CaFe, respectively, as shown in Fig. 13. The minimum return loss achieved for CaFe is −13 dB and for CuFe is −16 dB, respectively. Indicating that the film coated with CuFe has a better absorption value than that coated with CaFe, and the coated films containing a high ratio of both nano-ferrites (e.g., 5%) offer the best absorption value. Therefore, the dielectric measurements in the range 8–12 GHz have been performed for 5% of both CaFe and CuFe.
Figure 12 represents the measured \(\varepsilon {^{\prime}}\), \(\varepsilon {^{\prime}}{^{\prime}}\) and \({\text{tan}}{\delta }_{\epsilon }\) of the two films coated with CaFe and CuFe at a high concentration of 5%. From this figure, it can be observed that the values of \(\varepsilon {^{\prime}}\) have a clear resonance at 10.75 GHz. Also, \({\text{tan}}{\delta }_{\epsilon }\) is an effective parameter for indicating the dielectric loss capability of the absorber. A moderate value of \({\text{tan}}{\delta }_{\epsilon }\) indicates a higher absorption rate. For \({\text{tan}}{\delta }_{\epsilon }\le 1\), good impedance matching will be obtained, indicating excellent microwave absorption performance. The measured \({\text{tan}}{\delta }_{\epsilon }\) obtained from the synthetic effect of \(\varepsilon {^{\prime}}\) and \(\varepsilon {^{\prime}}{^{\prime}}\) is indicated in Fig. 12c. It can be clearly seen from these two figures that the values of \(\varepsilon {^{\prime}}{^{\prime}}\) and \({\text{tan}}{\delta }_{\epsilon }\) of the films fluctuate in the operating band of 8–12 GHz.
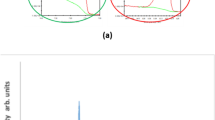
The relationship between \(\varepsilon {^{\prime}}\) and \(\varepsilon {^{\prime \prime}}\) of the two films of CaFe (5% wt) and CuFe (5% wt) is presented by the Cole–Cole plots in the frequency range 8 to 12 GHz, as shown in Fig. 13. It can be noted from these figures that the film coated with CuFe reveals a Debye relaxation process implied by a clear semicircle at the resonance frequency of 10.7 GHz, matching the resonance frequencies of the \(\varepsilon {^{\prime \prime}}\) curve.
The absorption mechanisms for the synthesized nano-CuFe2O4 and nano-CaFe2O4 can be explained based on their structural and electromagnetic properties. Nano-CuFe2O4 and nano-CaFe2O4 are both ferrite materials, which are known for their excellent electromagnetic properties and ability to absorb electromagnetic waves. These ferrites possess unique crystal structures that contribute to their absorption behavior. When exposed to microwave radiation, some electromagnetic microwaves transmit outside, and some waves interact with the nano-ferrite particles in the coatings. The absorption mechanism of both nano-CaFe2O4 and nano-CuFe2O4 involves a combination of dielectric loss, magnetic loss, multiple reflections, scattering, and interface effects. Figure 14 illustrates a schematic diagram of the mechanism of microwave absorption. The dielectric loss occurs because of the polarization of the electric dipoles within the material in response to the applied electric field of the electromagnetic wave. Both nano-CuFe2O4 and nano-CaFe2O4, exhibit dielectric properties that cause them to absorb and dissipate energy from the incident electromagnetic waves. Another absorption mechanism is magnetic loss. Nano-ferrites possess a high magnetic permeability, which allows them to interact strongly with the magnetic component of the electromagnetic wave. This interaction leads to the conversion of the magnetic energy into heat, resulting in energy absorption and attenuation of the electromagnetic wave. Also, the synthesized nano-ferrites contribute to multiple reflections and scattering of the incident electromagnetic waves. Due to their small size and unique morphology, the nano-ferrite particles can scatter the incoming waves in multiple directions. This scattering effect enhances the path length of the waves within the coatings, increasing the opportunity for absorption and dissipation of energy. The interfaces between the nano-ferrite particles and the surrounding matrix (such as epoxy resin) also play a role in the absorption mechanism. These interfaces can lead to additional absorption due to interfacial polarization and frictional losses. All these mechanisms collectively contribute to the effective absorption of microwave energy within the coatings, reducing the reflection of electromagnetic waves. It's important to note that the specific absorption characteristics of nano-CuFe2O4 and nano-CaFe2O4 can depend on factors such as their composition, particle size, morphology, and the surrounding matrix material9.
Conclusions
In summary, new and cost-effective electromagnetic absorbing coatings that may effectively minimize electromagnetic radiation were successfully developed by the introduction of nano-CuFe2O4 and nano-CaFe2O4 modified epoxy resin. The study examined the mechanical characteristics of coatings that absorb electromagnetic waves, as well as the impact of coating impermeability and EMW absorption qualities. From this investigation, the following results are drawn:
-
The addition of proper concentration of nano-CuFe2O4 and nano-CaFe2O4 can effectively improve the adhesion and hardness of epoxy resin coating. Among them, the optimized modified epoxy coating with nano-ferrites content of 5% exhibits the best enhancement effect, which can be attributed to the good arrangement of the nano-particles, which can block the whole voids in the matrix and form a tight film that could restrict the diffusion of aqueous solution through the film.
-
The microwave results illustrate that adding nano-ferrites with high content (e.g., 5%) enhances the absorption characteristics of the tested films.
-
From this result, it can be observed that adding nano-ferrites with high content enhances the absorption characteristics of the tested films, as the two films coated with 2.5% of nano-ferrites have a return loss of around −5 dB. However, the two films coated with (5% wt) of nano-ferrites have two absorption bands with RL < –10 dB ranged from 10.61 to 10.97 GHz for CuFe and from 10.25 to 11.2 GHz for CaFe, with centre frequencies of 10.75 GHz and 10.8 GHz for CuFe and CaFe, respectively. Indicating that the film coated with CuFe has a better absorption value than that coated with CaFe.
-
Thus, the novel electromagnetic-absorbing coatings synthesized in this work can be widely applied for the purpose of protecting the environment from building electromagnetic pollution and reducing electromagnetic radiation caused by health hazards to people. They can also be used in television stations, airports, docks, and navigation beacons to eliminate reflection interference and reduce electromagnetic radiation.
References
Zhao, T. et al. Multiphase interfacial regulation based on hierarchical porous molybdenum selenide to build anticorrosive and multiband tailorable absorbers. Nano-micro Lett. 16, 6 (2023).
Yin, P. et al. Structure regulation in N-doping biconical carbon frame decorated with CoFe2O4 and (Fe, Ni) for broadband microwave absorption. Chem. Eng. J. 446, 136975 (2022).
Lv, H. et al. Staggered circular nanoporous graphene converts electromagnetic waves into electricity. Nat. Commun. 14, 1982 (2023).
Wu, Y., Lan, D., Ren, J. & Zhang, S. A mini review of MOFs derived multifunctional absorbents: From perspective of components regulation. Mater. Today Phys. 36, 101178 (2023).
Guo, Y., Ruan, K., Wang, G. & Gu, J. Advances and mechanisms in polymer composites toward thermal conduction and electromagnetic wave absorption. Sci. Bull. 68, 1195–1212 (2023).
Pan, C., Kou, K., Zhang, Y., Li, Z. & Wu, G. Enhanced through-plane thermal conductivity of PTFE composites with hybrid fillers of hexagonal boron nitride platelets and aluminum nitride particles. Compos. Part B Eng. 153, 1–8 (2018).
Zhang, Y., Kong, J. & Gu, J. New generation electromagnetic materials: Harvesting instead of dissipation solo. Sci. Bull. (Beijing) 67, 1413–1415 (2022).
Pan, C. et al. Improved thermal conductivity and dielectric properties of hBN/PTFE composites via surface treatment by silane coupling agent. Compos. Part B Eng. 111, 83–90 (2017).
Mahmoud, D. S., Eldesouki, E. M. & Abd El-Gawad, W. M. Nano-lithium ferrite/nanosilica-filled butadiene-acrylonitrile rubber for microwave absorption. Mater. Chem. Phys. 313, 128673 (2024).
Sani, R. A. S., Azis, I., Ismail, Y. & Yaakob, J. Mohammed, Enhanced electromagnetic microwave absorbing performance of carbon nanostructures for RAMs: A review. Appl. Surf. Sci. Adv. 18, 100455 (2023).
Liu, P., Huang, Y., Yan, J. & Zhao, Y. Magnetic graphene@PANI@porous TiO2 ternary composites for high-performance electromagnetic wave absorption. J. Mater. Chem. C 4, 6362–6370 (2016).
Raveendra, R. S. et al. Synthesis, characterization and antibacterial activity of zinc ferrite nanopowder. Int. J. Sci. Res. 1, 543–547 (2015).
Wrzyszcz, J., Zawadzki, M., Trzeciak, A. M. & Ziółkowski, J. J. Rhodium complexes supported on zinc aluminate spinel as catalysts for hydroformylation and hydrogenation: Preparation and activity. J. Mol. Catal. A Chem. 189, 203–210 (2002).
Ahmed, N. M., Abd El-Gawad, W. M. & Souaya, E. M. R. Evaluation of the anticorrosive performance of epoxy coatings containing new core/shell pigments. Pigment Resin Technol. 47, 116–126 (2018).
Ahmed, N. M., Abd El-Gawad, W. M., Youssef, E. A. & Souaya, E. R. New eco-friendly anticorrosive core-shell pigments. Pigment Resin Technol. 44, 276–291 (2015).
Yin, P. et al. Recent progress in ferrite microwave absorbing composites. Integr. Ferroelectr. 211, 82–101 (2020).
Li, R., Qing, Y., Li, W. & Li, Y. The electromagnetic absorbing properties of plasma-sprayed TiC/Al2O3 coatings under oblique incident microwave irradiation. Ceram. Int. 47, 22864–22868 (2021).
Liu, T., Xie, X., Pang, Y. & Kobayashi, S. Co/C nanoparticles with low graphitization degree: A high performance microwave-absorbing material. J. Mater. Chem. C 4, 1727–1735 (2016).
Li, Y. et al. Unique nanoporous structure derived from Co3O4–C and Co/CoO–C composites towards the ultra-strong electromagnetic absorption. Compos. Part B Eng. 213, 108731 (2021).
Qing, Y., Yao, H., Li, Y. & Luo, F. Plasma-sprayed ZrB2/Al2O3 ceramics with excellent high temperature electromagnetic interference shielding properties. J. Eur. Ceram. Soc. 41, 1071–1075 (2021).
Lv, H. et al. A brief introduction to the fabrication and synthesis of graphene based composites for the realization of electromagnetic absorbing materials. J. Mater. Chem. C 5, 491–512 (2017).
Qiu, X., Wang, L., Zhu, H., Guan, Y. & Zhang, Q. Lightweight and efficient microwave absorbing materials based on walnut shell-derived nano-porous carbon. Nanoscale 9, 7408–7418 (2017).
Abouhaswa, A. S., Rammah, Y. S. & Turky, G. M. Characterization of zinc lead-borate glasses doped with Fe3+ ions: Optical, dielectric, and AC-conductivity investigations. J. Mater. Sci. Mater. Electron. 31, 17044–17054 (2020).
Heikal, S., Mokhtar, H. M. & Abd El-Ghany, W. A. Effect of CuO addition on the structural, electrical and dielectric properties of the sodium borate glasses. Phys. B Condens. Matter 667, 415142 (2023).
Li, Y. et al. Ni0.6Zn0.4Fe2O4/Ti3C2Tx nanocomposite modified epoxy resin coating for improved microwave absorption and impermeability on cement mortar. Construct. Build. Mater. 310, 125213 (2021).
Sulaiman, N. H., Ghazali, M. J., Majlis, B. Y., Yunas, J. & Razali, M. Superparamagnetic calcium ferrite nanoparticles synthesized using a simple sol–gel method for targeted drug delivery. Bio-med. Mater. Eng. 26(Suppl 1), S103-110 (2015).
Rahman, T., Borah, G. & Gogoi, P. K. Spinel structured copper ferrite nano catalyst with magnetic recyclability for oxidative decarboxylation of phenyl acetic acids. Catal. Lett. 150, 2267–2272 (2020).
Reddy, B. C. et al. Synthesis and characterization of barium ferrite nano-particles for X-ray/gamma radiation shielding and display applications. Prog. Nucl. Energy 147, 104187 (2022).
Chandrasekar, N., Kumar, K. M. M., Balasubramnian, K. S. & Varadharajan, K. K. R. Facile synthesis of iron oxide, iron-cobalt and zero valent iron nanoparticles and evaluation of their antimicrobial activity, free radicle scavenginging activity and antioxidant assay. Digest J. Nanomater. Biostruct. 8, 765–775 (2013).
Liu, Y., Zhang, H., Fu, W., Yang, Z. & Li, Z. Characterization of temperature sensitivity of V-modified CuFe2O4 ceramics for NTC thermistors. J. Mater. Sci. Mater. Electron. 29, 18797–18806 (2018).
Bai, R. et al. Rapid and efficient removal of naproxen from water by CuFe(2)O(4) with peroxymonosulfate. Environ. Sci. Pollut. Res. Int. 27, 21542–21551 (2020).
Li, J., Ren, Y., Ji, F. & Lai, B. Heterogeneous catalytic oxidation for the degradation of p-nitrophenol in aqueous solution by persulfate activated with CuFe2O4 magnetic nano-particles. Chem. Eng. J. 324, 63–73 (2017).
Kamali, M., Sheibani, S. & Ataie, A. Magnetic MgFe(2)O(4)-CaFe(2)O(4) S-scheme photocatalyst prepared from recycling of electric arc furnace dust. J. Environ. Manag. 290, 112609 (2021).
Obukuro, Y. et al. Formation of CaFe2O4 porous structure by addition of Zr in malic acid complex. J. Ceram. Soc. Jpn. 123, 995–998 (2015).
Guo, L., Okinaka, N., Zhang, L. & Watanabe, S. Molten salt-assisted shape modification of CaFe2O4 nanorods for highly efficient photocatalytic degradation of methylene blue. Opt. Mater. 119, 111295 (2021).
Mahdy, M. A., El Zawawi, I. K. & Turky, G. M. Lead telluride nanocrystalline thin films: Structure, optical characterization and a broadband dielectric spectroscopy study. Curr. Appl. Phys. 19, 787–793 (2019).
El-Bassyouni, G. T., Turky, G. M., Kenawy, S. H., Abd El-Aty, A. A. & Hamzawy, E. M. A. Effect of yttrium oxide in hydroxyapatite biocomposite materials: Electrical and antimicrobial evaluation. ECS J. Solid State Sci. Technol. 10, 123014 (2021).
Kolonelou, E., Papathanassiou, A. N. & Sakellis, E. Evidence of local softening in glassy poly(vinyl alcohol)/poly(vinyl pyrrolidone) (1/1, w/w) nano-graphene platelets composites. Mater. Chem. Phys. 223, 140–144 (2019).
Koops, C. G. On the dispersion of resistivity and dielectric constant of some semiconductors at audiofrequencies. Phys. Rev. 83, 121–124 (1951).
Kılıç, M., Kahya, N. D., Mısırlıoğlu, B. S., Çakır, Ö. & Özdemir, Z. G. Dielectric and magnetic properties of CuFe2O4/CuO nanocomposites. Ferroelectrics 571, 183–199 (2021).
Yadav, R. S. et al. Structural, dielectric, electrical and magnetic properties of CuFe2O4 nanoparticles synthesized by honey mediated sol–gel combustion method and annealing effect. J. Mater. Sci. Mater. Electron. 28, 6245–6261 (2017).
Mabrouk, M., Mousa, S. M., Abd El-Ghany, W. A., Abo-elfadl, M. T. & El-Bassyouni, G. T. Bioactivity and cell viability of Ag+- and Zr4+-co-doped biphasic calcium phosphate. Appl. Phys. A 127, 34 (2021).
Lal, G. et al. Rietveld refinement, Raman, optical, dielectric, Mössbauer and magnetic characterization of superparamagnetic fcc-CaFe2O4 nanoparticles. Ceram. Int. 45, 5837–5847 (2019).
Bindu, K., Ajith, K. M. & Nagaraja, H. S. Influence of cations on the dielectric properties of spinel structured nanoferrites. Mater. Res. Exp. 6, 045011 (2019).
Selvan, R. K. et al. Synthesis and characterization of CuFe2O4/CeO2 nanocomposites. Mater. Chem. Phys. 112, 373–380 (2008).
Taha, T. A., Azab, A. A. & El-Khawas, E. H. Comprehensive study of structural, magnetic and dielectric properties of borate/Fe3O4 glass nanocomposites. J. Electron. Mater. 49, 1161–1166 (2019).
Mubasher, A. et al. Comparative study of frequency-dependent dielectric properties of ferrites MFe2O4 (M = Co, Mg, Cr and Mn) nanoparticles. Appl. Phys. A 11, 126 (2020).
Naz, K. et al. Structural, dielectric, impedance and electric modulus analysis of Ni substituted copper spinel ferrites nanoparticles for microwave device applications. Mater. Chem. Phys. 285, 126091 (2022).
Liu, Y. & Hsu, J. Study of the electromagnetic properties of nano (MxZn1−x)Fe2O4 (M=Cu, Ni) as a function of the sintering temperature. Appl. Sci. 8, 605 (2018).
Thakur, P., Chahar, D., Taneja, S., Bhalla, N. & Thakur, A. A review on MnZn ferrites: Synthesis, characterization and applications. Ceram. Int. 46, 15740–15763 (2020).
Ali, M. A. et al. Yttrium-substituted Mg–Zn ferrites: Correlation of physical properties with Yttrium content. J. Mater. Sci. Mater. Electron. 30, 13258–13270 (2019).
Hafez, A. I., Ali, H. M., Sabry, R. M., El-Masry, H. M. & AbdEl-Gawad, W. M. Generation of novel, hygienic, inhibitive, and cost-effective nanostructured core-shell pigments. Prog. Organ. Coat. 175, 107325 (2023).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Dr. W.M. Abd El-Gawad was responsible for the synthesis and characterization of nano-ferrites and their interpretations. Also, Dr. W.M. Abd El-Gawad prepared the coatings and investigated their adhesion and mechanical properties. Dr. W.A. Abd El-Ghany was responsible for the determination of dielectric, electrical, and magnetic properties of nano-ferrites. E.M. Eldesouki was responsible for evaluating the microwave absorption of the prepared coatings. All authors shared the responsibility of writing and revising the manuscript and analyzing the obtained data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abd El-Gawad, W.M., Eldesouki, E.M. & Abd El-Ghany, W.A. Development of high performance microwave absorption modified epoxy coatings based on nano-ferrites. Sci Rep 14, 5190 (2024). https://doi.org/10.1038/s41598-024-55571-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55571-y
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.