Abstract
Nitrogen (N) and phosphorus (P) are vital for crop growth. However, most agricultural systems have limited inherent ability to supply N and P to crops. Biochars (BCs) are strongly advocated in agrosystems and are known to improve the availability of N and P in crops through different chemical transformations. Herein, a soil-biochar incubation experiment was carried out to investigate the transformations of N and P in two different textured soils, namely clay loam and loamy sand, on mixing with rice straw biochar (RSB) and acacia wood biochar (ACB) at each level (0, 0.5, and 1.0% w/w). Ammonium N (NH4-N) decreased continuously with the increasing incubation period. The ammonium N content disappeared rapidly in both the soils incubated with biochars compared to the unamended soil. RSB increased the nitrate N (NO3–N) content significantly compared to ACB for the entire study period in both texturally divergent soils. The nitrate N content increased with the enhanced biochar addition rate in clay loam soil until 15 days after incubation; however, it was reduced for the biochar addition rate of 1% compared to 0.5% at 30 and 60 days after incubation in loamy sand soil. With ACB, the net increase in nitrate N content with the biochar addition rate of 1% remained higher than the 0.5% rate for 60 days in clay loam and 30 days in loamy sand soil. The phosphorus content remained consistently higher in both the soils amended with two types of biochars till the completion of the experiment.
Similar content being viewed by others
Introduction
Nitrogen and phosphorous are indispensable for plant growth. The supply of significant amounts of nitrogen and phosphorus in farmlands is a common practice by farmers to augment the growth of crops, responsible for non-point source pollution and bringing an indisputable soil environment1. Despite the excess of these nutrients, most agricultural systems cannot supply nitrogen and phosphorus to crops2. Sustainable approaches are in demand to utilize the excessive nutrient stores of soils for better assimilation in plant biota. Biochar application for augmenting soil fertility is gathering momentum globally to achieve desired agronomic outcomes3,4. Biochar is a carbon-rich material produced by pyrolysis under minimal or zero oxygen supply for its application to the soil and is recognised as a sustainable methodology to improve soil properties and nutrient availability5. Its production and application to soils have been in advocation for a long as an effective method for recycling biomass along with benefiting soil carbon (C) sequestration, soil moisture and nutrient retention, and alleviating nutrient leaching6. Biochars, diversly heterogeneous in their form and reactivity in soil, are reported to affect soil N and P dynamics. Various biochar studies have focused on row crop agricultural systems associated with external nutrient inputs7,8, along with the effect of variously prepared biochars on nitrogen (N) and phosphorus (P) transformations in soils. Adsorption of phosphate and ammonium N (NH4-N) ions on biochars eventually leads to less N availability for nitrification9,10. Some reports state that biochar addition increased net nitrification rates in temperate and boreal forest soils11 as it stimulates or inhibits soil microbial activity.
P application and availability in agricultural soils is a matter of immense pertinence to maintaining high crop productivity. Alteration in the concentration of P on biochar-amended soil is often used as a possible explanation for biochar effects on improved crop growth12,13, which is reportedly due to biochar anion exchange capacity, different adsorption effects14 or by influencing the activity of cations that interacts with soil P. The study15 found that the addition of biochar reduced P leaching from manured soil due to the sorption of both orthophosphate and organic P by biochar. The information available in the literature on the effects of biochar on nutrient transformation in soil needs to be more consistent. The mechanisms and processes of P reaction dynamics in biochar-amended soils need further investigation16,17.
Though various reports are there on the effect of adding biochars on N and P, the information is limited to N and P transformations in some agricultural soil types. Moreover, the effects of biochar on N and P transformations depend on the type of biochar and soil2. Laboratory incubation studies of biochar application on N and P transformations in soils (varying in texture) is a pertinent concept that needs a critical examination. In this contextthe effect of rice straw biochar (RSB) and acacia wood biochar (ACB) are put on incubation trails on clay loam and loamy sand soils to study the transformation pattern of nitrogen and phosphorous. Such fundamental knowledge is crucial for developing guidelines for the selection and appropriate use of biochar and evaluating the potential sustainable use of biochar in agricultural soils.
Materials and methods
Biochar production
Rice (Oryza sativa L.) straw was collected from research farms at Punjab Agricultural University, Ludhiana, Punjab. The straw was air-dried at room temperature and ground to pass through a 10-mesh sieve. The biochar was prepared as per the previously reported methodology18 at a temperature of 380 °C for an incubation time of 3 h.
The ACB was prepared using the procedure outlined in Gupta et al.19, rationally, a pyramid-shaped structure (earth kiln) of wood is piled for its preparation. Vents are provided from the top to downwards to allow the combustion products to escape. When smoke production is ceased, the cooling process is initiated by pouring a layer of moist earth on this structure. The cooling process is completed in two to three days. Then, the biochar is separated from the surrounding carbonized portions before removing the earth, finely grounded and analyzed for basic properties.
Laboratory incubation study
Effects of biochar on nitrogen and phosphorus transformations were determined through a laboratory incubation study with loamy sand and clay loam soils. Bulk soil samples (0–15 cm) representing clay loam and loamy sand were obtained from two distinct fields 1.5 km apart at the Punjab Agricultural University's experimental farm in Ludhiana, Punjab, India. The samples were further air-dried, passed through a 0.5 mm sieve and analyzed for initial physical and chemical properties using standard analytical methods.
Effect of biochar type and application rates on nitrogen transformation in soils
Treatments included RSB and ACB, each mixed with soil at three rates (0, 0.5 and 1% on a w/w basis). Nitrogen as urea was applied at 0 and 100 mg Nkg−1. The treated pots followed a completely randomized design (CRD) in three factorial combinations: (a) type of biochar, (b) rates of biochar, and (c) two N-rates. Each treatment was replicated thrice. A known weight of biochar was thoroughly mixed with 750 g of soil (on a dry weight basis). To one set of treated soil, 100 mg N kg−1 was applied in solution form, while the other set was kept as no-N control. In total, there were 12 treatments for each soil type. All the treatments were thoroughly mixed with soil, and distilled water was adjusted to 75% of field capacity (16% for clay loam and 11% for loamy sand; dry weight basis). Thereafter, treated soil samples were transferred into plastic containers (10 cm internal diameter). Sealed containers were arranged in CRD in the incubator at 25 °C for 60 days. Soil sub-samples from each container were drawn at 1, 3, 5, 7, 15, 30, and 60 days after incubation to determine soil wetness, ammonium-N and nitrate–N contents.
Effect of biochar type and application rates on phosphorus transformation in soils
The two biochar types and similar application rates were used. The P as KH2PO4 was applied at 0 and 30 mg P kg−1. The treated pots were placed in triplicate in a CRD with three factorial combinations: (a) type of biochar, (b) biochar rates, and (c) two P rates. Soil subsamples from each container were drawn at 1, 3, 5, 7, 15, 30, and 60 days after incubation to determine soil moisture and Olsen-P contents.
Analytical procedures
Biochar analysis
The pH, electrical conductivity (EC), total carbon (C), nitrogen (N), ammonium N (NH4–N), nitrate N (NO3–N), phosphorus (P), and potassium (K) concentrations of biochar samples were determined. Total C and N contents were determined by a CHN analyser (Elementar Vario EL). The pH and EC of the biochar were determined in the solution having a 1:5 biochar to water ratio. The NH4-N and NO3-N contents in the 2 M KCl extracts were calculated as described by Ref.20. Total P and K contents in di-acid digests were determined on Inductively Coupled Argon Plasma (ICAP). The chemical composition of the biochars is illustrated in Table 1.
Soil analysis
The International Pipette Method described by Ref.21 was used to determine the particle size distribution of soil samples. The USDA textural triangle was then used to determine the textural class. Soil pH and EC were measured using a glass electrode pH meter and a conductivity meter in a 1:2 soil-to-water ratio. Wet digestion was used to calculate organic carbon22. Total N was determined by digesting soil samples with concentrated H2SO4 digests, as described in Ref.23. The available P was determined in 0.5M NaHCO3 soil extracts as outlined by Ref.24. The amount of available K in 1N NH4OAc extracts was determined using a flame photometer25. A MicroKjeldahl distillation method was used to detect inorganic N (NH4−N and NO3−N) in 2M KCl extracts. The selected properties of the two soils used in the incubation study are given in Table 2. The pH of clay loam and sandy loam soil was 7.62 and 6.95, respectively, and both soils were non-saline. Clay loam soil contained 6.0 g kg−1 of organic carbon and was high in both available P (34.2 kg ha−1) and K (336 kg ha−1). In contrast, loamy sand soil tested low in organic carbon (2.93 g kg−1), medium in available P (13.6 kg ha−1) and available K (275 kg ha−1).
Statistical analysis
Analysis of variance (ANOVA) was used to assess the data statistically. To examine the significance of changes in treatments, the least significant difference (LSD) at a 0.05 level of probability was utilized.
Results
Biochar characterization
RSB with pH 9.09 contained 0.52% total N, 0.37% total P and 9.2% total K (Table 1). The ACB had a pH value of 7.26 with 0.20% total N, 0.11% total P, and 0.85% total K. RSB contained a high concentration of K due to high K contents in rice straw used as a source for biochar production. It also contained a higher concentration of P compared to ACB. The RSB was observed to be more alkaline compared to ACB. The EC of RSB and ACB were measured to be 0.85 dS m−1 and 0.13 dS m−1, respectively.
Ammonical nitrogen
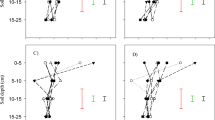
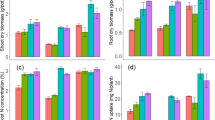
At all sampling periods, except 15 and 60 days after incubation in clay loam and 30 and 60 days after incubation in loamy sand soil, a significant interaction of type and rate of biochar was detected (Fig. 1). Starting on day one and continuing until the end of the 60-day incubation, the NH4-N level was considerably lower in both soils supplemented with biochar compared to unamended soil (Fig. 1). Increasing the biochar rate from 5 to 10g kg−1 soil resulted in generally non-significant effect of NH4-N content on different sampling dates. In clay loam soil, NH4–N contents one day after incubation were similar (62.5 and 62.3 mg kg−1) with the mixing of (0.5 and 1%, w/w) biochar in soil and decreased gradually to 0.1 mg kg−1 at the end of the 60-day incubation period (Fig. 1a). The corresponding values of NH4-N for loamy sand soil were markedly lower (27.9 and 26.9 mg kg−1), which declined to 0.2 mg kg−1 at 30 days after incubation (Fig. 1b). Biochar type was not able to affect the NH4–N content. Biochars showed a greater effect on NH4–N content in loamy sand compared to clay loam soil. The concentration of NH4–N in the soil at 30 days after incubation was decreased to 8.68 and 6.62% of the concentration at day 1 after incubation when RSB was mixed with clay loam soil at the rate of (0.5 and 1.0%, w/w), respectively. In clay loam, the corresponding values of NH4-N concentration with the addition of ACB were 9.72 and 8.59%, respectively. The concentration of NH4-N at 30 days after incubation decreased more rapidly when RSB was applied compared to ACB, suggesting that RSB had a greater effect on increasing the nitrification rate in clay loam, while similar effects were observed at 15 days after incubation in loamy sand soil.
A significant interaction between N levels and rates of biochar (averaged across two types of biochar) was observed in NH4-N concentration in both the soil types on all the sampling dates (Fig. 2). The concentration of NH4-N was more in clay loam than in loamy sand soil due to higher initial content of NH4-N. As expected, the application of 100 mg urea-N kg-1 soil resulted in a significantly higher concentration of NH4-N compared to no-N control treatment on all the sampling dates. The NH4-N concentration in N-amended clay loam was significantly lower when biochar was added at 1% level as compared to 0 and 5% levels. However, no significant effect of biochar rate was observed in no-N treatment in clay loam (Fig. 2a). Generally, similar effects were observed in loamy sand soil (Fig. 2b). The NH4–N concentration in urea-amended soil decreased at a faster rate between 1 and 7 days of incubation compared to that between 7 to 15 days of incubation and thereafter, mainly due to nitrification. The decrease in NH4–N concentration in N-amended soil was significantly higher when 1% biochar was mixed with soil as compared to 0.5% level. At 30 and 60 days after incubation in N-supplemented loamy sand and clay loam, the concentration of NH4–N in loamy sand was reduced to a very low level (0.2 mg kg−1) with adding 0.5 or 1% biochar nitrate nitrogen.
Significant interaction effects of types and rates of biochar on NO3-N content were observed at all sampling periods in both soils. The NO3-N contents continued to increase throughout the 60-day incubation period in both soils due to the nitrification of applied N (Fig. 3a,b). Except for 60 days after incubation, all subsequent sampling dates in clay loam soil showed increased NO3-N content with a higher biochar rate. The contents of NO3-N in loamy sand soil increased with increasing biochar rate until 15 days after incubation. However, they decreased when 1% biochar was combined compared to 0.5 per cent biochar treatment at 30 and 60 days after incubation. On all sampling dates in both soils, RSB treated at 0.5 or 1.0% induced a considerably larger rise in NO3–N content than ACB. While the application of RSB at 1.0% level compared to 0.5% resulted in a significant increase in NO3–N content on all sampling dates, however, the reverse trend was observed in the case of ACB. Increasing the rate of ACB from 5 to 10 g kg−1 soil resulted in a significant decrease in NO3–N contents on all sampling dates in both soils. The NO3–N contents (averaged over biochar types) were 8.4, 8.0 and 8.9 mg kg-1 with the addition of 0, 0.5 and 1.0% biochar in clay loam soil one day after incubation. They increased to 44.9, 56.5 and 58.1 mg kg-1 30 days after incubation, respectively. The corresponding values of NO3-N content in loamy sand were 7.8, 13.1 and 13.4 mg kg−1 with adding 0, 0.5 and 1.0% biochar at 1 day and increased to 50.2, 67.8 and 66.1 mg kg−1 at 30 days after incubation. The increase in the concentration of NO3–N at 15 days after incubation was 18.3 and 18.9% of the concentration observed one day after incubation with the addition of 0.5 and 1.0% RSB in clay loam soil. The corresponding increase in NO3-N concentration in loamy sand was 37.8 and 40.5%, respectively.
The effect of biochar addition in increasing NO3–N concentration was lower initially and then increased faster on or seven days until 60 days after incubation in both soils. This was primarily because loamy sand had a greater starting concentration of NO3–N than clay loam soil. There were no significant interaction effects of biochar rate and N-rate on NO3–N contents in both soils. As expected, the application of urea caused a significant increase in NO3-N contents at all the sampling dates in both soils (Table 3). The increase in NO3-N contents in soil treated with 100 mg urea-N kg-1 soil over No-N control was 33.8 mg and 51.0 mg kg−1 in clay loam and 24.2 mg and 38.2 mg kg−1 in loamy sand at 5 and 30 days after incubation, respectively (Table 3). The NO3–N contents in No-N control soil increased from 8.0 mg at 1 day to 27.4 mg kg−1 30 days after incubation in clay loam and 10.6 mg to 38.7 mg kg−1 in loamy sand soil. Thus, an increase in NO3–N contents during the incubation is more in loamy sand compared to clay loam soil.
RSB resulted in significantly higher nitrification than ACB throughout the 60-day incubation period in both soils (Table 4). A significant increase in nitrification with biochar was observed up to mixing of 0.5%. There was no further increase in nitrification rate at the 1.0% level of biochar mixing 5 days after incubation and after that in both soils (Table 4). At 15 days after incubation, nitrification of applied N with no biochar treatment was 31.5%, which increased to 53.5% with 0.5% biochar, irrespective of the type of biochar in clay loam. Compared to clay loam soil, the nitrification rate of administered N was substantially slower during the first three days after incubation in loamy sand. However, the nitrification rate in loamy sand was substantially quicker than in clay loam for the remaining incubation duration. The nitrification rate in clay loam was 30 days, and 15 days in loamy sand soil. The nitrification in biochar amended treatment decreased markedly at 30 and 60 days compared to 15 days after incubation in loamy sand soil. The decrease in nitrification in loamy sand soil may be due to the loss of NO3–N or immobilization of NO3-N during incubation.
Available phosphorus
RSB resulted in a significant increase in Olsen-P content in soil compared to ACB at 3 (in loamy sand) and 7 (clay loam soils) days after incubation (Figs. 4 and 5). At the end of the 60-day incubation period, Olsen-P remained consistently higher in both soils amended with RSB than the ACB. The increase in the mean concentration of Olsen-P (averaged over rates of biochar) at 60 days after incubation was 68.5 and 63.6% higher for RSB and ACB, respectively, compared to that at one day after incubation in the clay loam soil. The corresponding increase in Olsen-P in loamy sand soil was 58.0 and 55.7%. The addition of 0.5 and 1.0% biochar significantly increased (averaged over two types of biochar) Olsen-P content over No-P control during the entire period of incubation starting from 3 days after incubation. The addition of 1.0% biochar significantly increased P availability as compared to 0.5%. At 60 days after incubation, the increase in Olsen-P was 42.7 and 56.7% with the addition of 0.5 and 1.0% biochar (averaged over two types) compared to No-P control in clay loam soil and 26.9 and 36.6% in loamy sand soil, respectively. The application of 1.0% biochar significantly increased P availability as compared to the addition of 0.5% biochar in both soils. The rise in Olsen-P concentration in soil was greater in loamy sand soil than in clay loam soil.
Principal component analysis (PCA)
Principal component analysis (PCA) of type and application rate of biochar on NH4–N content (mg kg−1) during a 60-day incubation period in clay loam and loamy sand soils are given in Supplementary Table S1 and Fig. S1 and application of different rates of biochar and N levels on NH4-N content (mg kg−1) during a 60-day incubation period in clay loam and loamy sand soil are given in Table S2 and Fig. S2.
Discussion
The effectiveness of biochar on soil properties is a multifactor phenomenon and depends on biochar synthetic methodology, soil type, biochar levels and so on26,27,28. The biochar applications have been known to influence nutrient applications, and the results are optimum if the BCs are prepared under the best conditions. The present experiment had the biochars prepared at a moderate temperature (380 °C), at which they were expected to show the maximum cation exchange capacities (CEC)29. The augmented pyrolysis temperatures are reported for the decline in CEC as explained by the decrease of some functional groups, such as –COOH and –OH groups30, along with a decrease in aliphatic, amide, and aromatic amines31.
The applications of optimally produced BC herein resulted in significantly lower NH4–N in both soils amended with biochar compared to unamended soil starting at one day until the end of 60-day incubation (Fig. 1), on line with32,33. This is attributed to the functional groups, such as carboxyl and ketone groups, which play an important role in NH4+ retention owing to hydrogen bonding and electrostatic interaction. Additionally, BC application decreased the ammonia volatilization from the soil because of ammonium ions retention by increasing the soil cation exchange capacity34,35, resulting in a reduction of cumulative NH4+–N losses, which varied with biochar application in a dose-dependent manner36. Another report37 illustrated that biochar could adsorb NH4+-N predominantly through its high cation exchange capacity, resulting in a 15.2% reduction in cumulative NH4+-N losses. Amin38 quantitatively showed the kinetic evidence of lower ammonia volatilization with a higher half-life affecting total available nitrogen on BC application. In the present experiment, the NH4-N concentration in N-amended clay loam was significantly lower when biochar was added at 10 g kg−1 soil compared to 0 and 5 g kg−1 soil with no significant effect of biochar rate was observed in no-N treatment in clay loam (Fig. 2a).
The NO3-N contents continued to increase throughout the 60-day incubation period in both soils due to the nitrification of applied N (Fig. 3a,b) in the present experiment, as supported by Refs.33,39. The increase in the rate of ABC might have caused the immobilization of soil N due to a higher C/N ratio than RSB27,28,40. Contrarily, Dempster et al.36 reported a significant decrease in net nitrification by applying Eucalyptus biochar (wood biochar) with increasing biochar addition in coarse-textured soils. Nitrogen mineralization is usually more rapid in sandy soils than in loam or clay soils due to the physical protection of organic matter and microbial biomass by clay layers41,42.
Char materials are reported to influence soil P availability by altering P sorption capacity43. A significant augmentation in the Olsen-P content in soil with RSB was observed as compared to ACB at 3 (in loamy sand) and 7 (clay loam soils) days after incubation (Figs. 4 and 5). Then, its consistently higher contents in both the soils amended with RSB compared to the ACB at the end of the 60-day incubation period is well supported by the findings of44,45,16,46. Soil retained phosphorus in its particles in addition to biochar, leading to phosphorus supply enhancement and the reduction of environmental pollution47,48. These studies reported that the content of Olsen-P increased significantly with the application of maize stalk biochar compared to the control. The concentration of Olsen-P in soil increased further with the increase in the biochar application rate because of its higher potential for unlocking P3.
Conclusions
The study concluded that adding 5 g rice straw biochar kg -1 soil on a weight basis in low N soils can support the growth of crops by continuously supplying NO3-N in both soils by increasing nitrification significantly. It also offers an alternative for soils rich in P content for better bioavailability of P because of its higher potential for unlocking P. ACB shows the most potent effect for externally supplied P in a clay loam system by mixing 5 g ACB kg−1 soil. It can help farmers to apply appropriate doses of locally available biochar as a soil amendment.
Ethics
All the authors abide by the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Ruhl, J. B. Farms, their environmental harms, and environmental law. Ecol. Law Q. 27, 263 (2000).
Gul, S. & Whalen, J. K. Biochemical cycling of nitrogen and Phosphorus in biochar-amended soils. Soil Biol. Biochem. 103, 1–15 (2016).
Gupta, R. K. et al. Optimisation of rice straw and acacia biochar doses in two soils for phosphorus availability. Acta Agriculturae Scandinavica Sect. B Soil Plant Sci. 73(1), 161–169. https://doi.org/10.1080/09064710.2023.2248998 (2023).
Jirka S, Tomlinson T (2015) State of the biochar industry 2014. A Survey of Commercial Activity in the Biochar Sector. International Biochar Initiative. https://biochar-international.org/wp-content/uploads/2018/11/ibi_state_of_the_industry_2014_final.pdf.
Zhang, Y., Wang, J. & Feng, Y. The effects of biochar addition on soil physicochemical properties: A review. Catena 202, 105284. https://doi.org/10.1016/j.catena.2021.105284 (2021).
Gao, S. & DeLuca, T. H. Biochar alters nitrogen and phosphorus dynamics in a western range land ecosystem. Soil Biol. Biochem. 148, 107868 (2020).
El-Eyuoon, A., Amin, A. Z. & Eissa, M. A. Biochar effects on nitrogen and phosphorus use efficiencies of zucchini plants grown in a calcareous sandy soil. J. Soil Sci. Plant Nutr. https://doi.org/10.4067/S0718-95162017000400006 (2017).
Munera-Echeverri, J. L., Martinsen, V., Strand, L. T., Cornelissen, G. & Mulder, J. Effect of conservation farming and biochar addition on soil organic carbon quality, nitrogen mineralization, and crop productivity in a light textured Acrisol in the sub-humid tropics. PLoS One 15(2), e0228717 (2020).
Ameloot, N., Sleutel, S., Das, K. C., Kanagaratnam, J. & De Neve, S. Biochar amendment to soils with contrasting organic matter level: Effects on N mineralization and biological soil properties. Glob. Change Biol. Bioenergy 7(1), 135–144 (2015).
DeLuca, T. H., Gundale, M. J., MacKenzie, M. D. & Jones, D. L. Biochar effects on soil nutrient transformations. In Biochar for Environmental Management Vol. 1 (eds Lehmann, J. & Joseph, S.) 453–486 (Routledge, 2015).
He, L., Shan, J., Zhao, X., Wang, S. & Yan, X. Variable responses of nitrification and denitrification in a paddy soil to long-term biochar amendment and short-term biochar addition. Chemosphere 234, 558–567 (2019).
Glaser, B. & Lehr, V. I. Biochar effects on phosphorus availability in agricultural soils: A meta-analysis. Sci. Rep. 9(1), 1–9 (2019).
Nelson, N. O., Agudelo, S. C., Yuan, W. & Gan, J. Nitrogen and phosphorus availability in biochar-amended soils. Soil Sci. 176(5), 218–226 (2011).
Bashir, S. et al. Efficiency of different types of biochar to mitigate Cd stress and growth of sunflower (Helianthus; L.) in wastewater irrigated agricultural soil. Saudi J. Biol. Sci. 28(4), 2453–2424. https://doi.org/10.1016/j.sjbs.2021.01.045 (2021).
Lawrinenko, M., Laird, D. A., Johnson, R. L. & Jing, D. Accelerated ageing of biochars: Impact on anion exchange capacity. Carbon 103, 217–227 (2016).
Li, F. et al. Chapter two—Effects of biochar amendments on soil phosphorus transformation in agricultural soils. Editor(s): Donald L Sparks. Adv. Agron. 158, 131–172. https://doi.org/10.1016/bs.agron.2019.07.002 (2019).
Li, F. et al. Effects of biochar amendments on soil phosphorus transformation in agricultural soils. Adv. Agron. 158, 131–172 (2019).
Gupta, R. K. et al. Rice straw biochar improves soil fertility, growth, and yield of rice–wheat system on a sandy loam soil. Exp. Agric. 56(1), 118–131 (2020).
Gupta, R. K., Kang, J. S. & Singh, V. Management of rice straw to enhance crop productivity. Prog. Fmg 53(10), 15–17 (2017).
Mulvaney, R. L. Nitrogen-inorganic forms. In Methods of Soil Analysis, Part 3-Chemical Methods (ed. Sparks, D. L.) 1123–1184 (American Society of Agronomy, 1996).
Day, R. P. Pipette method of particle size analysis. Methods Soil Anal. Agron. 9, 553–562 (1965).
Walkley, A. J. & Black, I. A. Estimation of soil organic carbon by the chromic acid titration method. Soil Sci. 37, 29–38 (1934).
Jackson, M. L. Soil Chemical Analysis 498 (Prentice Hall of India Pvt. Ltd., 1973).
Olsen SR, Cole C, Watanabe CV, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate, USDA Circular No. 939.
Knudsen, D., Peterson, G. A. & Pratt, P. F. Potassium. In Methods of Soil Analysis Part 2 (2nd Edition), Chemical and Microbiological Properties (eds Miller, R. H. & Keeney, D. R.) 225–246 (Wiley, 1982).
Abagandura, G. O., Bansal, S., Karsteter, A. & Kumar, S. Soil greenhouse gas emissions, organic carbon and crop yield following pinewood biochar and biochar–manure applications at eroded and depositional landscape positions: A field trial in South Dakota, USA. Soil Use Manag. 38(1), 487–502 (2022).
Amin, A. E. Comparative effects of different kinds of biochar on ammonia volatilization and chemical properties of saline soil. Arch. Agron. Soil Sci. 69(9), 1600–1613 (2023).
Amin, A. E. E. A. Z. Effects of saline water on soil properties and red radish growth in saline soil as a function of co-applying wood chips biochar with chemical fertilizers. BMC Pl Biol. 23(1), 382 (2023).
Karimi, A., Moezzi, A., Chorom, M. & Enayatizamir, N. Chemical fractions and availability of Zn in a calcareous soil in response to biochar amendments. J. Soil Sci. Plant Nutr. 19, 851–864 (2019).
Eduah, J. O., Nartey, E. K., Abekoe, M. K., Breuning-Madsen, H. & Andersen, M. N. Phosphorus retention and availability in three contrasting soils amended with rice husk and corn cob biochar at varying pyrolysis temperatures. Geoderma 341, 10–17 (2019).
Zhang, C. et al. Biochar for environmental management: Mitigating greenhouse gas emissions, contaminant treatment, and potential negative impacts. Chem. Eng. J. 373, 902–922 (2019).
Miller, M. T. P. & Sohi, S. P. Biochar-root interactions are mediated by biochar nutrient content and impact soil nutrient availability. Eur. J. Soil Sci. 65, 173–185 (2014).
Nelissen, V. et al. Maize biochars accelerate short-term soil nitrogen dynamics in a loamy sand soil. Soil Biol. Biochem. 55, 20–27 (2012).
Mandal, S. et al. Biochar-induced concomitant decrease in ammonia volatilization and increase in nitrogen use efficiency by wheat. Chemosphere 142, 120–127 (2016).
Sun, X., Zhong, T., Zhang, L., Zhang, K. & Wu, W. Reducing ammonia volatilization from paddy field with rice straw derived biochar. Sci. Total Environ. 660, 512–518 (2019).
Dempster, D. N., Jones, D. L. & Murphy, D. M. Clay and biochar amendments decreased inorganic but not dissolved organic nitrogen leaching in soil. Soil Res. 50, 216–221 (2012).
Ding, Y., Liu, Y., Wu, W., Shi, D. & Yang, M. Evaluation of biochar effects on nitrogen retention and leaching in multi-layered soil columns. Water Air Soil Poll. 213, 47–55 (2010).
Amin, A. E. E. A. Z. Carbon sequestration, kinetics of ammonia volatilization and nutrient availability in alkaline sandy soil as a function on applying calotropis biochar produced at different pyrolysis temperatures. Sci. Total Environ. 726, 138489 (2020).
Xiao, Z. et al. The effect of biochar amendment on N-cycling genes in soils: A meta-analysis. Sci. Total Environ. 696, 133984. https://doi.org/10.1016/j.scitotenv.2019.133984 (2019).
Anderson, C. R. et al. Biochar induced soil microbial soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and Phosphorus. Pedobiologia 54, 309–320 (2011).
Hassink, J. Effects of soil texture and grassland management on soil organic C and N and rates of C and N mineralization. Soil Biol. Biochem. 26, 1221–1231 (1994).
Verberne, E. L. J., Hassink, J., de Willigen, P., Groot, J. J. R. & van Veen, J. A. Modelling organic matter dynamics in different soils. Neth. J. Agric. Sci. 38, 221–238 (1990).
Laird, D., Fleming, P., Wang, B. Q., Horton, R. & Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 158, 436–442 (2010).
Deluca, T. H., MacKenzie, M. D. & Gundale, M. J. Biochar effects on soil nutrient transformations. In Biochar for Environmental Management (eds Lehmann, J. & Joseph, S.) 251–270 (Earthcan, 2009).
Zhai, L. et al. Short-term effects of maize residue biochar on phosphorus availability in two soils with different phosphorus sorption capacities. Biol. Fertil. Soils 51, 113–122 (2014).
Lu, Y. et al. Effects of biochar addition on the abundance, speciation, availability, and leaching loss of soil phosphorus. Sci. Total Environ. 758, 143657. https://doi.org/10.1016/j.scitotenv.2020.143657 (2020).
Amin, A. E. Phosphorus dynamics and corn growth under applications of corn stalks biochar in a clay soil. Arab. J. Geosci. 11(14), 379 (2018).
Mihoub, A., Amin, A. E., Motaghian, H. R., Saeed, M. F. & Naeem, A. Citric acid (CA)–modified biochar improved available phosphorus concentration and its half-life in a P-fertilized calcareous sandy soil. J. Soil Sci. Pl. Nutr. 1, 1 (2022).
Acknowledgements
The authors to the Deanship of Scientific Research, King Saud University, for funding through the Vice Deanship of Scientific Research Chairs, Research Chair of Prince Sultan Bin Abdulaziz International Prize for Water.
Funding
Open access funding provided by Lulea University of Technology. This research was funded by the Deanship of Scientific Research, King Saud University through the Vice Deanship of Scientific Research Chairs, Research Chair of Prince Sultan Bin Abdulaziz International Prize for Water.
Author information
Authors and Affiliations
Contributions
Conceptualization, supervision, methodology, formal analysis, writing—original draft preparation, writing—review and editing, R.K.G., M.V., R.K.N., N.D., N.A.-A.; data curation, project administration, investigation, writing—review and editing, M.S.S., P.K.S., N.R., N.A.-A., A.A., A.Z.A., M.A.M. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gupta, R.K., Vashisht, M., Naresh, R.K. et al. Biochar influences nitrogen and phosphorus dynamics in two texturally different soils. Sci Rep 14, 6533 (2024). https://doi.org/10.1038/s41598-024-55527-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55527-2
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.