Abstract
While novel oral anticoagulants are increasingly used to reduce risk of stroke in patients with atrial fibrillation, vitamin K antagonists such as warfarin continue to be used extensively for stroke prevention across the world. While effective in reducing the risk of strokes, the complex pharmacodynamics of warfarin make it difficult to use clinically, with many patients experiencing under- and/or over- anticoagulation. In this study we employed a novel implementation of deep reinforcement learning to provide clinical decision support to optimize time in therapeutic International Normalized Ratio (INR) range. We used a novel semi-Markov decision process formulation of the Batch-Constrained deep Q-learning algorithm to develop a reinforcement learning model to dynamically recommend optimal warfarin dosing to achieve INR of 2.0–3.0 for patients with atrial fibrillation. The model was developed using data from 22,502 patients in the warfarin treated groups of the pivotal randomized clinical trials of edoxaban (ENGAGE AF-TIMI 48), apixaban (ARISTOTLE) and rivaroxaban (ROCKET AF). The model was externally validated on data from 5730 warfarin-treated patients in a fourth trial of dabigatran (RE-LY) using multilevel regression models to estimate the relationship between center-level algorithm consistent dosing, time in therapeutic INR range (TTR), and a composite clinical outcome of stroke, systemic embolism or major hemorrhage. External validation showed a positive association between center-level algorithm-consistent dosing and TTR (R2 = 0.56). Each 10% increase in algorithm-consistent dosing at the center level independently predicted a 6.78% improvement in TTR (95% CI 6.29, 7.28; p < 0.001) and a 11% decrease in the composite clinical outcome (HR 0.89; 95% CI 0.81, 1.00; p = 0.015). These results were comparable to those of a rules-based clinical algorithm used for benchmarking, for which each 10% increase in algorithm-consistent dosing independently predicted a 6.10% increase in TTR (95% CI 5.67, 6.54, p < 0.001) and a 10% decrease in the composite outcome (HR 0.90; 95% CI 0.83, 0.98, p = 0.018). Our findings suggest that a deep reinforcement learning algorithm can optimize time in therapeutic range for patients taking warfarin. A digital clinical decision support system to promote algorithm-consistent warfarin dosing could optimize time in therapeutic range and improve clinical outcomes in atrial fibrillation globally.
Similar content being viewed by others
Stroke killed an estimated 6,190,000 people in 2019, making it the 2nd leading cause of death worldwide1. A leading cause of stroke is atrial fibrillation, a heart arrythmia that imposes a five-fold increase in the risk of ischemic stroke2. While novel oral anticoagulants are increasingly used to reduce risk of stroke in patients with atrial fibrillation, vitamin K antagonists such as warfarin continue to be commonly used for stroke prevention across the world3,4, and remains the only oral option for patients with conditions such as mechanical heart valves.
While effective in reducing the risk of stroke, vitamin K antagonists like warfarin are difficult to use clinically. They interact with a range of foods and drugs5, and thus require ongoing laboratory monitoring and frequent dose adjustments to maintain adequate anticoagulation. In clinical practice, this is accomplished by monitoring plasma International Normalized Ratio (INR), which evaluates coagulation. The established therapeutic range for atrial fibrillation is an INR of 2.0–3.0, and extended time in therapeutic range (TTR) is associated with a lower risk of stroke6. However, given the complex pharmacodynamics of warfarin, achieving a high TTR can be difficult and thus many patients on warfarin experience inadequate anticoagulation7.
Clinical and computerized algorithms have been developed to support clinicians in addressing the challenges of managing warfarin, and both have been shown to improve TTR8,9,10,11,12. While effective, most of these algorithms are relatively simple, typically providing clear decision rules based on INR thresholds (such as increasing the dose of warfarin by 10% if INR = 1.5–2.0). This approach has the advantage of being highly interpretable and easy to implement, but the potential disadvantage of being ‘one-size fits all’, in that the same set of rules are applied irrespective of a patient’s age, ethnicity, concurrent medications, and other factors that may be associated with warfarin metabolism. Indeed, there is evidence that more data-driven approaches to warfarin dosing could provide incremental gains in TTR over existing algorithms, further reducing the risk of stroke in patients with atrial fibrillation11,12.
Warfarin dosing for atrial fibrillation can be framed as a dynamic treatment regime problem13. Dynamic treatment regimes are sets of rules or policies that describe how treatments are to be assigned in response to a patient’s state, including dynamically changing factors like INR14,15. Initial approaches to optimizing dynamic treatment regimes employed statistical methods16, but there is growing interest in reinforcement learning (RL) for this application17,18. RL is a branch of machine learning in which an agent learns an optimal policy through interactions with an environment, aiming to maximize a reward function. RL has been applied with varying levels of success to dynamic treatment regime problems in sepsis19, cancer20, epilepsy21, diabetes22, schizophrenia23, anemia24, and HIV25.
We used a novel semi-Markov decision process26 formulation of the Batch-Constrained deep Q-learning27 algorithm to develop an RL model that can dynamically recommend optimal warfarin dosing for patients with atrial fibrillation (Fig. 1). We developed and evaluated this model on four large non-overlapping data sets made up of patients in the control groups of the pivotal randomized clinical trials of dabigatran (RE-LY)28, edoxaban (ENGAGE AF-TIMI 48)29, apixaban (ARISTOTLE)30 and rivaroxaban (ROCKET AF)31, made available to the research team as part of the COMBINE AF collaborative32. Each of these clinical trials compared a different anticoagulant to warfarin, providing us with a total sample (following data cleaning) of 28,232 patients on warfarin with INR monitoring, followed for a median of 22.2 months, for a total of 892,333 dose–response pairs for training and evaluation. The use of randomized control trial data for reinforcement learning model development is novel and provides several notable advantages over most routinely collected data sets, including dedicated data collection and on-site monitoring to ensure high data quality, a clinically representative set of baseline patient features for accurate modeling of the state space, a more statistically robust approach to evaluation, and more generalizability thanks to a study population that includes patients living across six continents.
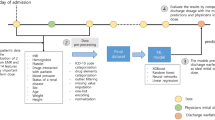
Warfarin dosing optimization as a sequential reinforcement learning task using semi-Markov Decision Processes. The figure illustrates the first several time intervals of a patient trajectory. The highlighted values in the top part of the figure illustrate a single patient trajectory. Each observed time step (T = 0, T = 1, etc.) has a corresponding International Normalized Ratio (INR) test result. The action space is illustrated in purple and is defined as percent changes in warfarin dose—the arrows illustrate that the effect of the change in dose is observed at the following time step. The dynamic elements of the state space are INR test results. We employ a semi-Markov Decision Process framework to handle the inconsistent time intervals between observations inherent to warfarin management, illustrated in the bottom part of the figure. Linear interpolation (Rosendaal’s method) is used to generate INR values for intermediate unobserved time steps. We then calculate intermediate rewards based on interpolated INR values (rewarded when INR is in the range of 2–3) and apply a cumulative discounted rewards to each observed time step. The cumulative discounted reward for an observed time step is applied to the action taken at the previous observed time step (e.g., the dosing action observed at T = 1 is rewarded based on the INR value observed at T = 2). We do not model warfarin initiation, so there is no reward function at T = 0. Observed time step are displayed in darker shades; unobserved interpolated time steps are displayed in lighter shades.
The primary objective of this study was to assess whether a reinforcement learning model for warfarin dosing could achieve equivalent time in therapeutic range and event reduction compared to a previously published clinical algorithm9 (referred to throughout as the “benchmark algorithm”). The secondary objective was to assess whether the inclusion of variables associated with warfarin metabolism (such as age, ethnicity, concurrent medications, etc.) improve performance over the traditional approach of focusing more strictly on INR.
Methods (online supplement)
Data sources
We developed and validated our model using data from randomized control trials that compared novel oral anticoagulants vs. warfarin. These trials were:
-
ARISTOTLE: patients randomized to apixaban (n = 9120) or warfarin (n = 9081) with a median follow up of 21.7 (16.1–28.0) months30.
-
ENGAGE AF-TIMI 48: patients randomized to higher dose edoxaban regimen (n = 7035), lower dose regimen edoxaban (n = 7034), or warfarin (n = 7036) with a median follow up of 33.6 (29.4–38.4) months29.
-
ROCKET AF: patients randomized to rivaroxaban (n = 7131) or warfarin (N = 7133) with a median follow up for 22.00 (15.8–27.8) months31.
-
RE-LY: patient randomized to higher dose dabigatran (n = 6076), lower dose dabigatran (n = 6015), or warfarin (n = 6022) with a median follow up for 24.0 months28.
Patient cohort
Patients in all four trials had atrial fibrillation confirmed by electrocardiogram. All trials excluded patients who had rheumatic mitral stenosis, mechanical prosthetic valves, severe renal impairment, or recent major bleeding, or whose atrial fibrillation was due to a reversible condition. All four trials used an INR target of 2–3 for their warfarin arm. Patients on warfarin were followed for a median of 22.2 months. A difference between patients across the trials was the number of risk factors for stroke required for inclusion32.
Exclusion criteria
We began with 29,272 patients in our combined data set. We excluded all patients who had no recorded dose(warfarin)-response(INR) pairs, as well as all patients with a weekly dose of > 140 mg (well beyond the reasonable range and indicative of data entry errors). For the external validation set, we also excluded any patients who were missing covariates required for performance estimation (described below), leaving a total of 28,232 patients.
Data preprocessing
For all trials, we extracted patients in the warfarin arms. To ensure each warfarin dose recorded would have a corresponding INR measurement, we removed all warfarin doses recorded before a patient’s first INR measurement and after their last INR measurement.
We extracted a subset of data from the trials based on clinical expert input about the features most relevant to warfarin dosing decisions. We extracted the following features for inclusion in our study: age, sex, weight, region, smoking status, concomitant medications (aspirin/amiodarone/thienopyridines), diabetes status, history of congestive heart failure, history of hypertension, history of myocardial infarction, current absolute warfarin dose, current INR, previous 4 INRs, whether the patient experienced a stroke during the study period, whether the patient experienced a major bleed during the study period, and whether the patient was hospitalized during the study period.
We discretized some continuous variables, which is known to aid in the learning process. These include age, weight, and warfarin dose, with the resulting categorical variable one-hot encoded with the first level dropped (i.e. we created binary dummy variables for each level of the category).
There were differences across the trials in how warfarin doses were recorded, so we normalized to weekly doses since this is reflective of how warfarin is prescribed in clinical practice.
The action space was constructed by categorizing warfarin dose adjustments based on clinical expert input and reference to existing clinical algorithms8,9. Possible actions were: decrease > 20%, decrease 10–20%, decrease ≥ 10%, no change, increase ≤ 10%, increase 10–20%, and increase > 20%.
Patient trajectories were organized as longitudinal sequences of dose (warfarin)-response (INR) pairs. We elected to split patient trajectories in a few scenarios. The first is when a significant adverse event was recorded (ischemic stroke, hemorrhagic stroke, major bleeding, hospitalization), since our RL model is meant to inform the management of atrial fibrillation in the community, not acute stroke or hemorrhage. We also split trajectories in cases where more than 90 days passed between clinical visits, since in these cases patients may be receiving care outside of the clinical trial (due to international travel, for example) and thus we deemed imputation unreliable in this context. Thanks to the rigor of data collection for randomized control trials, these data sets required minimal data cleaning, however some missing or impossible values for warfarin dose and INR were identified. In these cases, we elected to split patient trajectories rather than to impute values for the missing data, because imputing values for either feature could result in cases where our model received incorrect rewards, introducing noise into our learned policy. Given the length of patient trajectories and low level of missingness in this data set, we determined that splitting trajectories in these cases would not negatively impact the learning process. Impossible values (such as INR = 0) were treated as missing. After implementing these trajectory splits, we were left with 42,384 patient trajectories for development and evaluation.
Model development
Our RL model was developed with the Batch Constrained Q-learning algorithm27 using a formulation designed for discrete action spaces33. The Batch Constrained Q-learning algorithm employs double deep Q-learning34,35, accompanied by a generative network to constrain the action space to actions previously observed. We selected this algorithm for its robustness in an “offline” setting where there are suboptimal clinical decisions in the observed data set and where complex pharmacodynamics of warfarin introduce a degree of uncertainty in predicting patient response to dose changes.
Reinforcement learning models, including the Batch Constrained Q-learning algorithm, typically structure the interaction between an agent and its environment as a Markov decision process, which assumes that state transitions occur at regularly spaced timesteps. However, in clinical practice warfarin dosing decisions are made at irregular timesteps. To account for this, we model our decision problem as a semi-Markov decision process26,36.
In regular clinical practice, warfarin dose decisions are made during a clinic or virtual visit, and that dose is maintained until the next visit, when a new INR result is available. Using the semi-Markov decision process framework, we model these clinical decisions as “options”, which are sequences of “primitive actions” which we model as daily dose adjustments. For example, if a clinician prescribes a dose adjustment of "Increase by > 20%" on day 5, and the next visit does not occur until day 10, we model this as an option consisting of the primitive action sequence “Increase > 20%” on day 5, and “no change” on days 6–9.
In this framework, the reward for each option is derived from rewards for each primitive action. We use Rosendaal’s linear interpolation method37 to generate INR values to reward each primitive action in an option (+ 1 for INR in range, 0 for INR out of range), which are used to calculate a cumulative discounted reward for each option (Fig. 1).
Using this framework, the goal of the Batch Constrained Q-learning algorithm is to the learn the policy
which maps the current patient state \(s\) to the best available option \(o\) according to its expected return \(Q\left(s,o\right)\). The \({\text{argmax}}\) function returns the option \(o\) from the set \(\mathcal{O}\left({\text{s}}\right)\) with the maximum value of \(Q\left(s,o\right)\), where \(\mathcal{O}\left({\text{s}}\right)\) is the set of options provided by the generative network for state \(s\). The policy \(\uppi \left(s\right)\) is learned by optimizing the estimate of the expected return via stochastic gradient descent. The learning process was governed by the following hyperparameters: batch size, learning rate, threshold, number of hidden layers, dimensionality of hidden layers, and the moving average parameter for the value estimator. Hyperparameters were optimized using grid search.
Using this RL framework, we developed a model using current and previous INR values and warfarin doses as model inputs (ie. patient state). For the secondary objective we developed a second model that made use of all of the clinical features described in the previous section.
Implementation of the benchmark algorithm and non-optimal policies
Comparing a RL policy against observed clinician behavior can lead to overly optimistic assessments of model performance33,34. To address this, we implemented three additional policies to evaluate our RL policy against.
The first of these was a clinical algorithm that provides clear warfarin dosing rules based on INR thresholds: if INR is less than 1.5 then increase warfarin dose by 15%, if INR 1.5–1.99 then increase by 10%; if INR 2.0–3.0 then no change, if INR 3.0–4.0 then reduce dose by 10%, if INR 4.0–4.99 then hold the dose for one day and then decrease by 10%, and if the INR was greater than 5.00 the dose was to be held until the INR was therapeutic and then decrease by 15%. Use of this type of algorithm has been shown to provide significant improvement in TTR over un-assisted clinician decision making9,35, and thus we refer to it throughout as the “benchmark algorithm.”
We also implemented two intentionally non-optimal policies against which to compare both the benchmark algorithm and our RL policy. One of these is a “no change” policy, which always maintains the current warfarin dose, regardless of changes in INR. The second is a “random” policy, which randomly selects a dose change at each observed time step, with the available options weighted based on their observed frequency in our data set.
Performance evaluation
To evaluate the relationship between algorithm-consistent dosing and TTR, we fit weighted linear regression models at the center level. Center-level algorithm-consistency was calculated by averaging the difference between the observed warfarin dose and the dose recommended under each policy. Center-level TTR was calculated by averaging the values obtained from patients in each center. Models were weighted by the number of patients per center, and were developed with mean center algorithm-consistency as a predictor and mean center TTR as the outcome. A coefficient of determination (R2) was reported for each model.
To estimate the relationship between center-level algorithm-consistency and TTR while controlling for patient, center and country-level factors, we developed multilevel, multivariable linear regression models, with patients nested in centers, and centers nested in countries. Patient-level characteristics included age (years); weight (kg); sex (male vs. female); ethnicity (white vs. other); current smoking status (yes vs. no); history of heart failure (yes vs. no), hypertension (yes vs. no), diabetes mellitus (yes vs. no), stroke (yes vs. no); previous warfarin use (yes vs. no); current amiodarone use (yes vs. no); and current insulin use (yes vs. no). Center-level characteristics included specialty (anticoagulation clinic vs. other), setting (secondary/tertiary hospital vs. primary), and center-level mean algorithm-consistent warfarin dosing (%). Country-level characteristics included the 2006 country Gross Domestic Product (high income vs. medium/low income), Disability Adjusted Life Expectancy (years), and Health System Performance Index. We also included random intercept for center and country.
Finally, to estimate the association between algorithm-consistent dosing and the composite outcome of stroke, systemic embolism, or major hemorrhage we developed multilevel, multivariable Cox proportionate hazard models. The Cox models were adjusted for the same patient-, center-, and country-level characteristics as in the previous multilevel model, including random intercepts. Additionally, four medication variables were included, which were baseline use of aspirin (yes vs. no), β-blockers (yes vs. no), ACE-inhibitors (yes vs. no), or statins (yes vs. no). Because the effect of warfarin dosing on clinical outcomes is mediated through INR control, TTR was not included as a covariate in the Cox models. Patients who experienced stroke, systemic embolism, or major hemorrhage were censored when the first adverse event happened, and algorithm-consistency was calculated using warfarin doses before their first adverse event; for patients who did not experience an event, algorithm consistency was calculated using all warfarin doses throughout the study. Algorithm-consistency was calculated for each center and was analyzed as a center-level variable.
To evaluate whether algorithm consistent dosing at the center-level was a marker of generalized high quality care, independent of its influence on warfarin control, we refit the multilevel Cox proportional hazards model on patients taking dabigatran to assess whether center-level algorithm consistency could independently predict center-level outcomes for dabigatran patients.
Ethics
This paper is a sub-study of COMBINE AF32, approved by the Duke University Institutional Review Board. Additionally, each of the four randomized control trials used in this study had institutional review board approval28,29,30,31. All participants in these trials provided informed consent, which included consent for secondary use of trial data in future research.
Results
A total of 28,232 patients with atrial fibrillation from the warfarin arms of four clinical trials of novel oral anticoagulants vs. warfarin were included in our study. The development set was made up 22,502 patients from 52 countries enrolled in the ARISTOTLE, ENGAGE AF-TIMI 48 and ROCKET AF trials. External validation set was made up of 5,730 patients from 44 countries enrolled in the RE-LY trial. All countries included in the external validation set were represented in the development set. The studies were well balanced with respect to most baseline patient characteristics (Table 1). Studies varied slightly in the distribution of continent (RE-LY had more patients from North America and Western Europe, for example) and ethnicity (RELY and ENGAGE have relatively few patients who identified as Hispanic). The most significant difference between the studies was the relatively higher rates of comorbidities in ENGAGE and ROCKET (mean CHADS2 score of 2.8 and 3.5 respectively), vs. ARISTOTLE and RE-LY (CHADS2 scores of 2.1), which was due to differences in the number of risk factors for stroke required for inclusion in each trial (1 for RE-LY and ARISTOTLE, 2 or more for ENGAGE and ROCKET).
Policies based on observed clinician behavior, the benchmark algorithm and our RL model are visualized in Fig. 2. Observed clinician behavior suggests some clinicians tend to maintain a dose even when INR well out of range (clinicians maintained the dose 32% of the time when INR was < 1.5 and 25% of the time when INR was > 3.5), while others will make relatively large dose adjustments in the same circumstances (36% of the time clinicians increased the dose by > 20% when INR was < 1.5 and 28% of the time decreased the dose by > 20% when the INR was > 3.5). Observed clinical behavior appears to include examples of clinically unintuitive behavior, such as cases where clinicians decrease the dose dramatically when INR was < 1.5 and increased it substantially when INR was > 3.5. Upon reviewing these cases, we found them to be clinical scenarios in which a clinician had temporarily suspended and then resumed a patient’s warfarin dose in response to an extreme INR or a clinical event such as an emergency surgery. The benchmark algorithm provides dosing recommendations based purely on INR thresholds, and so produces a very clean policy with no apparently unintuitive recommendations. The benchmark algorithm was designed to minimize dramatic swings in INR and so does not recommend any dose changes greater than 15%, even when INR is well out of range. The RL policy appears to be more aggressive than the others, rarely maintaining a dose when the INR is out of range and recommending dosage adjustments of > 20% relatively frequently (63% of cases where INR was < 1.5 and 20% of cases where INR was > 3.5). We conducted a manual post hoc review of cases where the RL policy appears to offer unintuitive dose adjustments when INR out of range and found that these were examples of the same temporary warfarin suspension/resumption scenarios described above.
Comparison of clinician, reinforcement learning (RL), and benchmark policies. The figure illustrates clinician, benchmark algorithm and RL policies using heatmaps, where the X axis illustrates INR result bins, and the Y axis illustrates the distribution of dose recommendations within each INR result bin. On average, the RL policy recommends larger dose changes in the context of very high and very low INR values than either the clinician or benchmark policies.
We evaluated the potential effectiveness of the RL policy to improve TTR using a weighted linear regression of the association between mean center algorithm agreement and mean center TTR. This analysis was conducted on our external validation set, which included 5730 patients from 903 centers spread across 44 countries. This analysis showed a positive association between RL-consistent dosing and TTR at the center-level (R2 = 0.56). We carried out the same analysis for the benchmark algorithm and found a similarly strong association between algorithm consistent dosing and TTR (R2 = 0.53) that is consistent with previous research9 (Fig. 3).
Weighted linear regression of the association between mean center algorithm-consistency and mean center time in therapeutic range (TTR). Mean center algorithm-consistent dosing and TTR were calculated by averaging the values obtained from patients in each center. The regression model was weighted by the number of patients per center. Each data point represents a single center, and the size of the data point represents the number of patients in that center.
We further explored the relationship between algorithm consistent dosing and TTR using a multilevel, multivariable linear regression, with patients nested in centers, and centers nested in countries (Table 2). After adjusting for patient, center, and country characteristics, center-level RL-consistent dosing was the strongest predictor of TTR, with each 10% increase in algorithm-consistent dosing at the center level predicting a 6.78% improvement in TTR (95% CI 6.29, 7.28, p < 0.001). We repeated this analysis for the benchmark algorithm and found comparable results, with each 10% increase in algorithm-consistent dosing predicting a 6.10% increase in TTR (95% CI 5.67, 6.54, p < 0.001).
To examine the degree to which the improvement in TTR associated with RL-consistent dosing might translate into reductions in stroke and other events, we developed a multilevel, multivariable Cox proportional hazard model to examine the relationship between center-level algorithm-consistency and a composite outcome of stroke, systemic embolism, or major hemorrhage (Table 3). We found that each 10% increase in RL-consistent dosing was associated with a 11% decrease in the composite outcome (HR 0.89; 95% CI 0.81, 0.98, p = 0.015). We repeated this analysis for the benchmark algorithm and found that every 10% increase in agreement was associated with an 10% decrease in the composite outcome (HR 0.90; 95% CI 0.83, 0.98, p = 0.018).
The association between algorithmically consistent dosing and the composite clinical outcome suggests a potential causal relationship, but a lower event rate at centers with better anticoagulation management could alternately be the result of generally higher quality care at those centers. However, if the lower composite clinical event rate were the result of higher quality of care at the center-level, then we would expect to see a similarly lower rate of events among dabigatran patients at the same centers. To assess this, we refit the Cox model for dabigatran patients in the evaluation set (N = 9467), but found no association between center-level algorithm consistent dosing and the composite clinical event rate for patients on dabigatran (HR 0.96; 95% CI 0.90, 1.02, p = 0.16).
To assess whether the inclusion of variables associated with warfarin metabolism provided any improvement in performance over a more strict focus on INR and previous dose, we used the same multilevel, multivariable regression models to evaluate a second RL model that included clinical features such as age, ethnicity, and concurrent medications in addition to INR and previous warfarin doses. There was very little difference in the performance of these two models, with each 10% increase in algorithm-consistent dosing predicting a 6.78% improvement in TTR in the case of the INR/dose only model and 6.49% in the case of the model that included the additional clinical features. The difference was slightly larger in the case of events, with each 10% increase in algorithm-consistent dosing associated with a 10% decrease in the composite outcome for the INR/dose only model and 7% for the model that included the additional clinical features.
Discussion
In this study of patients with atrial fibrillation receiving vitamin K antagonist therapy, we demonstrate that a RL model could achieve TTR improvement and event reduction equivalent to an established rules-based warfarin dosing algorithm designed by clinical experts. This data driven model was developed using 3 international randomized control trials, and externally validated on a fourth trial of patients living in 44 countries across 6 continents. In addition to potentially improving warfarin dosing for atrial fibrillation (still being used commonly around the world despite the rise of novel oral anticoagulants) the methodology described in this paper may have important applications in other clinical domains, including dosing of warfarin for other indications (such as patients with mechanical valves) as well as optimization of other medications where dosing is based on sequential dose–response relationships, such as heparin, insulin, oral antihyperglycemic drugs, and phenytoin.
This study helps address some of the existing knowledge gaps with respect to the use of machine learning to improve warfarin dosing. A number of machine learning-based systems have been developed, but many of these have been focused on patients within a single jurisdiction or ethnic group, which may limit their generalizability41,42,43,44,45,46. A number of machine learning studies have leveraged International the Warfarin Pharmacogenetics Consortium dataset of 5700 patients from 21 countries, however many of these have incorporated genetic markers associated with warfarin metabolism46,47,48,49,50, which may offer valuable performance improvements, but cannot be easily integrated into routine clinical practice (while single nucleotide polymorphisms CYP2C9 and VKORC1 were available for a subset of our patients, we elected not to use them in order to enhance the generalizability of our model for real world clinical settings, especially low and middle income countries where warfarin is still routinely used). Ours is also the first study we are aware of to evaluate the use of machine learning for the long-term dynamic management of warfarin in the community, versus warfarin initiation or short-term management during a hospital stay42,43,44,45,46,47. Our study is the largest its kind by a considerable margin, which is relevant given the data-hungry nature of machine learning methods.
Our study also reveals that clinical features related to warfarin metabolism do not appear to provide sufficient information to justify their inclusion in a model. While one of the strengths of a machine learning approach is to be able to incorporate high dimensional data, in this case the established clinical approach of including only INR and previous warfarin doses to drive decision making produced the best performing model. While are unable to say definitively what was responsible for this phenomenon, given the nature of neural networks it is likely that it is due to a form of overfitting. In particular, the personalized model may have been learning spurious correlations between demographic features and INR that do not generalize between our development data set and the external validation data set (Table S1).
A common limitation of most reinforcement learning research in the health domain has been the lack of rigorous statistical evaluation. Unlike supervised learning approaches that can be evaluated using standard techniques for classification and regression, evaluation of RL models on observational data have typically been limited to importance sampling or ad hoc methods (such as U-curves), both of which have limitations that can produce performance estimates that are overly-optimistic19,38,39. We were able to address this limitation by taking advantage of the multicenter structure of our data to apply statistically robust techniques developed in a previous study of warfarin dosing algorithms9,40. This approach allowed us to provide a rigorous estimate the relationship between algorithmically consistent dosing, TTR, and patient outcomes.
Our study has a number of important advantages over some other reinforcement learning applications in health care, owing to the structure of the warfarin dosing problem. In many clinical domains where RL has been applied, such as sepsis, data sets have not always contained all the data relevant to clinical decision making, which can lead to estimates confounded by spurious correlation38. In addition, RL studies in domains such as sepsis can suffer from a sparsity of rewards, where events of interest can be so infrequent that it becomes challenging to assess the value of an individual treatment decision39. In our case, however, the data set contained all the relevant data points used to manage warfarin in routine clinical practice. Moreover, the causal connection between INR and clinically relevant outcomes is well-established, which allowed us to use INR results to reward (or not) every decision the model observed during training.
Finally, it is important to note that while our study establishes that RL can be used to learn an optimal policy for warfarin dosing, it provides a relatively small improvement over that of a rules-based clinical algorithm. While our study suggests promise for the future of artificial intelligence to learn directly from clinical data to enhance care, it is important to recognize just how effective a well-researched, interpretable rule-based algorithm developed by clinical experts can be in supporting better clinical practice.
Limitations
Our study is retrospective, so prospective evaluation through a randomized control trial should be undertaken prior to adoption of our model into routine clinical practice. Because our study is based on data from the setting of clinical trials with strict inclusion/exclusion criteria and selected centers, our results may underestimate real-world variation in TTR between centers51,52 as well as the association between warfarin dosing and clinical outcomes. While our evaluation methods account for some social factors such as country-level GDP, we were not able to consider factors such as income or education level, which can include warfarin dose variability, TTR and outcomes. Our methodology employs deep neural networks, whose inner workings are black boxes – while explainable machine learning techniques may go some way to increasing the clinical acceptability of black box models, these techniques have some limitations of their own53.
Summary
In summary, we trained a deep reinforcement learning model on longitudinal data from 22,502 patients on warfarin, and externally validated this model on a further 5730 warfarin patients from 44 countries across 6 continents. Our findings suggest that a machine learning algorithm is capable of optimizing time in therapeutic range for patients taking warfarin. A digital clinical decision support system to promote algorithm-consistent warfarin dosing could optimize time in therapeutic range and improve clinical outcomes in atrial fibrillation globally.
Data availability
Access to the RCT data used for this study is governed by the COMBINE AF steering committee. Investigators interested in working with this data set should contact a member of the steering committee to discuss potential collaborations32. The complete source code for this study is publicly available on GitHub54. We have deployed the final model for demonstration and validation purposes as a publicly available web tool55.
References
World Health Statistics 2021: Monitoring Health for the SDGs, Sustainable Development Goals. (World Health Organization, Geneva, 2021).
Wolf, P. A., Abbott, R. D. & Kannel, W. B. Atrial fibrillation as an independent risk factor for stroke: The framingham study. Stroke 22, 983–988 (1991).
Lippi, G., Mattiuzzi, C., Cervellin, G. & Favaloro, E. J. Direct oral anticoagulants: Analysis of worldwide use and popularity using Google Trends. Ann. Transl. Med. 5, 322 (2017).
Pirmohamed, M. Warfarin: The end or the end of one size fits all therapy?. J. Pers. Med. 8, 22 (2018).
Wadelius, M. & Pirmohamed, M. Pharmacogenetics of warfarin: Current status and future challenges. Pharmacogenom. J. 7, 99–111 (2007).
Jones, M. et al. Evaluation of the pattern of treatment, level of anticoagulation control, and outcome of treatment with warfarin in patients with non-valvar atrial fibrillation: A record linkage study in a large British population. Heart 91, 472–477 (2005).
Connolly, S. J. et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 118, 2029–2037 (2008).
Nieuwlaat, R. et al. Randomised comparison of a simple warfarin dosing algorithm versus a computerised anticoagulation management system for control of warfarin maintenance therapy. Thromb. Haemost. 108, 1228–1235 (2012).
van Spall, H. G. C. et al. Variation in warfarin dose adjustment practice is responsible for differences in the quality of anticoagulation control between centers and countries: An analysis of patients receiving warfarin in the randomized evaluation of long-term anticoagulation the. Circulation 126, 2309–2316 (2012).
Kim, Y. K. et al. Effect of a simple two-step warfarin dosing algorithm on anticoagulant control as measured by time in therapeutic range: A pilot study. J. Thromb. Haemost. 8, 101–106 (2010).
Gage, B. F. et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin. Pharmacol. Ther. 84, 326–331 (2008).
WarfarinDosing. http://www.warfarindosing.org/Source/Home.aspx.
Rosthøj, S., Fullwood, C., Henderson, R. & Stewart, S. Estimation of optimal dynamic anticoagulation regimens from observational data: A regret-based approach. Stat. Med. 25, 4197–4215 (2006).
Mahar, R. K. et al. A scoping review of studies using observational data to optimise dynamic treatment regimens. BMC Med. Res. Methodol. 21, 1–13 (2021).
Chakraborty, B. & Murphy, S. A. Dynamic treatment regimes. Annu. Rev. Stat. Appl. 1, 447–464 (2014).
Murphy, S. A. et al. Optimal dynamic treatment regimes. J. R. Stat. Soc. Ser. B Stat. Methodol. 65, 331–355 (2003).
Zhang, Z. Reinforcement learning in clinical medicine: A method to optimize dynamic treatment regime over time. Ann. Transl. Med. 7, 345–345 (2019).
Coronato, A., Naeem, M., De Pietro, G. & Paragliola, G. Reinforcement learning for intelligent healthcare applications: A survey. Artif. Intell. Med. 109, 101964 (2020).
Komorowski, M., Celi, L. A., Badawi, O., Gordon, A. C. & Faisal, A. A. The Artificial Intelligence Clinician learns optimal treatment strategies for sepsis in intensive care. Nat. Med. 24, 1716–1720 (2018).
Liu, Y. et al. Deep Reinforcement Learning for Dynamic Treatment Regimes on Medical Registry Data. in Proceedings - 2017 IEEE International Conference on Healthcare Informatics, ICHI 2017 380–385 (Institute of Electrical and Electronics Engineers Inc., 2017). https://doi.org/10.1109/ICHI.2017.45.
Pineau, J., Guez, A., Vincent, R., Panuccio, G. & Avoli, M. Treating epilepsy via adaptive neurostimulation: A reinforcement learning approach. Int. J. Neural. Syst. 19, 227–240 (2009).
Wang, Y., Fu, H. & Zeng, D. Learning optimal personalized treatment rules in consideration of benefit and risk: With an application to treating Type 2 diabetes patients with insulin therapies. J. Am. Stat. Assoc. 113, 1–13 (2018).
Shortreed, S. M. et al. Informing sequential clinical decision-making through reinforcement learning: An empirical study. Mach. Learn. 84, 109–136 (2011).
Escandell-Montero, P. et al. Optimization of anemia treatment in hemodialysis patients via reinforcement learning. Artif. Intell. Med. 62, 47–60 (2014).
Parbhoo, S., Bogojeska, J., Zazzi, M., Roth, V. & Doshi-Velez, F. Combining kernel and model based learning for HIV therapy selection. AMIA Jt Summits Transl. Sci. Proc. 2017, 239–248 (2017).
Sutton, R. S., Precup, D. & Singh, S. Between MDPs and semi-MDPs: A framework for temporal abstraction in reinforcement learning. Artif. Intell. 112, 181–211 (1999).
Fujimoto, S., Meger, D. & Precup, D. Off-policy deep reinforcement learning without exploration. in 36th International Conference on Machine Learning, ICML 2019 vols 2019-June 3599–3609 (2019).
Connolly, S. J. et al. Dabigatran versus warfarin in patients with atrial fibrillation. New Engl. J. Med. 361, 1139–1151 (2009).
Giugliano, R. P. et al. Edoxaban versus warfarin in patients with atrial fibrillation. New Engl. J. Med. 369, 2093–2104 (2013).
Granger, C. B. et al. Apixaban versus warfarin in patients with atrial fibrillation. New Engl. J. Med. 365, 981–992 (2011).
Patel, M. R. et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. New Engl. J. Med. 365, 883–891 (2011).
Carnicelli, A. P. et al. Individual patient data from the pivotal randomized controlled trials of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation (COMBINE AF): design and rationale: from the COMBINE AF (A Collaboration between Multiple institutio. Am Heart J 233, 48–58 (2021).
Fujimoto, S., Conti, E., Ghavamzadeh, M. & Pineau, J. benchmarking batch deep reinforcement learning algorithms. 1–13 (2019).
Van Hasselt, H., Guez, A. & Silver, D. Deep reinforcement learning with double Q-Learning. 30th AAAI Conference on Artificial Intelligence, AAAI 2016 2094–2100 (2016).
Mnih, V. et al. Human-level control through deep reinforcement learning. Nature 518, 529–533 (2015).
Fatemi, M. et al. Semi-Markov Offline Reinforcement Learning for Healthcare. in Proceedings of Machine Learning Research 119–137 (2022).
Rosendaal, F. R., Cannegieter, S. C., van der Meer, F. J. & Briët, E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb. Haemost. 69, 236–239 (1993).
Gottesman, O. et al. Guidelines for reinforcement learning in healthcare. Nat. Med. 25, 16–18 (2019).
Gottesman, O. et al. Evaluating Reinforcement Learning Algorithms in Observational Health Settings. (2018).
Rose, A. J. Improving the management of warfarin may be easier than we think. Circulation 126, 2277–2279 (2012).
Roche-Lima, A. et al. Machine learning algorithm for predicting warfarin dose in caribbean hispanics using pharmacogenetic data. Front. Pharmacol. 10, (2020).
Nguyen, V. L. et al. Comparison of multivariate linear regression and a machine learning algorithm developed for prediction of precision warfarin dosing in a Korean population. J. Thromb. Haemost. 19, 1676–1686 (2021).
Steiner, H. E. et al. Machine learning for prediction of stable warfarin dose in US Latinos and Latin Americans. Front. Pharmacol. 12, (2021).
Asiimwe, I. G. et al. Stable warfarin dose prediction in sub-Saharan African patients: A machine-learning approach and external validation of a clinical dose-initiation algorithm. CPT Pharmacometrics Syst. Pharmacol. 11, 20–29 (2022).
Lee, H. et al. Development of a system to support warfarin dose decisions using deep neural networks. Sci. Rep. 11, (2021).
Li, Q. et al. Warfarin maintenance dose Prediction for Patients undergoing heart valve replacement— a hybrid model with genetic algorithm and Back-Propagation neural network. Sci. Rep. 8, (2018).
Grossi, E. et al. Prediction of optimal warfarin maintenance dose using advanced artificial neural networks. https://doi.org/10.2217/pgs.13.21215,29-37 (2013).
Ma, Z., Wang, P., Gao, Z., Wang, R. & Khalighi, K. Ensemble of machine learning algorithms using the stacked generalization approach to estimate the warfarin dose. PLoS One 13, (2018).
Consortium & T. I. W. P,. Estimation of the warfarin dose with clinical and pharmacogenetic data. New Engl. J. Med. 360, 753–764 (2009).
Liu, R., Li, X., Zhang, W. & Zhou, H. H. Comparison of nine statistical model based warfarin pharmacogenetic dosing algorithms using the racially diverse international warfarin pharmacogenetic consortium cohort database. PLoS One 10, (2015).
Rose, A. J. et al. Prompt repeat testing after out-of-range INR values: A quality indicator for anticoagulation care. Circ. Cardiovasc. Qual. Outcomes 4, 276–282 (2011).
Rose, A. J. et al. Gaps in monitoring during oral anticoagulation: Insights into care transitions, monitoring barriers, and medication nonadherence. Chest 143, 751–757 (2013).
Petch, J., Di, S. & Nelson, W. Opening the Black Box: the promise and limitations of explainable machine learning in cardiology. Can. J. Cardiol. 38, 204–213 (2022).
Warfarin study · GitHub · GitHub. https://github.com/hamilton-health-sciences/warfarin.
Warfarin Dosing Algorithm. https://warfarin.herokuapp.com/.
Author information
Authors and Affiliations
Contributions
J.P., W.N., M.W., M.G., A.B., M.F., S.D., A.C., and S.J.C. contributed to study design, data analysis and manuscript drafting. C.G., R.G., H.H., M.P., L.W., and J.E. contributed to data acquisition, data analysis and provided critical feedback on the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr. Petch reports research support from Roche Canada. Mr. Nelson has nothing to disclose. Ms. Wu has nothing to disclose. Ms. Di has nothing to disclose. Dr. Carnicelli is supported by grant funding from the National Institutes of Health (5T32HL069749-17). Dr. Ghassemi has nothing to disclose. Dr. Benz has nothing to disclose. Dr. Fatemi is an employee of Microsoft Research. Dr. Granger reports research grants/contracts from AKROS, Apple, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Duke Clinical Research Institute, US Food and Drug Administration, Glaxosmithkline, Janssen Pharmaceutica, Medtronic Foundation, Novartis, and Pfizer; Consulting from Abbvie, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, CeleCor Therapeutics, Correvio, Espero BioPharma, Janssen, Medscape, Medtronic LLC, Medtronic Inc, Merck, National Institute of Health, Novo Nordisk, Novartis, Pfizer, Rhoshan Pharmaceuticals, and Roche Diagnostics. Dr. Giugliano reports research support from Amgen and Anthos Therapeutics; Honoraria for CME lectures from Amgen, Daiichi Sankyo, and Servier; Consultant fees from Amarin, American College of Cardiology, Amgen, Astra Zeneca, CryoLife, CVS Caremark, Daiichi Sankyo, Esperion, Gilead, Glaxosmithkline, SAJA Pharmaceuticals, Samsung, and Servier. Dr. Hong has nothing to disclose. Dr. Patel reports receiving research grants/contracts from AstraZeneca, Bayer, and Janssen Research and Development; Funding through Duke for educational activities from AstraZeneca and Janssen Research and Development; Consulting from AstraZeneca, Bayer, and the Thrombosis Research Institute. Dr. Wallentin reports grants from AstraZeneca, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline, Merck & Co, Boehringer Ingelheim, and Roche Diagnostics; Personal fees from Abbott. Dr. Wallentin has a patent (EP2047275B1) licensed to Roche Diagnostics and a patent (US8951742B2) licensed to Roche Diagnostics. Dr. Eikelboom reports consulting/honoraria support from Astra-Zeneca, Bayer, Boehringer-Ingelheim, Bristol-Myer-Squibb, Daiichi-Sankyo, Eli-Lilly, Glaxo-Smith-Kline, Pfizer, Janssen, Sanofi-Aventis, Servier and grant support from Astra-Zeneca, Bayer, Boehringer-Ingelheim, Bristol-Myer-Squibb, Glaxo-Smith-Kline, Pfizer, Janssen, Sanofi-Aventis. Dr. Connolly reports research support and honoraria for consulting and lectures from Portola, BMS, Pfizer, Javelin, Boehringer Ingelheim, Bayer, Daiichi Sankyo, and Abbott.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Petch, J., Nelson, W., Wu, M. et al. Optimizing warfarin dosing for patients with atrial fibrillation using machine learning. Sci Rep 14, 4516 (2024). https://doi.org/10.1038/s41598-024-55110-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55110-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.