Abstract
While the importance of human milk in shaping infant immune function is well established, the impact of at-the-nipple (ATN) breastfeeding on maternal immune status has been understudied. Since lactation evolved to support infant survival and boost maternal fitness, we predict that ATN breastfeeding will confer benefits on maternal immune function. We measure the absolute and relative frequency of different infant feeding methods (ATN breastfeeding, pumping, donated milk, other supplementation) used by postpartum women in Seattle, WA (USA). We implement Bayesian modeling to estimate the effects of ATN breastfeeding on diurnal change in secretion rate of “pro-inflammatory” salivary cytokines and C-reactive protein (CRP). Our results show that most mothers in our sample used a variety of infant feeding methods, with pumping as the most common alternative to ATN breastfeeding. We find that ATN breastfeeding is associated with non-linear effects on diurnal IL-8 and CRP. Furthermore, we find that women who report zero versus ubiquitous ATN breastfeeding exhibit opposing diurnal patterns in CRP secretion rate. This study provides evidence that variation in maternal lactation practices corresponds to differences in maternal immune responses, highlighting how measuring lactation as a continuous variable can further enhance understanding of postpartum maternal physiology.
Similar content being viewed by others
Introduction
Overview
The well-documented benefits of human milk for infant immune function (e.g., reduced short-term morbidity and mortality from infectious disease)1,2, especially in regions without access to clean drinking water, form the basis for current public health guidance on breastfeeding. Considering lactation evolved as a key form of maternal investment crucial for both infant survival and maternal fitness, lactation may also confer benefits on maternal immune function, especially in the early postpartum period when maternal mortality risk is highest3. To date, however, testing this hypothesis in humans has been complicated by both a relative scarcity of data on postpartum maternal immune function and predominance of infant-centered and binary/categorical measures of breastfeeding behavior (e.g., simple presence/absence of supplementation)4,5,6. Perhaps most critically, we know of very few studies that have separated the immunological effects of at-the-nipple (ATN) breastfeeding from breastfeeding-by-pumping, an evolutionarily novel infant feeding method that preserves many, but not all, of the physiological aspects of ATN breastfeeding. To address these gaps in knowledge, we measure the absolute and relative frequency of ATN breastfeeding used by postpartum mothers during a 24-h collection period and estimate the effects of ATN breastfeeding (modeled as a continuous variable) on salivary markers of inflammation.
Lactation and immune function
The transition from pregnancy (a phase of female reproduction requiring immunological tolerance of a semi-allogeneic fetus7) to the postpartum period is marked by metabolic and hormonal recalibration8,9 and potentially unique demands on immune competence (e.g., heightened pathogen clearance)10. Prior research suggests that the hormonal milieu involved in sustained lactation (e.g., elevated oxytocin and prolactin, reduced progesterone) may bolster immune competence while suppressing hyper-inflammation11,12,13,14,15,16,17,18. In humans, greater energetic throughput and tighter regulation of metabolic “resetting” induced by regular lactation19,20 may also exert anti-inflammatory effects, given the immunological benefits associated with exercise21, regulated caloric restriction22 and reduced adiposity23. To date, however, much of the research on potential mechanisms through which lactation may regulate inflammatory processes has been done outside the context of the postpartum period (e.g., ex vivo studies, use of animal models, exogenous oxytocin dosage in men). Of the studies that have focused specifically on postpartum women, evidence is mixed: some research reports that breastfeeding is associated with heightened and activated innate and specific immune defenses24 while other studies show no effect of lactation on inflammation25. Greater intensity of breastfeeding has been associated with reduced long-term risk of breast26 and ovarian cancer27. However, such observational studies (commonly conducted in economically developed populations) are often plagued by similar confounds (e.g., higher socioeconomic status and better health care access) as those demonstrating long-term benefits of breastfeeding for infants28. There is some evidence, at the proximate level, that breastfeeding activates tumor immune response, but such findings are complicated by categorical measures of breastfeeding (e.g., presence/absence of any reported breastfeeding)29.
Infant feeding behavior from the maternal perspective
A growing number of women worldwide are provided with access to evolutionarily novel alternatives to at-the-nipple breastfeeding (e.g., electric breast pumps, formula) while simultaneously directed to provide only human milk to infants six months of age and younger. Current World Health Organization (WHO) guidelines recommend “exclusive breastfeeding for the [infant’s] first 6 months of life”. Similarly, the United States Centers for Disease Control (CDC) recommends a minimum of six months exclusive breastfeeding, defined as a situation in which an infant is fed “only breast milk—no solids, water, or other liquids”, even though complete lack of supplementation is rare even in natural fertility populations30,31.
One key feature of these guidelines is that they do not differentiate between breastfeeding at-the-nipple and lactation-by-pumping (henceforth referred to as pumping). Pumping, especially using modern pumps with customizable settings, effectively mimics the mechanical aspects of infant suckling32, thus encouraging a similar positive feedback loop that sustains milk production while removing direct nipple contact between mother and infant. Reduced at-the-nipple contact might alleviate risk of direct microbial transfer from infant to mother, but breast pumps themselves can easily become contaminated, presenting an alternative source of pathogen exposure33. Pumping is a particularly useful infant feeding method for when it may be inconvenient, difficult or impossible for a mother to engage in at-the-nipple breastfeeding. Additionally, pumping helps maintain milk supply if the infant is born prematurely and/or has problems latching and more time is needed to successfully establish breastfeeding33,34. On the other hand, pumping may attenuate the predicted benefits of lactation on maternal physiology by disrupting the rhythmicity of milk production and ejection elicited by on-demand ATN breastfeeding, increasing risk of milk stasis (a primary cause of breast inflammation)35 and removing or attenuating important signaling pathways between mother and infant (e.g., hormonal regulation, retrograde flow effects of microbiota)36,37. Given the growing prevalence of pumping as a feasible alternative to ATN breastfeeding38, it is critical to (1) develop more fine-grained measures of lactation that differentiate between these two methods and (2) use these measures to separate out the effects of ATN breastfeeding versus pumping on maternal physiology.
Objectives and predictions
For this study, we developed a novel method for quantifying infant feeding behavior that (1) differentiated between lactation that occurred at-the-nipple versus lactation using a pump, (2) allowed women to report multiple infant feeding methods used for each feeding bout, and (3) produced continuous rather that categorical/binary measures of infant feeding behavior. We then used these data to estimate the effects of at-the-nipple (ATN) breastfeeding on evening-to-morning change in “pro-inflammatory” maternal salivary cytokines (IL-1ß, IL-6, IL-8, TNF-α) and C-reactive protein (CRP), accounting for the separate effects of pumping. Data collection occurred during the COVID-19 pandemic stay-at-home order, which provided a rare ‘natural control’ for concurrent pathogen exposure.
We chose to measure IL-1ß, IL-6, IL-8, TNF-α, and C-reactive protein (CRP) because these markers can be reliably detected in saliva, provide information on both specific inflammatory pathways (cytokines) and non-specific systemic inflammation (CRP), and should therefore provide a snapshot of current inflammatory status39 (Table 1, Table S1). Most salivary cytokines peak at time of waking40,41,42,43, with adverse conditions (e.g., sleep deprivation, PTSD, sleep apnea, obesity, arthritis, cardiovascular disease, domestic abuse) commonly associated with higher morning peaks in inflammatory cytokine levels44,45,46,47. Based on this knowledge, we predicted that morning peak in inflammatory markers would be comparatively attenuated among mothers reporting high incidence of ATN breastfeeding. In other words, we predicted that ATN breastfeeding would be negatively associated with evening-to-morning change in IL-1ß, IL-6, IL-8, TNF-α, and CRP secretion rate.
Results
The demographics of our sample were roughly similar to those of the general Seattle population reported in the 2022 Census (https://www.census.gov/quickfacts/fact/table/WA/PST045222): 75.00% of our participants reported a household income over $100,000 (Table S2), 55.21% reported holding a professional degree (Table S3), 89.58% were married or in a domestic partnership (Table S4), and 67.71% were of European descent (Table S5). Table S6 shows the number and percent of participants by reported employment status. The median age at time of data collection was 34 but ranged from 25 to 42 (Table 2). The median number of days since delivery was 70, ranging from 8 to 212 days (Table 2). Only one participant reported giving birth to twins, while all other participants reported a singleton birth. 27.08% of mothers reported giving birth via Cesarean section (slightly more than the 21% rate reported for Washington by the CDC in 2021) and 44.79% reported having a birth complication of some kind (e.g., pre-eclampsia, delivery 3+ weeks before due date, gestational diabetes, gestational hypertension, breech presentation, manual removal of placenta) (Table 3).
COVID-19 pandemic created unique environment
All data were collected during COVID-19 stay-at-home order. Most women (86.46%) reported at least one “adverse” effect of the pandemic, with 78.12% of participants reporting social isolation and 61.46% reporting heightened anxiety (Table 4). Conversely, our participants reported very low incidence of infectious disease symptoms (likely because of social isolation). Only 4 individuals (4.17%) reported having “a minor cold that made [them] feel uncomfortable but did not keep [them] sick in bed” at some point in the week prior to data collection, while one participant (1.04%) reported incidence of a “stomach bug due to infection (i.e., diarrhea, vomiting)” or “respiratory infection more severe than a minor cold” in the preceding week (Table 3).
Most mothers employed a mixture of infant feeding methods
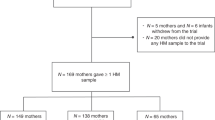
During the 24-h collection period, only 11.46% (n = 11) of participants in our sample reported a complete absence of ATN breastfeeding, 38.54% (n = 37) individuals indicated exclusive ATN breastfeeding, and 50% (n = 48) indicated using a mix of ATN breastfeeding and other infant feeding methods (Table S7). When combined with reported long-term history of infant feeding behavior, only two participants in our sample had never breastfed their infant at-the-nipple. Both these individuals reported reliance on pumped milk, therefore no participants in our sample exhibited exclusive reliance on infant formula. Only 8.33% (n = 8) of participants reported a history of using exclusive at-the-nipple breastfeeding with no supplementation or replacement. In sum, 3.12% of participants (n = 3) reported ever using donated breast milk, 15.62% (n = 15) indicated use of solid foods, 32.29% (n = 31) reported using non-breastmilk liquids at some point, and 85.42% (n = 82) reported either current or past use of expressed/pumped milk.
Morning rise in IL-8 highest among women who reported partial reliance on ATN breastfeeding
As shown in Table S8, median evening IL-8 secretion rate was 127.79 pg/mL/min (IQR = 216.83 pg/mL/min) while median morning IL-8 secretion rate was 269.24 pg/mL/min (IQR = 457.38 pg/mL/min). We found a strong non-linear effect of % ATN breastfeeding on evening-to-morning change (i.e., delta) in IL-8 secretion rate (Variance = 359.42; 95% CI = 25.3, 986.82) (Fig. 1). As described in Table 5, median evening-to-morning rise in IL-8 secretion rate was similar among mothers reporting lowest and highest reliance on ATN feeding, with the maximum predicted rise in IL-8 secretion rate (Median: 538.20 pg/ml/min; 95% CI: 189.85, 925.39) occurring among mothers reporting a moderate rate of 46% ATN breastfeeding. While there was no effect of % pumping on diurnal IL-8 secretion rate (Table S9, Fig. S3), time since delivery was positively associated with morning-to-evening rise in IL-8 secretion rate (Table S9, Fig. S4). Figure 2 shows the effect of increasing time since delivery on predicted values for delta IL-8 secretion rate. Conversely, % ATN breastfeeding, % pumping, and time since delivery were not associated with any robust change in evening-to-morning IL-1ß, IL-6, or TNF-α secretion rate (Table S9, Fig. 1, Figs. S3, S4).
Predicted evening-to-morning change in CRP, IL-8, IL-6, IL-1ß, and TNF-α secretion rates by % ATN breastfeeding. Solid lines: point estimates for predicted median value. Shaded regions: 95% credible intervals. Dotted lines: zero difference between evening and morning secretion rate. Values above the horizontal dotted line: secretion rate is higher in the morning. Values below the horizontal dotted line: secretion rate is higher in the evening.
Predicted median evening-to-morning change in IL-8 by % ATN breastfeeding and time since delivery. Solid lines: point estimates for predicted median value. Shaded regions: 95% credible intervals. Dotted lines: zero difference between evening and morning secretion rate. Values above the horizontal dotted line: secretion rate is higher in the morning. Values below the horizontal dotted line: secretion rate is higher in the evening.
Heavy reliance on ATN breastfeeding associated with evening peak in CRP secretion rate, a pattern exacerbated by concurrent reliance on pumping
As shown in Table S8, median evening CRP secretion rate was 145.02 pg/mL/min (IQR = 287.09 pg/mL/min) while median morning CRP secretion rate was 164.87 pg/mL/min (IQR = 460.40 pg/mL/min). As shown in Fig. 1 and Table 5, we observed a strong non-linear effect of % ATN breastfeeding on evening-to-morning change in CRP secretion rate (Variance = 411.54; 95% CI = 14.28, 1371.3). Among mothers reporting 0% ATN breastfeeding, estimated evening-to-morning change in CRP secretion rate was positive (median: 377.95 pg/ml/min; 95% CI: −41.86, 801.37), indicating CRP secretion rate peaked in the morning. Among mothers reporting 100% reliance on ATN breastfeeding, estimated evening-to-morning change in CRP secretion rate was negative (median: −352.97 pg/ml/min; 95% CI: −682.15, −24.56), indicating CRP secretion rate peaked in the evening. As Fig. 1 shows, the highest predicted evening-to-morning change in CRP secretion rate (Median: 495.16 pg/ml/min; 95% CI: 75.08, 1026.14) occurred among mothers reporting 25% ATN breastfeeding. We also found a negative linear effect of % pumping (Table S9, Fig. S3). Figure 3 shows the effect of different % pumping values on predicted values for the change (delta) in evening to morning CRP secretion rate.
Predicted median evening-to-morning change in CRP by % ATN breastfeeding at 0%, 50% and 100% pumping. Solid lines: point estimates for predicted median value. Shaded regions: 95% credible intervals. Dotted lines: zero difference between evening and morning secretion rate. Values above the horizontal dotted line: secretion rate is higher in the morning. Values below the horizontal dotted line: secretion rate is higher in the evening.
Discussion
In our sample of 96 mothers in the Seattle metro area, most women utilized at-the-nipple (ATN) breastfeeding in addition to other infant feeding methods, with pumping as the most common alternative method. Exclusive formula feeding was nonexistent while long-term exclusive ATN breastfeeding (with no history of supplementation) was reported by less than 10% of participants. These results show that, even in a sample of women reporting various markers of high socioeconomic status (e.g., high household income, high educational attainment, and presence of spouse/domestic partner), most women report mixed feeding in the first 6 months postpartum.
We did not observe any robust effects of ATN breastfeeding (or pumping) on evening-to-morning change in IL-1ß, IL-6, or TNF-α secretion rate. We did, however, find a strong non-linear effect of ATN breastfeeding on diurnal IL-8 secretion rate. Predicted evening-to-morning rise in IL-8 was highest among mothers in the later postpartum period who reported using ATN breastfeeding for roughly a quarter of all infant feeding bouts. We also observed a robust non-linear effect of ATN breastfeeding on diurnal CRP, wherein evening-to-morning rise in CRP secretion rate was highest among women who reported using ATN breastfeeding for approximately half of all infant feeding bouts (with no concurrent use of pumping). On a proximate level, the estimated effects of ATN breastfeeding on CRP (a non-specific measure of systemic inflammation) may be at least partially driven by the downstream effects of ATN breastfeeding on IL-8 activity. IL-8 is a strong chemoattractant with high specificity for neutrophils, a type of granulocyte that induces inflammatory cascades14,48,49,50. Neutrophils may therefore act as key mediators between changes in IL-8 and CRP secretion rates. We strongly recommend that subsequent research investigate the prevalence and activation status of circulating neutrophils among postpartum women practicing different infant feeding methods.
Given that morning rise in IL-8 and CRP secretion rates were highest among women who reported using ATN breastfeeding for less than half of all infant feeding bouts, our results suggest that “part-time” ATN breastfeeding may induce and/or be a consequence of relatively heightened inflammatory activation, with potential implications for maternal response to infectious disease challenges. Such “part-time” ATN breastfeeding might induce inflammation due to longer intervals between breastfeeding bouts, increased milk stasis, altered hormone production, and less energetic throughput. On the other hand, upstream factors that limit ATN breastfeeding (e.g., psychosocial stress, infant-led weaning, return to work) may drive the association between proportionally less frequent use of ATN breastfeeding and comparatively higher evening-to-morning rise in IL-8 and CRP secretion rates. We attempted to control for as many of these factors as possible, but the cross-sectional nature of this study precludes a definitive statement on causality. In reality, these relationships are probably complex and arrows of effect may go in both directions (e.g., reduced time spent ATN breastfeeding may cause stress about breastfeeding goals, exacerbating existing inflammation).
In addition to our observation that evening-to-morning rise in CRP secretion rate was highest among women who reported moderate rates of ATN breastfeeding, we also found that evening-to-morning change in CRP secretion rate was negative among women who engaged in proportionally more ATN breastfeeding and/or pumping: in this group, CRP secretion rate peaked in the evening. The existing literature indicates that, in most sample populations, “normal” diurnal salivary CRP is characterized by a morning peak in CRP, with adverse conditions being associated with a relatively greater increase in morning CRP levels40,43,44. Our results therefore suggest that sustained lactation, via either ATN breastfeeding or pumping, induces an entirely unique immunological context that blunts or reverses this “normal” diurnal pattern.
Sleeping through the night is often regarded as the “ideal” version of infant sleep in contemporary Western populations. However, human lactation evolved under the circumstances of on-demand breastfeeding and waking throughout the night (‘breastsleeping’) 51, reflecting the highly digestible nature of human milk (compared to alternatives)52. It is possible that CRP peaking in the evening (as opposed to the morning) may be due, in part, to a higher number of sleep–wake cycles among women who are regularly breastfeeding at-the-nipple and/or using pumps. Disruption in sleep–wake cycles corresponds to altered cortisol, melatonin, and human growth hormone production. Considering the well-established effects of these hormones on regulating diurnal shifts in immune function53,54, changes in the diurnal production of these hormones may mediate the relationship we find between heavy reliance on lactation and diurnal CRP.
It is also possible that on-demand lactation throughout the day enhances early nighttime immune activity (e.g., phagocytosis, wound healing) as an adaptive response, to the direct benefit of the mother. Prolactin, a hormone produced at particularly high levels during regular lactation16, has already been shown to induce beneficial pro-inflammatory cascades during slow wave sleep (which occurs during the first few hours of sleep) in non-postpartum populations55,56,57,58. It is therefore tempting to speculate that regular lactation via either ATN breastfeeding or pumping is directly and uniquely beneficial for nighttime immune recovery. While we controlled for the presence/absence of night feeding, we did not collect detailed data on the frequency, timing, or duration of night feedings. Future research is therefore needed to parse out the effects of altered sleep patterns versus lactation itself on both hormone levels (e.g., cortisol, melatonin) and immune status among mothers who report similar lactation practices but varying sleep duration/quality.
While we believe that the results we present here can serve as a crucial springboard for future research, we acknowledge the limitations. We used data from a relatively homogeneous sample of women in the USA and therefore these results need to be replicated across diverse populations. Additionally, our findings shed light on the first 6 months of the postpartum only and we make no claims regarding long-term effects. Given budgetary and time constraints, our sample was limited to postpartum women; future research would benefit from comparisons with pregnant and regularly menstruating women to further contextualize the results we report here. Lastly, the method we used to measure the absolute and relative frequency of lactation (either at-the-nipple or via pumping) did not consider the specifics of each breast emptying episode (e.g., which breast was used, the intervals between episodes). We therefore recommend that future research include these more fine-grained measures.
Despite these limitations, this study introduces a novel method for measuring the complexities of maternal lactation practices (and infant feeding behavior more broadly), shows that such behavior is far more complex than most existing metrics are able to capture, and provides strong evidence that variation in maternal lactation practices corresponds to robust differences in postpartum maternal immune status. The first step towards understanding variation is exposing it. We therefore hope this study encourages and informs future research mapping variation in maternal lactation practices and postpartum immune status to specific health outcomes and supports continuing efforts to understand and eliminate maternal morbidity and mortality.
Methods
Ethics declaration
This study was approved by the Institutional Review Board at the University of California, Santa Barbara [IRB #1-20-0574] and was conducted in accordance with all corresponding guidelines and regulations. Each participant read and signed an online informed consent form, participation in the study was completely voluntary and anonymous (thus ensuring privacy and autonomy), and all participants were compensated accordingly for their time.
Participants
Our sample was limited to postpartum women between the ages of 25 and 42 who lived in Seattle, WA, USA, had given birth within the last 6 months, and who reported an absence of previous cancer diagnosis, periodontal disease, and regular tobacco use/vaping. All participants (n = 96) completed a single 24-h collection period, beginning at 12 PM on day one and ending at 12 PM on day two. Because data for this study were collected during the height of the COVID-19 pandemic (September 29th, 2020–July 28th, 2021), research protocols were specifically designed to reduce in-person contact. Saliva was selected for its minimally invasive nature, ease of at-home collection, and similarly detectable levels of cytokines compared to plasma59. Collection kits were mailed to each participant before the start of the 24-h collection period and all data were collected from home.
Infant feeding behavior
Participants were asked to keep an infant feeding record noting the method(s) used during each infant feeding bout and the total number of feedings completed during the 24-h time frame. Possible infant feeding methods included use of (1) other liquids, (2) semi-solid/solid foods, (3) pumped/expressed milk given to infant without storing, (4) participant’s own previously refrigerated/frozen pumped/expressed milk given to infant, (5) donated milk, and (6) breastfeeding at-the-nipple. Based on initial participant feedback, pumping/expressing milk for later use was added as an option part way through the study. When applicable, participants were able to indicate multiple methods used in a single feeding bout. Our final measure of ATN breastfeeding was calculated as the percentage of total infant feedings occurring via at-the-nipple breastfeeding during the 24-h collection period (% ATN breastfeeding). Pumping was calculated as the percentage of total infant feedings that involved the use of fresh or frozen expressed/pumped milk and any reported instances of pumping/expressing for future use (% pumping). We decided to calculate each infant feeding method as a percentage rather than use absolute frequency in order to account for differences in infant demand, considering our sample included infants ages 0–6 months old and that the number of total feedings in the 24-h period ranged from 5 to 20. Infant feeding behavior reported during the 24-h sampling interval generally corresponded to infant feeding behavior reported for the two weeks preceding data collection (Fig. S1).
Salivary immune markers
Each participant was asked to collect two passive drool saliva samples during the 24-h collection period. Participants were instructed to collect the first sample before going to bed on day one and the second at the time of waking the following morning. To ensure sample integrity, participants were instructed to place saliva samples in a home freezer immediately after collection. At the end of the 24-h collection period, saliva samples were transported on ice and stored at −80 °C.
Saliva samples were then shipped on dry ice to the Salimetrics SalivaLab (Carlsbad, CA) where they were tested for IL-1β, IL-6, TNF-α, and IL-8 cytokines and C-reactive protein (CRP) (Table 1). The cytokine panel was performed using a proprietary electrochemiluminesence method developed and validated for saliva by Salimetrics, while the CRP assay was performed using a high sensitivity enzyme immunoassay (Cat. No. 1-2102: https://salimetrics.com/wp-content/uploads/2017/05/c-reactive-protein-saliva-elisa-kit.pdf). All tubes were weighed before analysis, all assays were run in duplicate, and the resulting mean values were used in all final analyses. Samples with initial values greater than the maximum value of the standard curve were diluted and rerun for accurate results according to manufacturer guidelines. For the cytokine panel, the average coefficient of variation for all samples tested was < 15%, which meets Salimetric’s criteria for accuracy and repeatability in Salivary Bioscience and exceeds the applicable NIH guidelines for Enhancing Reproducibility through Rigor and Transparency. For the CRP assay, the average intra-assay coefficient of variation was 3.20% and the average inter-assay coefficient of variation was 2.61%, which meets the manufacturers’ criteria for accuracy and repeatability in Salivary Bioscience and exceeds the applicable NIH guidelines for Enhancing Reproducibility through Rigor and Transparency. For assay sensitivity and standard curve range values, see Table S1.
To account for the effects of salivary flow rate, absolute concentrations of all salivary biomarkers were transformed into secretion rates before analysis. For each saliva sample, there was a recorded duration (in seconds) that it took for the participant to collect the sample as well as a corresponding weight (in grams) of the final sample. Since saliva is approximately 99% water, it is standard practice to use a 1:1 conversion rate for grams to milliliters60. We therefore converted each sample weight to milliliters and the duration into minutes and calculated the flow rate (mL/min) for each sample. Salivary flow rate did not vary by infant feeding method (% ATN breastfeeding or % pumping) or sampling time (bedtime versus waking) (Fig. S2). Secretion rate of each analyte for each sample was calculated as the raw concentration (unit/mL) multiplied by the flow rate (mL/min).
To account for individual variation in baseline immune status61 and better quantify the effects of ATN breastfeeding on immune response patterns, we used absolute morning and evening salivary cytokine secretion rates to calculate each participant’s evening-to-morning change (i.e., delta) in secretion rate. Delta secretion rate, which we used as the outcome variable in all models described below, was calculated by subtracting evening secretion rate from morning secretion rate: positive delta values indicated that secretion rate was higher in the morning, negative delta values indicated secretion rate was lower in the morning, and a delta value of zero indicated no difference between evening and morning secretion rate. Due to issues with several saliva samples (e.g., outside detection threshold), evening-to-morning change in CRP secretion rate was successfully calculated for 92 out of 96 participants.
Statistical analyses
All analyses were executed in R 4.3.1 (https://cran.r-project.org). Using the brms package62, we employed Bayesian regression models to estimate the effects of % ATN breastfeeding on evening-to-morning change in IL-1β, IL-6, IL-8, TNF-α, and CRP secretion rate. To explicitly separate the effects of ATN breastfeeding from pumping, all models accounted for the effects of % pumping. All models also accounted for the fixed effects of days since delivery, time between saliva sample collections, maternal age, pre-pregnancy BMI, income, educational attainment, delivery mode (C-section versus vaginal birth), presence/absence of reported pregnancy complications, and presence/absence of co-sleeping, night feeding, and alcohol consumption during the 24-h collection period. We also controlled for whether a participant completed the study before or after the addition of “pumping/expressing milk for later use” as an infant feeding method. Lastly, all models accounted for “pandemic score”, an integer variable created by summing the number of adverse effects reported as a result of the COVID-19 pandemic (i.e., loss of employment, financial stress, loss of childcare, heightened anxiety, social isolation, reduced access to postpartum support, and reduced access to mental health services). These data were collected via a “Select all that apply” survey question designed by the researchers to approximate the perceived effects of the rapid societal changes induced by the COVID-19 pandemic and associated stay-at-home order. Finally, all categorical covariates were converted into dummy numerical variables before modeling to allow for standardization of posterior predicted values.
Because the effects of % ATN breastfeeding on CRP and IL-8 were non-linear (as determined by comparing variance parameter values to linear coefficients), spline regression model formulas were used for these outcome variables. All other outcome variables were modeled using linear regression formulas. All models were run using two chains for between 10,000 and 25,000 iterations and used loose priors and Gaussian distributions. All models had R-hat values of 1, indicating appropriate chain mixing and convergence. Predicted values were standardized by the mean value for % pumping (18.71), median values for all continuous covariates (Table 2), and middle values for all dummy categorical covariates (e.g., 0.5 for a binary dummy variable) (Table 3).
Data availability
Data and R code for the statistical analyses are published on GitHub (https://github.com/carmenhove/sphs)63.
References
Chen, A. & Rogan, W. J. Breastfeeding and the risk of postneonatal death in the United States. Pediatrics 113, e435–e439 (2004).
Ip, S. et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid. Technol. Assess. (Full Rep.) 153, 1–186 (2007).
Ronsmans, C. & Graham, W. J. Maternal mortality: Who, when, where, and why. Lancet 368, 1189–1200 (2006).
Alayón, S. et al. Exclusive breastfeeding: Measurement to match the global recommendation. Matern. Child Nutr. 18, e13409 (2022).
Greiner, T. Exclusive breastfeeding: Measurement and indicators. Int. Breastfeed. J. 9, 18 (2014).
Mulol, H. & Coutsoudis, A. Limitations of maternal recall for measuring exclusive breastfeeding rates in South African mothers. Int. Breastfeed. J. 13, 19 (2018).
Von Rango, U. Fetal tolerance in human pregnancy. A crucial balance between acceptance and limitation of trophoblast invasion. Immunol. Lett. 115, 21–32 (2008).
Ladyman, S. R., Augustine, R. A. & Grattan, D. R. Hormone interactions regulating energy balance during pregnancy. J. Neuroendocrinol. https://doi.org/10.1111/j.1365-2826.2010.02017.x (2010).
Magon, N. & Kumar, P. Hormones in pregnancy. Nigerian Med. J. 53, 179 (2012).
Coss, S. L. et al. CD4+ T cell restoration and control of hepatitis C virus replication after childbirth. J. Clin. Invest. 130, 748–753 (2020).
Clodi, M. et al. Oxytocin alleviates the neuroendocrine and cytokine response to bacterial endotoxin in healthy men. Am. J. Physiol.-Endocrinol. Metab. 295, E686–E691 (2008).
Costanza, M., Binart, N., Steinman, L. & Pedotti, R. Prolactin: A versatile regulator of inflammation and autoimmune pathology. Autoimmun. Rev. 14, 223–230 (2015).
Hannah, M. E. et al. Maternal colonization with group B Streptococcus and prelabor rupture of membranes at term: The role of induction of labor. Am. J. Obstet. Gynecol. 177, 780–785 (1997).
İşeri, S. Ö. et al. Oxytocin protects against sepsis-induced multiple organ damage: Role of neutrophils. J. Surg. Res. 126, 73–81 (2005).
Matthiesen, A.-S., Ransjo-Arvidson, A.-B., Nissen, E. & Uvnas-Moberg, K. Postpartum maternal oxytocin release by newborns: Effects of infant hand massage and sucking. Birth 28, 13–19 (2001).
Neville, M. C. Anatomy and physiology of lactation. Pediatr. Clin. N. Am. 48, 13–34 (2001).
Yu-Lee, L. Y. Prolactin modulation of immune and inflammatory responses. Recent Prog. Horm. Res. 57, 435–455 (2002).
Deing, V. et al. Oxytocin modulates proliferation and stress responses of human skin cells: Implications for atopic dermatitis. Exp Dermatol. 22, 399–405 (2013).
Baker, J. L. et al. Breastfeeding reduces postpartum weight retention. Am. J. Clin. Nutr. 88, 1543–1551 (2008).
Stuebe, A. M. Duration of lactation and incidence of type 2 diabetes. Jama 294, 2601 (2005).
Nieman, D. C. & Wentz, L. M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 8, 201–217 (2019).
Almeneessier, A. S. et al. The effects of diurnal intermittent fasting on proinflammatory cytokine levels while controlling for sleep/wake pattern, meal composition and energy expenditure. PLOS ONE 14, e0226034 (2019).
Bianchi, V. E. Weight loss is a critical factor to reduce inflammation. Clin. Nutr. ESPEN 28, 21–35 (2018).
Groer, M. W. et al. Immunity, inflammation and infection in post-partum breast and formula feeders. Am. J. Reprod. Immunol. 54, 222–231 (2005).
Kuzawa, C. W., Adair, L. S., Borja, J. & Mcdade, T. W. C-reactive protein by pregnancy and lactational status among Filipino young adult women. Am. J. Hum. Biol. 25, 131–134 (2013).
Beral, V., Bull, D., Doll, R., Peto, R. & Reeves, G. Breast cancer and breastfeeding: Collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50 302 women with breast cancer and 96 973 women without the disease. Lancet 360, 187–195 (2002).
Kotsopoulos, J. et al. Breastfeeding and the risk of epithelial ovarian cancer among women with a BRCA1 or BRCA2 mutation. Gynecol. Oncol. 159, 820–826 (2020).
Kramer, M. S. et al. Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: Evidence from a large randomized trial. Am. J. Clin. Nutr. 86, 1717–1721 (2007).
Mongiovi, J. et al. Abstract 3017: Breastfeeding and ovarian cancer risk by tumor immune profiles. Cancer Res. 83, 3017–3017 (2023).
Martin, M. A., Garcia, G., Kaplan, H. S. & Gurven, M. D. Conflict or congruence? Maternal and infant-centric factors associated with shorter exclusive breastfeeding durations among the Tsimane. Soc. Sci. Med. 170, 9–17 (2016).
Sellen, D. W. Comparison of infant feeding patterns reported for nonindustrial populations with current recommendations. J. Nutr. 131, 2707–2715 (2001).
Gardner, H. et al. Milk ejection patterns: An intra-individual comparison of breastfeeding and pumping. BMC Pregnancy Childb. 15, 1–6 (2015).
Leiter, V., Agiliga, A., Kennedy, E. & Mecham, E. Pay at the pump?: Problems with electric breast pumps. Soc. Sci. Med. 292, 114625 (2022).
Meier, P. P., Engstrom, J. L., Janes, J. E., Jegier, B. J. & Loera, F. Breast pump suction patterns that mimic the human infant during breastfeeding: Greater milk output in less time spent pumping for breast pump-dependent mothers with premature infants. J. Perinatol. 32, 103–110 (2012).
Fetherston, C. Risk factors for lactation mastitis. J. Hum. Lactation 14, 101–109 (1998).
Zinaman, M. J., Queenan, J. T., Labbok, M. H., Albertson, B. & Hughes, V. Acute prolactin and oxytocin responses and milk yield to infant suckling and artificial methods of expression in lactating women. Pediatrics 89, 437–440 (1992).
Moossavi, S. et al. Composition and variation of the human milk microbiota are influenced by maternal and early-life factors. Cell Host Microbe 25, 324-335.e4 (2019).
Loewenberg Weisband, Y., Keim, S. A., Keder, L. M., Geraghty, S. R. & Gallo, M. F. Early breast milk pumping intentions among postpartum women. Breastfeed. Med. 12, 28–32 (2017).
Riis, J. L., Ahmadi, H., Hamilton, K. R., Hand, T. & Granger, D. A. Best practice recommendations for the measurement and interpretation of salivary proinflammatory cytokines in biobehavioral research. Brain Behav. Immun. 91, 105–116 (2021).
Izawa, S., Miki, K., Liu, X. & Ogawa, N. The diurnal patterns of salivary interleukin-6 and C-reactive protein in healthy young adults. Brain Behav. Immun. 27, 38–41 (2013).
Reinhardt, É. L., Fernandes, P. A. C. M., Markus, R. P. & Fischer, F. M. Short sleep duration increases salivary IL-6 production. Chronobiol. Int. 33, 780–782 (2016).
Sarkar, A., Kuehl, M. N., Alman, A. C. & Burkhardt, B. R. Linking the oral microbiome and salivary cytokine abundance to circadian oscillations. Sci. Rep. 11, 1–13 (2021).
Wetterö, J. et al. Pronounced diurnal pattern of salivary C-reactive protein (CRP) with modest associations to circulating CRP levels. Front. Immunol. 11, 607166 (2021).
Hernández, L. M. & Taylor, M. K. Diurnal pattern of salivary C-reactive protein and associations with biobehavioral correlates in military men. Med. Sci. Sports Exerc. 49, 96 (2017).
Irwin, M. R. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch. Intern. Med. 166, 1756 (2006).
Pervanidou, P. et al. Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology 32, 991–999 (2007).
Out, D., Hall, R. J., Granger, D. A., Page, G. G. & Woods, S. J. Assessing salivary C-reactive protein: Longitudinal associations with systemic inflammation and cardiovascular disease risk in women exposed to intimate partner violence. Brain Behav. Immun. 26, 543–551 (2012).
Amulic, B., Cazalet, C., Hayes, G. L., Metzler, K. D. & Zychlinsky, A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 30, 459–489 (2012).
Hosoki, K., Itazawa, T., Boldogh, I. & Sur, S. Neutrophil recruitment by allergens contribute to allergic sensitization and allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 16, 45–50 (2016).
Palmblad, J. The role of granulocytes in inflammation. Scand. J. Rheumatol. 13, 163–172 (1984).
McKenna, J. J. & Gettler, L. T. There is no such thing as infant sleep, there is no such thing as breastfeeding, there is only. Breastsleep. Acta Paediatr. 105, 17–21 (2016).
Ball, H. L. Breastfeeding, bed-sharing, and infant sleep. Birth 30, 181–188 (2003).
Fong, T. C. T., Ho, R. T. H. & Yau, J. C. Y. Longitudinal associations between salivary cortisol to C-reactive protein ratios and psychological well-being in Chinese adults. Psychoneuroendocrinology 143, 105824 (2022).
Dumbell, R., Matveeva, O. & Oster, H. Circadian clocks, stress, and immunity. Front. Endocrinol. 7, 11 (2016).
Besedovsky, L., Lange, T. & Born, J. Sleep and immune function. Pflügers Arch. Eur. J. Physiol. 463, 121–137 (2012).
Gala, R. R. Prolactin and growth hormone in the regulation of the immune system. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. (New York, N.Y.) 198, 513–527 (1991).
Lange, T., Dimitrov, S., Bollinger, T., Diekelmann, S. & Born, J. Sleep after vaccination boosts immunological memory. J. Immunol. (Baltimore, Md.: 1950) 187, 283–290 (2011).
Reinoso Suárez, F. The neurobiology of slow-wave sleep. Anal. Real Acad. Nacl. Med. 116, 209–224 (1999) (discussion 224–226).
La Fratta, I. et al. The plasmatic and salivary levels of IL-1 β, IL-18 and IL-6 are associated to emotional difference during stress in young male. Sci. Rep. 8, 3031 (2018).
De Almeida, P. D. V., Grégio, A. M. T., Machado, M. A. N., de Lima, A. A. S. & Azevedo, L. R. Saliva composition and functions: A comprehensive review. J. Contemp. Dent. Pract. 9, 72–80 (2008).
Brodin, P. & Davis, M. M. Human immune system variation. Nat. Rev. Immunol. 17, 21–29 (2017).
Bürkner, P.-C. Brms : An R package for Bayesian multilevel models using stan. J. Stat. Softw. 80, 1–27 (2017).
Hove, C. Carmenhove/SPHS: Seattle Postpartum Study Dataset and Associated Code. https://doi.org/10.5281/zenodo.10480687 (2024).
Acknowledgements
We extend deep gratitude to all our participants, who spit into tubes and diligently filled out surveys during a global pandemic. Many thanks to Eleanor Brindle for her guidance on laboratory methods. Thank you to Michael Gurven, Aaron Blackwell, and David Lawson for providing critical feedback during various stages of this project. This research was generously funding by the National Science Foundation [Dissertation Research Improvement Grant #1945759] and the Wenner-Gren Foundation [Dissertation Fieldwork Grant #10004].
Author information
Authors and Affiliations
Contributions
C.H.: grant proposals, study design, participant recruitment, data collection, data analysis, paper writing; K.C.: paper writing; M.M.: study design, paper writing; M.H.: participant recruitment, data collection, paper writing; A.B.: grant proposals, study design, paper writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hove, C., Chua, K.J., Martin, M.A. et al. Variation in maternal lactation practices associated with changes in diurnal maternal inflammation. Sci Rep 14, 4376 (2024). https://doi.org/10.1038/s41598-024-54963-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54963-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.