Abstract
Recent biological surveys of ancient inselbergs in southern Malawi and northern Mozambique have led to the discovery and description of many species new to science, and overlapping centres of endemism across multiple taxa. Combining these endemic taxa with data on geology and climate, we propose the ‘South East Africa Montane Archipelago’ (SEAMA) as a distinct ecoregion of global biological importance. The ecoregion encompasses 30 granitic inselbergs reaching > 1000 m above sea level, hosting the largest (Mt Mabu) and smallest (Mt Lico) mid-elevation rainforests in southern Africa, as well as biologically unique montane grasslands. Endemic taxa include 127 plants, 45 vertebrates (amphibians, reptiles, birds, mammals) and 45 invertebrate species (butterflies, freshwater crabs), and two endemic genera of plants and reptiles. Existing dated phylogenies of endemic animal lineages suggests this endemism arose from divergence events coinciding with repeated isolation of these mountains from the pan-African forests, together with the mountains’ great age and relative climatic stability. Since 2000, the SEAMA has lost 18% of its primary humid forest cover (up to 43% in some sites)—one of the highest deforestation rates in Africa. Urgently rectifying this situation, while addressing the resource needs of local communities, is a global priority for biodiversity conservation.
Similar content being viewed by others
Introduction
Tropical ecosystems of great antiquity are known to harbour exceptionally high levels of biodiversity and endemism1. In Africa, mountains typically host relict forests that are remnants of a widespread forest belt, which, prior to the uplift and long-term aridification of the East African plateaus, stretched across most of the continent2,3,4. As the global climate began to cool in the Early Oligocene, the pan-African rainforests began to fragment leading to significant forest reduction throughout the Miocene4,5. Much of the original forest in eastern Africa became confined to isolated montane patches that persisted due to orographic rainfall4. This caused forest-dependent species with low vagility to become trapped in upland refugia where moisture-laden trade winds maintained a relatively stable climate6. Subsequent climatic fluctuations through the Late Cenozoic disrupted gene flow between adjacent mountains7, driving allopatric speciation and establishing the unique biotic assemblages that now characterise African montane systems4,8.
To integrate conservation planning at relevant scales, it is helpful to recognise groups of mountains with shared geology, evolutionary history, and characteristic species assemblages as distinct ecoregions9. One of the best documented examples is the Eastern Arc ecoregion in Tanzania and Kenya10, long recognised as a global priority for conservation11,12. Much less known, however, are a series of granitic inselbergs (‘island mountains’) 600 km south of the Eastern Arc, stretching from southern Malawi across northern Mozambique. We propose that these inselbergs constitute an evolutionarily distinct montane ecoregion, characterised by high levels of endemism and ongoing threats from human activity.
Over the past century, numerous biological surveys have been undertaken on the mountains in southern Malawi. For example, Mt Mulanje, the second highest free-standing mountain in southern Africa (3002 m), is known to host a suite of endemic animal species13,14 and high levels of botanical endemism15, including the endemic Mulanje Cedar (Widdringtonia whytei)—Malawi's national tree16. In contrast, due to a protracted war for independence (1964–1974) followed by a civil war (1977–1992), the mountains in northern Mozambique remained largely unstudied by biologists until recently. Some mountains in northern Mozambique were surveyed in the late-19th through mid-twentieth centuries17,18,19,20, but only in the last 20 years have biological surveys begun to uncover the full extent of the region’s uniqueness. These more recent surveys began with ad hoc visits by ornithologists21 and herpetologists22. Then, following ecological surveys on Mt Mulanje, questions arose as to the degree of biological similarity, between Mt Mulanje and neighbouring inselbergs in Mozambique23,24,25. A series of scientific expeditions26,27,28,29,30,31,32 targeted sites in Mozambique above 1500 m, uncovering many species new to science and elucidating levels of shared endemism between these sites33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53. These findings prompted further surveys of other mountains in the region and resulted in new species descriptions, including many from Mt Mabu, which is now recognised to be the most extensive mid-elevation rainforest in southern Africa54.
Preliminary evidence for a new biogeographically distinct montane ecoregion was first proposed54 in 2014, and subsequently corroborated55,56,57,58. The name ‘South East Africa Montane Archipelago’ (SEAMA) was suggested in 2019, at the Annual General Meeting of the Transglobe Expedition Trust, Royal Geographical Society, London59, and formally proposed in 2022 at the 1st Southern African Mountain Conference in South Africa60. Here, we present a formal definition of the SEAMA ecoregion based on endemic species, geology, topography and climate, and place this in context through comparison with surrounding ecoregions. We synthesise and summarise all available records for taxa unique to the SEAMA, and thus report the degree of overlapping endemism across multiple taxa (plants, mammals, birds, reptiles, amphibians, crabs, and butterflies). Other taxonomic groups, such as most invertebrate groups, fungi and bryophytes await expert assessments and are therefore not included in our key estimates. Future assessments of these groups will invariably yield many more species new to science, thus further raising levels of endemism within the SEAMA. We assess potential drivers of speciation in different taxa, and suggest mechanisms for forest fragmentation that have produced the distinct ‘islands in the sky’ that harbour the unique biodiversity we see today16,61. Finally, we identify threats to the ecoregion and assess prospects for future conservation.
Results
Definition, extent and context
We recognise at least 30 sites in the core of the SEAMA ecoregion (nine in Malawi, 21 in Mozambique; Fig. 1 and Table 1), each reaching an elevation of at least 800 m (elevation range is 500–3002 m) above sea level, and with high humidity (aridity index > 0.65). These sites host remnants of humid evergreen forest and upland grasslands, have ancient substrates, share similar climatic influences, and exhibit high levels of shared and single-site endemism compared with the ecosystems that directly surround them. More precisely, we define the SEAMA ecoregion as “a range of ancient granitic inselbergs in southern Malawi and northern Mozambique, climatically isolated by topography and trade winds, hosting humid evergreen forest, montane grassland and shrublands notable for their high levels of endemism across multiple taxa.”
Location and extent of the South East Africa Montane Archipelago (SEAMA) showing core sites in red, and an outline boundary of the convex hull of the ecoregion (created using QGIS version 3.28.12 LTR https://qgis.org/en/site/).
Most mountains within the SEAMA were formed ca. 600–126 million years ago (Mya) as a result of igneous, rock-forming batholiths intruding into older, softer metamorphic rocks that subsequently weathered away62,63. The intrusions are mostly granitic and syenitic in composition63,64. Their mineralogy is characterised by low (typically < 10%) ferromagnesian mineral and high quartz and feldspar contents, rendering them relatively resistant to erosion. One aspect that contributes to this is the low degree of jointing in the rocks: SEAMA intrusions are characterised by homogeneous, randomly orientated crystals, which render them much less prone to jointing, water ingress and weathering, compared with, for example, some nearby gneisses (typically of planar fabric resulting from deformation and strain at an earlier time).
The distinct nature of the SEAMA ecoregion is further clarified through contemporary patterns of rainfall and humidity, which clearly show the ecoregion falling under its own climatic envelope with a break to the north and connections to the coast (Fig. 2). This suggests that a defining factor is the south-east trade winds, funnelled up through the Mozambique Channel, carrying moisture to the mountains throughout the year. Mountains further north outside of the SEAMA (including Mts Njesi, Yao, and Mecula) are in the rain shadow of Madagascar, and historically have been more influenced by fluctuations in the water level of Lake Malawi. Further south (e.g., Mt Gorongosa), the air is cooler and so holds less moisture, especially in the dry season.
Contemporary macro-climatology of the South East Africa Montane Archipelago. Also labelled is the Eastern Arc Mountains (EAM) ecoregion to the north, and the Eastern Highlands of Zimbabwe to the southwest (EHZ). Annual rainfall (a) is measured in mm. Rainfall seasonality (b) is the coefficient of variation across months65. Aridity index (c) is the ratio of annual rainfall to potential evapotranspiration66 with values above 0.65 considered humid67. Maximum water deficit (d) is a measure of water stress defined across the most arid months of the year, with lower values conducive of potentially dense, evergreen canopy structure68. Bioclimatic layers were extracted from WorldClim version2 (https://www.worldclim.com/version2) and presented in QGIS version 3.28.12 LTR (https://qgis.org/en/site/).
The SEAMA has distinctly higher annual rainfall and humidity, especially in the dry season, compared to surrounding regions (Fig. 2). This is similar to the Eastern Arc Mountains (EAM) and also the Eastern Highlands of Zimbabwe (EHZ). For plants, this equates to a shorter period of physiological stress, and hence a potentially longer growing season and the existence of mesic vegetation types such as humid evergreen forest. Moreover, since montane forests also extract moisture directly from the air via local orographic precipitation, the estimates of macroclimate presented here (Fig. 2) likely underestimate how much moisture the SEAMA forests receive relative to the lowlands69.
The convex hull of the ecoregion is defined by the farthest extents of the core sites identified through the selection criteria outlined by the methodology, plus other inselbergs at the periphery where elevations exceed 800 m and the aridity index exceeds 0.65 (humid climate). The interior of the convex hull also spans arid lowland habitats which, during cooler climes, would have periodically connected the now isolated inselberg habitats, as well as smaller inselbergs that no longer support humid evergreen forest, either due to lower elevation (< 800 m) or because the forests on these sites have already been cleared by humans (some of the core sites have also been largely deforested, e.g., Chiradzulu, Thyolo).
Accordingly, the SEAMA ecoregion has a core area (sum of the areas of the 30 individual sites) of 336,200 ha with a total extent of occurrence (convex hull) close to 10 million ha (Table 1). In relation to the WWF Global 200 ecoregions9,70,71, the SEAMA has a smaller core area than some of its neighbours, such as the Eastern Arc Montane Forests EAM (2.4 million ha), the Southern Rift Montane Forest-grassland Mosaic SRMFM (3.3 million ha), the East African Montane Forest ecoregion EAMF (6.5 million ha), and the Ethiopian Montane Forests EMF (24.9 million ha). However, it is a similar size to other mountain ecoregions such as the East African Montane Moorlands EAMM (330,000 ha) and the Knysna-Amatole Montane Forests KAMF (310,000 ha). The SEAMA incorporates the South Malawi Montane Forest-grassland Mosaic, which represents the Malawi component on this larger SEAMA ecoregion, and the Mulanje-Namuli-Ribáuè sub-Centre of Plant Endemism55. It also forms part of the Africa-wide Afromontane Archipelago botanical Centre of Endemism2. In relation to Key Biodiversity Areas (KBAs) in the Eastern Afromontane Biodiversity Hotspot72, the SEAMA is located between the Northern Lake Nyassa Catchments (Southern Rift montane forest-grassland mosaic) and the Chimanimani-Nyanga Catchments (Eastern Zimbabwe montane forest-grassland mosaic). The SEAMA ecoregion encompasses nine Important Bird Areas (IBAs), eight Important Plant Areas (IPAs) from Mozambique73 (yet to be determined for Malawi), and 12 Key Biodiversity Areas (KBAs) in Malawi and Mozambique74 (Table 1).
Comparative ecoregion endemism
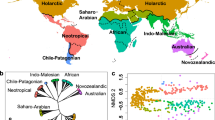
Compared to five neighbouring mountain ecoregions, levels of endemism in the SEAMA are higher than most in the taxonomic groups surveyed. Notably the number of strictly endemic reptile species is higher than the much larger Albertine Rift (AlbRft) ecoregion (Table 2). When area is taken into consideration using a species-area function (see “Methods”), the SEAMA is a close third in ecoregion endemism to the EAMs and the AlbRft across most taxa, and second in comparative endemism for reptiles and crabs (Fig. 3).
Comparative ecoregion endemism according to the species-area function. Within each taxonomic group and ecoregion, we plot the number of endemics divided by Az, where A is the core area (ha) of the ecoregion and z = 0.25 is taken as a representative value of the slope of the species-area curve. This yields a metric of endemism to estimate the number of endemic species per hectare, which diminishes the impact of larger areas.
Relative survey effort per site
Historically, relative survey effort (defined as sampling intensity relative to mountain blocks and site area75, see “Methods”) has been much greater in Malawi than in Mozambique (Fig. 4). Overall, the mean relative survey effort for the whole of the SEAMA is 0.22, while the Malawi sites have a mean relative survey effort of 0.41, compared to 0.12 for Mozambique. The most comprehensively surveyed site in Malawi is Mount Mulanje, followed by Mangochi, Ndirande, Soche, and Thyolo. In Mozambique, the most comprehensively surveyed sites are Mabu, Namuli, and Lico, and nine Mozambique sites have never been scientifically surveyed.
Relative survey effort across core sites in the SEAMA. Bars plot a comparative measure of sampling intensity, relative to the area of each site75. Score varies between 0 and 1, where 0 is the minimum (no sampling at all) while 1 is the maximum effort possible to obtain.
Biological uniqueness
The SEAMA hosts endemic genera and species assemblages (Fig. 5a−k), which characterise the ecoregion (Table SI1). Currently, 217 endemic taxa (192 strictly endemic species, plus 25 subspecies and races) are recognised, with a greater number of endemic plants than animals (127 vs 90 taxa, Table 3). The number of endemic taxa is notably high for such a limited geographic area. We expect the number of known endemics, especially amongst the fauna, to grow substantially with additional research, given the low sampling effort for some groups (e.g., although reptiles have been relatively well surveyed, small mammals have not)76. Overall, of the groups surveyed, levels of endemism are proportionally highest among reptiles, amphibians, mammals, crabs, and butterflies. Most endemic animals are forest specialists, as opposed to endemic plants which are typically restricted to high-elevation grasslands and lithophyte communities. Taxonomic influences are evident from all surrounding ecoregions, however there is greater influence from the north (Tanzania) amongst the faunal groups, which suggests a historical continuous humid forest belt stretching from eastern to southern Africa.
Examples of SEAMA endemics. (a) Rhinolophus mabuensis (AM), (b) Chamaetylas choloensis (JB), (c) Nothophryne inagoensis (WC), (d) Atheris mabuensis (WRB), (e) Epamera malaikae (TCEC), f) Rhampholeon maspictus (JB), (g) Nadzikambia baylissi (WRB), (h) Maritonautes namuliensis (JB), (i) Euphorbia mlanjeana (ID), (j) Widdringtonia whytei (JB), (k) Encephalartos gratus (JB).
Plants
A subset of the SEAMA sites has recently been recognised as forming a distinct botanical sub-centre of endemism55, part of the Africa-wide Afromontane archipelago-like Centre of Endemism, as defined by White2. Among the best studied sites in the SEAMA are Mts Mulanje16,77,78, Zomba79,80, Namuli30,34,81, Mabu29,54,82, Chiperone31,34, and Ribáuè55,83. Plants account for 117 strictly endemic species and a total of 127 endemic taxa (Table 3, Fig. 5i−k, and Table S1), with the main single areas being Mts Mulanje (48 endemic taxa) and Namuli (19 endemic taxa) (Table S1). Of those assessed for the IUCN Red List, 37 (29%) are listed as threatened (VU, EN, CR). Approximately 69% of endemic plant taxa occur on rocky crags and/or grassland, and only 28% are limited to forest or forest margins. Only three endemic plant taxa show any link to woodland. It is likely that endemic plant taxa remain to be scientifically discovered in the SEAMA from sites currently not surveyed, and from the forests and forest margins of Mt Mabu.
Mammals
Mammals account for four strictly endemic species (Table 3 and Table S1). All are small (< 250 g in mass), and all are forest-dependent. Of those assessed, two (50%) are listed as threatened (VU, EN, CR) (IUCN 2022), while the remainder await formal description. Given narrow ranges and high rates of forest loss, the remaining two mammals will likely be considered Threatened by the IUCN once they are formally described. Because of the relatively low sampling effort for small mammals, the expectation is that many species remain scientifically undiscovered, and so diversity and richness in this group is likely to be significantly higher than is currently known84.
Birds
Birds account for eight endemic taxa, three strictly endemic species and five endemic subspecies (Table 3 and Table S1). All endemic taxa are forest-dependent, and most are considered highly threatened. Four (50%) are listed as Near Threatened (NT) or threatened (VU, EN, CR) on the IUCN Red List. Sampling effort has been good on some mountains (Mts Mulanje, Zomba, Mangochi, Liwonde, Namuli, Mabu, and Chiperone) but most of the other mountains are yet to be surveyed by ornithologists. However, the chance of finding new endemic bird taxa is low, since the remaining forest fragments across unsurveyed sites are generally too small to expect new ornithological scientific discoveries.
Reptiles
There are 22 strictly endemic reptile species (Table S1). Of these, 19 (86%) are forest-dependent species, and the others occur mainly in upland grasslands and on rock faces. As they predominantly occur in forest, SEAMA’s endemic reptiles are highly threatened by habitat loss. Of those assessed for the IUCN Red List, 12 (55%) are listed as Near Threatened (NT) or threatened (VU, EN, CR), while at least six species await formal description, after which they will almost certainly qualify for an IUCN Threat category. Sampling effort has been adequate for certain sites (Mts Mulanje, Mabu, Namuli, Chiperone, Ribáuè), but many others remain unsampled. Given that new species have been scientifically discovered on each of the surveyed mountains, it is likely that other mountains harbour undescribed endemics, many of which will probably be considered threatened by ongoing forest loss.
Amphibians
There are 11 strictly endemic amphibian species (Table 3 and Table S1). Approximately half of these occur in forest and of those assessed for the IUCN Red List, seven (64%) are listed as Near Threatened (NT) or threatened (VU, EN, CR). Sampling effort has been adequate for some mountains (Mts Mulanje, Mabu, Namuli, Ribáuè) although some fossorial species are probably under-sampled. There are many other mountains that remain poorly sampled or unsampled. New species have been scientifically discovered on some of the recently surveyed mountains32,46,85, suggesting that further efforts on other mountains will likely reveal new species or new populations of endemic amphibians, and increased representation on the IUCN Red List.
Freshwater crabs
There are six strictly endemic freshwater crab species to the SEAMA ecoregion, two of which are undescribed species (Table 3 and Table S1). Currently, most of the recognised endemic diversity occurs in the newly established Maritonautes group (Fig. 5h). Four of the endemic species are found inside forest (67%), although none have yet been assessed for their IUCN threat status. Given that new species have been scientifically discovered on several mountains, there is a high likelihood of finding new species of freshwater crab on other mountains.
Butterflies
There are 39 endemic butterfly taxa in the SEAMA ecoregion including 30 strictly endemic species (Table 3, Fig. 5e, and Table S1). The majority (75%) occur in forest and given the degree of forest loss due to slash and burn agricultural expansion28,29,30,31; these species are under threat. Of those assessed for the IUCN Red List, seven (18%) are listed as threatened (VU, EN, CR). Sampling effort has been adequate in certain sites (Mts Mulanje, Mangochi, Zomba, Mabu, Namuli, and Lico), with single collecting visits made to some other sites (Mts Chiperone, Socone, Pewé, Nállume), leaving many others that remain scientifically unexplored. Given that new species have been scientifically discovered on most mountains, there is a high likelihood of finding more species of butterflies new to science.
Discussion
Origins, connections, and divergence
Many of the plant endemics, including several genera, have their closest relatives in the Southern Afromontane region (stretching from southern Tanzania to the Western Cape province of South Africa), as opposed to elsewhere in East Africa. Although there are some links between the endemic plants of Mt Mabu and the Southern Highlands of Tanzania (e.g., Helixanthera schizocalyx), there appear to be more links with areas to the south, such as the Nyanga-Chimanimani area, while a few appear to be more strongly linked to the East African coastal belt. Others have more general affinities with southern African forms (especially those of drier formations) or even the wider Afrotropics.
Within vertebrates, the closest relatives of the endemic mammals are mainly East African (e.g., with Rhinolophus mabuensis (Fig. 5a) close to R. hildebrandtii). Amongst birds, the affinities are mainly with Tanzania to the north and Zimbabwe to the west. For reptiles, the closest relatives are to the north or west depending on the genus, and so taxonomic influences are from all surrounding regions. The closest relatives of endemic amphibians are mainly from east Africa, with just two species associated with southern Africa.
Amongst invertebrates, the closest relatives of the endemic butterfly fauna are East African (88%), with just four species with affinities to central and southern Africa. Therefore, the influences are predominantly northern. The closest relatives of endemic crabs are mainly East African, with 40% connected to Zimbabwe. Therefore, the influences are from the north and the west. Divergence time estimation for endemic freshwater crab species suggest cladogenic activity was initiated during the late Miocene and continued to the Pliocene50,86.
Species-level divergences for some of the genera in the SEAMA have been estimated, with divergence estimates ranging from relatively recently (e.g., Plio-Peistocene) to fairly ancient (e.g., Mid-Miocene), Table SI2. As of yet, no common patterns have emerged, in part due to information on the timing of divergence being lacking for most genera. Nevertheless, for some of the small mammal species, especially the bats (Rhinolophous mabuensis, Fig. 5a), species-level divergences are relatively recent, e.g., 1–2 Mya. The divergence dates between butterfly species have yet to be examined, although a new species of Cymothoe diverged from sister taxa approximately 4 Mya49. Within the freshwater crabs (Potamonautes and Maritonautes) species-level divergence dates are estimated at 2.5–8 Mya50. In contrast, estimated divergence dates for reptiles and amphibians are more ancient, with the earliest diverging lineages dating to the Mid-Miocene. For example the bush viper Atheris mabuensis (Fig. 5d), the sole representative of the genus from the SEAMA, diverged from its East Africa sister taxa around 15 Mya33. The pygmy chameleons (Rhampholeon spp.) are better represented in the SEAMA, with eight of the 25 described species (plus probably several undescribed) occurring on isolated mountains. Six of these form a monophyletic clade, having diverged from the East African species more than 10 Mya37,87. The two remaining Rhampholeon species are sister to East/Central African species and likely diverged even earlier. Similarly, species-level diversification within the SEAMA endemic amphibian genus Nothophryne (Fig. 5g) are ancient, between 7.5 and 18 Mya85. Unlike the chameleons, Nothophryne shares a common ancestor with southern African taxa, not East African86 suggesting that the SEAMA has linkages to both East and southern Africa. Although additional studies are needed to assess common patterns, the reptile and amphibians essentially exhibit some of the oldest species known from the SEAMA (e.g., Nothophryne broadleyi, 18.65 Mya; Atheris mabuensis, 15 Mya).
Amongst the chameleons, Rhampholeon and Nadzikambia (Fig. 5f,g), most species are endemic to a single mountain, apart from R. tilburyi, which has been recorded from at least five mountains (and is therefore a SEAMA regional endemic). Mts Namuli, Inago, Mabu, Chiperone, Mulanje and the Malawi Hills all have an endemic Rhampholeon, with an endemic Nadzikambia recorded for each of Mabu (Fig. 5g), Chiperone, and Mulanje. Chameleons on these inselbergs probably shared a common ancestor in the Mid-Miocene37. Phylogenetic analysis has shown that R. bruessoworum is the oldest diverging lineage, dated to the Mid or Early-Miocene (ca. 11–30 Mya). This may have been followed by the isolation of R. platyceps on Mt Mulanje and R. tilburyi on Mts Namuli, Pico Mulli, Nallume, Ribáuè, and Pewe in the Mid to Late Miocene. Divergence times for Rhampholeon from Mts Mabu (R. maspictus, Fig. 5f), Chiperone (R. nebulauctor), and the Malawi Hills (R. chapmanorum) are more recent (4–9 Mya) within the Late Miocene or Early Pliocene, suggesting these forests remained connected until most recently37. The two most closely related species are R. nebulauctor from Mt Chiperone and R. chapmanorum from Malawi Hills, which are just 75 km apart and may have been connected through the Late Pliocene37. A recent phylogenetic study50 showed that initial diversification with the SEAMA endemic crabs (Potamonautes and Maritonautes) dates to just over 7 Mya, with species-level diversification mainly within the Pliocene, e.g., Maritonautes namuliensis, 5.4 Mya (Fig. 5h); Potamonautes mulanjeensis, 2.84 Mya; Maritonautes licoensis/Maritonautes choloensis, 2.5 Mya.
Overall across taxa, dating estimates suggest that the initiation of allopatric speciation through vicariance of forest patches began in the mid-Miocene for some groups but with other groups diversifying more recently. Thus, vicariance events do not appear to be coeval suggesting the SEAMA taxa have a complex evolutionary history that has melded to form the rich diversity of the region. For example, species-level divergences within the amphibian genus Nothophryne from Mts Mulanje, Namuli and Ribáuè can be dated to approximately 18 Myr, 13 Myr and 7 Myr, respectively85 and the one SEAMA endemic clade of Rhampholeon shows a similar pattern. Thus, vicariance of the forests on these mountains may have been sequential, resulting in isolated populations that diverged in allopatry. Forest specialists or non-vagile species may have been prone to early vicariance events, becoming isolated in forest patches. The overall patterns to date suggest that Mt Mulanje became isolated first, followed by the eastern SEAMA mountains such as Mts Namuli, Mabu, Chiperone and Mt Ribáuè. Clearly, the high species richness that defines the SEAMA was formed as an amalgamation of both ancient and also recently diverged lineages (crabs, butterflies, and bats), suggesting the processes that contribute to speciation and diversification have been ongoing over tens of millions of years.
Dated phylogenetic analyses show that the earliest SEAMA lineages date back to the Mid- or Early-Miocene. Therefore, we can assume that this ecoregion’s forest, started to become isolated from at least the Mid-Miocene. Given that species-level diversification is evident throughout the Mid-to-Late Miocene and into the Plio/Pleistocene, vicariance of these forests would have continued with subsequent isolation of species. These dates are similar to published data on the breakup of the continuous forest belt that covered this part of Africa at this time2. Throughout the Miocene, despite periods of wet and dry cycles, the climate became drier overall, resulting in a reduction of forest and an increase in woodland and open grassland. Forests in eastern and southern Africa were consequently confined to areas with higher precipitation, and especially to mountains1,11,78,88, resulting in a fragmented forest landscape with upland grasslands in which endemic species emerged as a result of the repeated isolation.
A threatened ecoregion
The major cause of montane forest loss in the SEAMA is slash and burn shifting agricultural practices, typically used for subsistence food production by local communities, along with charcoal production, for household cooking and as a source of revenue (sold on for use in urban areas)16. The fertility of the forest soil is valued, which results in smallholder plots inside or on the margins of forest being cleared for crops (especially maize, cassava, and Irish potatoes). Fire from such agriculture affects the forest edges around these cleared plots. Most of these forest patches are naturally small (< 1000 hectares) and edge effects therefore have a disproportionate impact on their ecological integrity. Where forest edges are proportionally high and degraded, the intact forest interior can be compromised due to drying effects. The upland grasslands are also threatened by increases in fire frequency, sometimes associated with flushing animals into traps. Conservation agriculture practices and sustainable alternative livelihoods are required to address these threats throughout the SEAMA ecoregion.
Official protection mechanisms vary between Malawi and Mozambique, as well as among the individual mountains. In Malawi, all the SEAMA sites are gazetted as national Forest Reserves under the management of the Department of Forestry, although this does not seem to afford adequate protection for either the forests or their natural resources, as regulated and unregulated deforestation for timber extraction and charcoal production is rife16. Thus, the wet forest on Thyolo Mountain (the type locality for several endemic taxa, including birds such as Chamaetylas choloensis (Fig. 5b) and the endemic race belcheri of Cryptolybia olivacea) was eliminated approximately 20 years ago, with the exception of a small (0.27 ha) forest patch on private land. The same fate affected Ndirande Mountain (in the 1990s) and Soche Mountain (2010s), as also most of Chiradzulu Mountain and the Malawi Hills89. In Mozambique, only one of the SEAMA sites, Mt Ribáuè (which includes Serra Mpàluwé) is a gazetted Forest Reserve. However, most forest reserves were established to regulate the harvesting of timber, not for establishing protected areas in terms of conservation90, and therefore Mt Ribáuè lacks any tangible, formal protection. Although all other sites lack any formal protection through national legislation, a project is currently underway to declare Mt Mabu as a ‘community-conservation’ protected area. Some SEAMA sites in Mozambique, such as Mt Lico, are protected due to their natural inaccessibility, while some others, such as Mt Mabu and Mt Pewé have remained in relatively good condition, possibly because these forests have spiritual value to the local communities. Overall, however, the majority of SEAMA sites lack meaningful national protection in contrast to conservation initiatives implemented in neighbouring ecoregions, such as the Eastern Arc Mountains in Tanzania.
At the level of taxonomic groups, extinction risks vary according to their reliance on the forest or upland grassland habitats (Table 2). Most of the small mammals, birds, reptiles, and amphibians, are forest-dependent and therefore the proportion of threatened species is generally high due to the acute forest destruction on most mountains. Therefore, many of these forest-dependant species are listed as Near Threatened (NT) or threatened (VU, EN, CR) on the IUCN Red List, for example the Atheris mabuensis (Fig. 5d), Rhinolophus mabuensis (Fig. 5a), Apalis lynesi, Rhampholeon chapmanorum, and Paraxerus vincenti—see Supplementary Material for a complete list. For the endemic plants (e.g., Fig. 5i−k) the majority (69%) are found in open habitat which are threatened to varying degrees by increased fire frequency30,91. However, forest plant species such as Helixanthera schizocalyx, the two new Polysphaeria species from Mts Ribáuè and Mabu42, and the forest tree Faurea racemosa, are all listed as Endangered on the IUCN Red List.
The greatest threat for the majority of sites is deforestation and increased fire frequency to the upland grasslands. Within the area defined (convex hull) for the SEAMA ecoregion, approximately 18% of primary humid forest above 800 m in elevation was lost between the years 2000 and 2022 (Table 1). This, for the same period, is far greater than in other African mountain ecoregions that contain primary humid montane forest91, including the Eastern Arc, Cameroonian Highlands, and Mt Cameroon and Bioko montane forests (< 5%), and the Albertine Rift and Guinean montane forests (ca. 10%). Therefore, the SEAMA is considerably more threatened by the rate of primary humid forest loss. Within the SEAMA, the extent and timing of forest loss varies between sites, with some such as Chiradzulu, Ndirande, and Thyolo Mountains in Malawi having lost all their forest cover prior to the year 2000, while the Malawi Hills are estimated89 to have lost nearly 80% of forest cover since the 1980s. Several other sites have suffered severe declines in the period 2000 to 2022 (Table 1), including Mts Nállume 43%, Inago 39%, Ribáuè 35%, Namuli 30%, Socone 18% and Chiperone 14%. The sites with the least loss of primary humid forest over the last 22 years are Mts Meluli and Mabu (both 3%), and Mt Lico (< 2%), this latter figure is because the Mt Lico forest is essentially inaccessible.. Note that these estimates only account for loss of primary humid forest, not secondary forest, or woodland, such that the actual habitat loss including the lower mountain slopes is likely to be much higher91,92,93. More generally, rates of montane forest loss in Mozambique, at close to 30% since 2000, are among the highest in tropical Africa91.
Conclusions
An ecoregion has shared biotic and abiotic characteristics that distinguish it from surrounding areas9,70. In the case of the SEAMA, a distinct climatic envelope clearly characterises a specific range of mountains, and defines its boundaries. Within these boundaries, we find unique species assemblages, characterised by an abundance of endemic lineages. Although survey effort has improved in recent decades for the SEAMA, compared to other neighbouring regions (e.g., Eastern Arc Mountains) its Relative Sampling Effort is still comparatively low (Table 1). Despite this, the SEAMA has more strictly endemic reptile species than the much larger and better-known Albertine Rift ecoregion. Where biological sampling has been relatively high within the SEAMA, e.g., Mt Mulanje, significant levels of endemism have been found, with a total of 48 endemic plant species (modified from Strugnell15) and a range of endemic fauna13,14,16.
The South East Africa Montane Archipelago (SEAMA)11,21,78,88 might be Africa’s newest and most threatened ecoregion with one of the highest deforestation rates (18%); however, the evidence base now exists for its global recognition as a priority site for conservation.
Methods
Definition, extent and context
For the purposes of data collation and mapping, we bounded the ecoregion in two ways. First as a collection of core sites, each individually named and spatially delimited. These are the sites of greatest known biological interest. Second, we defined a convex hull around the core sites, plus other inselbergs with the requisite elevation (> 800 m) and humidity (aridity index > 0.65). Thus, the convex hull encompasses the core sites, other inselbergs (with or without forest remaining), as well as lowland connections in between. We defined ‘endemic taxa’ as those found only within the convex hull of the ecoregion and associated with one or more of the 30 core sites (as per Fig. 1 and Table 1). Our definition of endemic taxa includes strictly endemic species, subspecies, and races. Our analysis is based on the taxonomic groups we have listed. It is recognised that levels of endemism will further increase when additional taxonomic groups are sampled.
We bounded the core sites as close to the base as possible, selecting the lowest 100-m elevational contour that distinguishes only the target features. Due to variation in base elevations across the SEAMA extent, different contours were appropriate for different sites (Table 1). The chosen contours were converted to polygons, buffered by 500 m and then smoothed to aggregate features and simplify the topology. We used digital elevation data from the Shuttle Radar Topography Mission (SRTM) v3 at 1 arc-second (~ 30 m) spatial resolution, gap-filled using a bilinear resampling of the 3 arc-second version of the same product (which has no gaps).
For the aridity index, we used the 30 arc-second (~ 1 km) surface provided by CGIAR-CSI66. The aridity index is defined as the ratio of mean annual precipitation to potential evapotranspiration, where values < 0.2 are indicative of an arid or hyper-arid environment, 0.2–0.5 semi-arid, 0.5–0.65 dry sub-humid, and > 0.65 humid67. Evapotranspiration was based on the FA0-56 Penman–Monteith Reference Evapotranspiration equation, using rainfall and temperature estimates from WorldClim version 265. In Fig. 2, we mapped this aridity index, and three other climatic variables that we expect to correlate with ecoclimatic stability94: annual rainfall (BIO12 in WorldClim2), rainfall seasonality (BIO15) defined as the coefficient of variation in rainfall across months, and maximum water deficit, a measure of dry season water stress67. We computed the maximum water deficit across consecutive months that experience rainfall < monthly ET0, over which the shortfall in rain was accumulated68.
Comparative ecoregion endemism
To place SEAMA (336,200 ha) in context, we compared the total number of strictly endemic species from various taxonomic groups with five other mountain ecoregions in east and southern Africa that are found within the Tropical & Subtropical Moist Broadleaf Forests category of the Afrotropical ecoregions according to the WWF Terrestrial Ecoregions Global 200 assessment. These are the Eastern Arc Mountains forests (EAM) at 2,380,000 ha; the Albertine Rift (AlbRft) at 10,390,000 ha; the East African Montane Forests (EAMF) at 6,563,700 ha; the Ethiopian Montane Forests (EMF) at 24,930,200 ha; and the Knysna-Amatole Montane Forests (KAMF) at 310,800 ha. In this context, in all taxonomic groups surveyed, ‘endemic species’ refer to strict endemic species only, while ‘endemic taxa’ refer to strict endemic species, endemic subspecies and endemic races.
Although direct comparisons between the absolute number of strictly endemic species can be made (Table 2), there are orders of magnitude differences in area between these ecoregions. To account for these differences in area, we applied a species-area function to estimate the number of endemic species per hectare95 by dividing richness (strict endemic species) by Az, where A is the core area (ha) of the ecoregion and z is the slope of the species-area curve (= 0.25), which diminishes the impact of larger areas.
Relative survey effort per site
We estimated relative survey effort per site using a composite index that takes into account the area of each SEAMA site (A), and sampling intensity per site (SI)75. Survey effort reflects the amount of time and resources allocated to assess the biophysical aspects of each site within SEAMA. As such it allows comparison of sampling effort between ecoregions which adds context to the known levels of endemism and the likelihood of increasing these levels. We scored sampling intensity using a 0–1 scale as follows: 0 = no known studies in that site; 0.2 = limited and non-systematic survey over few localities in the site; 0.5 = systematic survey over many localities in the site; and 1 = extremely thorough and systematic survey covering all habitats in the site.
We adapted the original formulae and calculated the mean sampling intensity (SI) for all the taxonomic groups studied at each site. In our case I indicates the number of mountain blocks per site thus including all habitat types to account for the endemic species found in the moist forests, upland grasslands, and rocky subtracts.
Primary forest loss since 2000
We estimated forest loss using a Global Forest Change dataset provided by the Global Land Analysis and Discovery (GLAD), University of Maryland93. These data have reasonable accuracy in large, dense evergreen forests, but accuracy decreases for seasonal forests, at low canopy density, and for earlier years (2001–2010). Loss rates are relative to estimated tree cover extent for the year 2000, including plantations. For these reasons, we restricted the estimates of forest loss to an estimate of primary humid tropical forest extent92. Nonetheless, we note that there may be inaccuracies owing to the global nature of these forest segmentation/classification algorithms, and that losses in secondary/disturbed are potentially higher.
Data availability
The raw data used in this study are provided in the supplementary materials, including endemic species data and site listings (Table SI1), a table of estimated divergence dates (Table SI2), and spatial data files for the delineation of core sites and convex hull (available at https://doi.org/10.6084/m9.figshare.24586941).
References
Lovett, J. C. & Wasser, S. K. Biogeography and Ecology of the Rain Forest of Eastern Africa (Cambridge University Press, 1993).
White, F. The Vegetation of Africa (UNESCO, 1983).
Couvreur, T. L., Chatrou, L. W., Sosef, M. S. & Richardson, J. E. Molecular phylogenetics reveal multiple tertiary vicariance origins of the African rain forest trees. BMC Biol. 6, 54 (2008).
Couvreur, T. L. P. et al. Tectonics, climate and the diversification of the tropical African terrestrial flora and fauna. Biol. Rev. 96, 16–51 (2021).
Jacobs, B. F. Palaeobotanical studies from tropical Africa: Relevance to the evolution of forest, woodland and savannah biomes. Philos. Trans. R. Soc. Lond. B 359, 1573–1583 (2004).
Fer, I., Tietjen, B., Jeltsch, F. & Trauth, M. H. Modelling vegetation change during Late Cenozoic uplift of the East African plateaus. Palaeogeogr. Palaeoclimatol. Palaeoecol. 467, 120–130 (2017).
Trauth, M. H., Maslin, M. A., Deino, A. & Strecker, M. R. Late cenozoic moisture history of East Africa. Science 1979(309), 2051–2053 (2005).
Fjeldsa, J. & Lovett, J. C. Geographical patterns of old and young species in African forest biota: The significance of specific montane areas as evolutionary centres. Biodivers. Conserv. 6, 325–346 (1997).
Olson, D. M. & Dinerstein, E. The Global 200: A representation approach to conserving the Earth’s most biologically valuable ecoregions. Conserv. Biol. 12, 502–515 (1998).
Platts, P. J. et al. Delimiting tropical mountain ecoregions for conservation. Environ. Conserv. 38, 312–324 (2011).
Lovett, J. Moist forests of eastern Tanzania. Swara 8, 8–9 (1985).
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B. & Kent, J. Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (2000).
Broadley, D. G. A new chameleon from Malawi. Arnoldia 31, 1–3 (1965).
Bayliss, J., Burrow, C., Martell, S. & Staude, H. An ecological study of the relationship between two living fossils in Malawi: the Mulanje Tiger Moth (Callioratis grandis) and the Mulanje Cycad (Encephalartos gratus). Afr. J. Ecol. 48, 472–480 (2009).
Strugnell, A. M. A checklist of the spermatophytes of Mount Mulanje, Malawi. Scr. Bot. Belg. 34, 1–199 (2006).
Bayliss, J., Makungwa, S., Hecht, J., Nangoma, D. & Bruessow, C. Saving the Island in the Sky: The plight of the Mount Mulanje cedar Widdringtonia whytei in Malawi. ORYX 41, 64–69 (2007).
Last, J. T. Journey of Mr. J. T. Last from Blantyre to the Namuli Hills. Proc. R. Geogr. Soc. Mon. Rec. Geogr. 9, 42 (1887).
Vincent, J. The Birds of Northern Portuguese East Africa. Comprising a list of, and observations on, the collections made during the British Museum Expedition of 1931-32. Ibis 13, 611–652 (1933).
Benson, C. W. A collection from Chiperoni Mountain, Portuguese East Africa. Bull. Br. Ornithol. Club 70, 51–51 (1950).
Poynton, J. C. Amphibia of northern Mozambique. Mem. Inst. Investig. Cien. Moçamb. A Cienc. B 8, 13–34 (1966).
Ryan, P. G. et al. The avifauna and conservation status of the Namuli Massif, northern Mozambique. Bird Conserv. Int. 9, 315–331 (1999).
Branch, W. R. & Ryan, P. G. Additions to the Mozambique Herpetofauna: Two new lizards from the Namuli Massif, Mozambique. Herpetol. Rev. 32, 281–282 (2001).
Bayliss, J. Danger and discoveries in northern Mozambique. Lepsoc News Afr. 4, 3–6 (2008).
Spottiswoode, C. N., Patel, I. H., Herrmann, E., Timberlake, J. & Bayliss, J. Threatened bird species on two little-known mountains (Chiperone and Mabu) in northern Mozambique. Ostrich 79, 1–7 (2008).
Smith, P. & Bayliss, J. Darwin project discovers virgin rainforest in Mozambique. Darwin News 1, 3 (2009).
Fishpool, L. D. C. & Bayliss, J. Brief notes on the birds of Mount Inago, northern Mozambique. Bull. ABC 17, 198–201 (2010).
Dowsett-Lemaire, F. Ornithological exploration of Namuli and Mabu Mountains. Bull. ABC 17, 2–159 (2010).
Bayliss, J. et al. Biodiversity and Conservation of Mount Inago, Mozambique. Report Produced Under Darwin Initiative Award 15/036. (2010).
Timberlake, J. et al. Mt Mabu, Mozambique: Biodiversity and Conservation. (Report Produced Under Darwin Initiative Award 15/036). (2012).
Timberlake, J. et al. Mt Namuli, Mozambique: Biodiversity and Conservation. (Report Produced Under Darwin Initiative Award 15/036). (2009).
Timberlake, J. et al. The Biodiversity and Conservation of Mount Chiperone, Mozambique (Report Produced Under Darwin Initiative Award 15/036). (2007).
Conradie, W. et al. Exploration into the hidden world of Mozambique’s sky island forests: new discoveries of reptiles and amphibians. Zoosyst. Evol. 92, 163–180 (2016).
Branch, W. R. & Bayliss, J. A new species of Atheris (Serpentes: Viperidae) from northern Mozambique. Zootaxa 2113, 41–54 (2009).
Harris, T., Darbyshire, I. & Polhill, R. New species and range extensions from Mt Namuli, Mt Mabu and Mt Chiperone in northern Mozambique. Kew Bull. 66, 241–251 (2011).
Branch, W. R. & Tolley, K. A. A new species of chameleon (Sauria: Chamaeleonidae: Nadzikambia) from Mount Mabu, central Mozambique. Afr. J. Herpetol. 59, 157–172 (2010).
Bayliss, J., Brattström, O., Bampton, I. & Collins, S. A new species of Leptomyrina Butler, 1898 (Lepidoptera: Lycaenidae) from Mts Mecula, Namuli, Inago, Nallume and Mabu in Northern Mozambique. Metamorphosis 30, 19–24 (2019).
Branch, W. R., Bayliss, J. & Tolley, K. A. Pygmy chameleons of the Rhampholeon platyceps compex (Squamata: Chamaeleonidae): Description of four new species from isolated ‘sky islands’ of northern Mozambique. Zootaxa 3814, 1–36 (2014).
Taylor, P. J. et al. Four New Bat Species (Rhinolophus hildebrandtii Complex) Reflect Plio-Pleistocene Divergence of Dwarfs and Giants across an Afromontane Archipelago. PLoS ONE 7, 41477 (2012).
Monadjem, A. et al. A recent inventory of the bats of Mozambique with documentation of seven new species for the country. Acta Chiropt 12, 371–391 (2010).
Bayliss, J., Congdon, T. C. E., Richardson, I. D. & Collins, S. C. A new species of Afriodinia d’Abrera, 2009 (Lepidoptera: Riodinidae) from Mount Lico, Northern Mozambique; the revision of the A delicata group; and the reinstatement of a species. Metamorphosis 33, 34–41 (2022).
Bayliss, J., Congdon, C. & Collins, S. A new subspecies of Papilio (Princeps) pelodurus Butler, 1896 (Lepidoptera: Papilionidae: Papilioninae) from several mountains in northern Mozambique. Metamorphosis 29, 132–136 (2018).
Darbyshire, I., Langa, C. & Romeiras, M. M. A synopsis of polysphaeria (Rubiaceae) in mozambique, including two new species. Phytotaxa 414, 1–18 (2019).
Darbyshire, I., Polhill, R. M., Magombo, Z. & Timberlake, J. R. Two new species from the mountains of southern Malawi and northern Mozambique. Kew Bull. 76, 63–70 (2021).
Bayliss, J. L., Collins, S. C. & Congdon, T. C. E. A new species of Iolaus Hübner, [1819] subgenus Epamera Druce, 1891 (Lepidoptera: Lycaenidae: Theclinae) from Mts Namuli and Mabu, Northern Mozambique. Metamorphosis 27, 21–28 (2016).
Daniels, S. R. & Bayliss, J. Neglected refugia of biodiversity: Mountainous regions in Mozambique and Malawi yield two novel freshwater crab species (Potamonautidae: Potamonautes). Zool. J. Linn. Soc. 164, 498–509 (2012).
Conradie, W. et al. New species of Mongrel Frogs (Pyxicephalidae: Nothophryne) for northern Mozambique inselbergs. Afr. J. Herpetol. 67, 61–85 (2018).
Downes, E. & Darbyshire, I. Coleus namuliensis and Coleus caudatus (Lamiaceae): a new species and a new combination in the Afromontane flora of Mozambique and Zimbabwe. Blumea Biodivers. Evol. Biogeogr. Plants 62, 168–173 (2018).
Branch, W. R. et al. A new species of tree snake (Dipsadoboa, Serpentes: Colubridae) from ‘sky island’ forests in northern Mozambique, with notes on other members of the Dipsadoboa werneri group. Zootaxa 4646, 541–563 (2019).
van Velzen, R., Collins, S. C., Brattström, O. & Congdon, T. C. E. Description of a new Cymothoe Hübner, 1819 from northern Mozambique (Lepidoptera: Nymphalidae: Limenitidinae). Metamorphosis 27, 34–41 (2016).
Daniels, S. R., Bittencourt-Silva, G. B., Muianga, V. & Bayliss, J. Phylogenetics of the freshwater crab (Potamonautes MacLeay, 1838) fauna from ‘sky islands’ in Mozambique with the description of a new species (Brachyura: Potamoidea: Potamonautidae). Eur. J. Taxon. https://doi.org/10.5852/ejt.2020.716 (2020).
Daniels, S. R., Phiri, E. E. & Bayliss, J. Renewed sampling of inland aquatic habitats in southern Africa yields two novel freshwater crab species (Decapoda: Potamonautidae: Potamonautes). Zool. J. Linn. Soc. 171, 356–369 (2014).
Portik, D. M., Travers, S. L., Bauer, A. M. & Branch, W. R. A new species of Lygodactylus (Squamata: Gekkonidae) endemic to Mount Namuli, an isolated ‘sky island’ of northern Mozambique. Zootaxa 3710, 415 (2013).
Safian, S., Bayliss, J. & Congdon, T. C. E. Description of four Iolaus Hübner, 1819 species in the subgenus Philiolaus Stempffer & Bennett, 1958 from East Africa, assigned to the proposed I. maritimus species group (Lepidoptera, Lycaenidae, Theclinae). Zootaxa 5099, 46–64 (2022).
Bayliss, J. et al. The discovery, biodiversity and conservation of Mabu forest: The largest medium-altitude rainforest in southern Africa. ORYX 48, 177–185 (2014).
Darbyshire, I. et al. The endemic plants of Mozambique: Diversity and conservation status. PhytoKeys 136, 45–96 (2019).
Bittencourt-Silva, G. B., Bayliss, J. & Conradie, W. First herpetological surveys of Mount Lico and Mount Socone, Mozambique. Amphib. Reptile Conserv. 14, 198–217 (2020).
Richardson, I. D. Revision of the genus Neptis Fabricius, 1807 (Lepidoptera, Nymphalidae) in the Afrotropical Region, Part 2: Two new species in the Agatha group. Metamorphosis 31, 84–93 (2020).
Jones, S. E., Jamie, G. A., Sumbane, E. & Jocque, M. The avifauna, conservation and biogeography of the Njesi Highlands in northern Mozambique, with a review of the country’s Afromontane birdlife. Ostrich 91, 45–56 (2020).
Bayliss, J. Mount Lico: Mountain of Mystery. Annual General Meeting of the Transglobe Expedition Trust. (2019).
SAMC2022. 1st Southern African Mountain Conference (SAMC2022), Champagne Sports Resort, Winterton, South Africa, 14–17 March 2022. (2022).
Fjeldså, J. & Bowie, R. C. K. Evolutionary and ecological explanations for the elevational flexibility of several East African bird species complexes. Front. Ecol. Evol. 9, 62 (2021).
Macey, P. H. et al. Mesoproterozoic geology of the Nampula Block, northern Mozambique: Tracing fragments of Mesoproterozoic crust in the heart of Gondwana. Precambrian Res. 182, 124–148 (2010).
Grantham, G. H., Marques, J. M., Wilson, M. G. C., Manhiça, V. & Hartzer, F. J. Explanation of the Geological Map of Mozambique, 1:1000000. (2010).
Bingen, B. et al. Geochronology of the Precambrian crust in the Mozambique belt in NE Mozambique, and implications for Gondwana assembly. Precambrian Res. 170, 231–255 (2009).
Fick, S. E. & Hijmans, R. J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
Zomer, R. J., Xu, J. & Trabucco, A. Version 3 of the global aridity index and potential evapotranspiration database. Sci. Data 9, 1–10 (2022).
UNEP. World Atlas of Desertification: Second Edition. https://wedocs.unep.org/20.500.11822/30300. (1997).
Pfeifer, M. et al. Tropical forest canopies and their relationships with climate and disturbance: Results from a global dataset of consistent field-based measurements. For. Ecosyst. 5, 7 (2018).
Los, S. O. et al. Sensitivity of a tropical montane cloud forest to climate change, present, past and future: Mt. Marsabit, N. Kenya. Quat. Sci. Rev. 218, 34–48 (2019).
Dinerstein, E. et al. An ecoregion-based approach to protecting half the terrestrial realm. Bioscience 67, 534–545 (2017).
Burgess, N. D. et al. Terrestrial Ecoregions of Africa and Madagascar: A Conservation Assessment (Island Press, 2004).
BirdLife International. Ecosystem Profile. Eastern Afromontane Biodiversity Hotspot. (2012).
Darbyshire, I. et al. Important Plant Areas of Mozambique (Royal Botanic Gardens, 2023).
WCS, G. de M. & U. Red List of Threatened Species, Ecosystems, Identification and Mapping of Key Biodiversity Areas (KBAs) in Mozambique. Final Report (Vol. I). (2021).
Rovero, F. et al. Targeted vertebrate surveys enhance the faunal importance and improve explanatory models within the Eastern Arc Mountains of Kenya and Tanzania. Divers. Distrib. 20, 1438–1449 (2014).
Farooq, H., Azevedo, J. A. R., Soares, A., Antonelli, A. & Faurby, S. Mapping Africa’s biodiversity: More of the same is just not good enough. Syst. Biol. 70, 623–633 (2021).
Strugnell, A. M. Endemics of Mt. Mulanje. The endemic spermatophytes of Mt. Mulanje, Malawi. Syst. Geogr. Plants 72, 11–26 (2002).
Dowsett-Lemaire, F. The flora and phytogeography of the evergreen forests of Malawi I: Afromontane and mid-altitude forests. Bull. Natl. Plant. Belg. 59, 3 (1989).
Dowsett-Lemaire, F. The flora and phytogeography of the evergreen forests of Malawi II: Lowland forests. Bull. Natl. Plant. Belg. 60, 9 (1990).
Chapman, J. D. & White, F. The Evergreen Forests of Malawi (University of Oxford, 1970).
Timberlake, J. R. A first plant checklist for Mt. Namuli, northern Mozambique. Kirkia 19, 191–225 (2021).
Timberlake, J. R. & Bayliss, J. Elaboration of a Conservation Management Plan for the Mt Mabu Region: Biophysical Attributes. CEPF Grant 379-CEP1. (2016).
McCoy, T. A., Rulkens, A. J. H. & Baptista, O. J. An extraordinary new species of aloe from the Republic of Mozambique. Cactus Succul. J. 86, 48–53 (2014).
Monadjem, A., Healy, K., Guillerme, T. & Kane, A. Dispersal ability is associated with contrasting patterns of beta diversity in African small mammal communities. J. Biogeogr. 50, 539–550 (2023).
Bittencourt-Silva, G. B. et al. The phylogenetic position and diversity of the enigmatic mongrel frog Nothophryne Poynton, 1963 (Amphibia, Anura). Mol. Phylogenet. Evol. 99, 89–102 (2016).
Cumberlidge, F. L. S. N. & Daniels, S. R. A new multilocus phylogeny reveals overlooked diversity in African freshwater crabs (Brachyura: Potamoidea): A major revision with new higher taxa and genera. Zool. J. Linn. Soc. 194, 1268–1311 (2022).
Tolley, K. A., Townsend, T. M. & Vences, M. Large-scale phylogeny of chameleons suggests African origins and Eocene diversification. Proc. R. Soc. B 280, 20130184 (2013).
Lovett, J. C. Classification and Status of the Moist Forests of Tanzania (Institute for General Botany, 1990).
Tolley, K. A. et al. Clinging to survival: Critically Endangered Chapman’s pygmy chameleon Rhampholeon chapmanorum persists in shrinking forest patches. Oryx 56, 451–456 (2022).
Müller, T., Sitoe, A. & Mabunda, R. Assessment of the Forest Reserve Network in Mozambique. (2005).
Cuni-Sanchez, A. et al. High aboveground carbon stock of African tropical montane forests. Nature 596, 536–542 (2021).
Turubanova, S., Potapov, P., Tyukavina, A. & Hansen, M. C. Ongoing primary forest loss in Brazil, Democratic Republic of the Congo, and Indonesia. Environ. Res. Lett. 13, 074028 (2018).
Hansen, M. C. et al. High-resolution global maps of 21st-century forest cover change. Science 1979(850), 850–854 (2013).
Platts, P. J. et al. Can distribution models help refine inventory-based estimates of conservation priority? A case study in the Eastern Arc forests of Tanzania and Kenya. Divers. Distrib. 16, 628–642 (2010).
Green, J. L. & Ostling, A. Endemics-area relationships: The influence of species dominance and spatial aggregation. Ecology 84, 3090–3097 (2003).
Acknowledgements
Research and collection permissions were provided by Museu de História Natural Maputo Moçambique; provinces of Zambézia and Nampula (Provincial Directorate of Land, Environment and Rural Development); Instituto de Investigação Agrária de Moçambique (IIAM); National Administration for Conservation Areas (ANAC) Moçambique, National Museums of Malawi, and the Forestry Research Institute of Malawi (FRIM). Specimens and samples were collected under institutional permits issued by the provinces of Zambezia, Nampula and Niassa (129-DPAN2014, 507DPAZ/SPFFB/420/2014, 710/DPTADER/NPL/REP/2017, 282/DPTADERZ/GD/SPFFB/420) and under export permits from the Museu de Historia Natural—Maputo (315/MHN/E.24/2014, No.08/2018, No.21/2018). Ethics clearance was also obtained from the South African National Biodiversity Institute (#001-2014 and #001-2015). The fieldwork was undertaken through the following grants: Darwin Initiative (Project ref: 15-036); Critical Ecosystem Partnership Fund-Eastern Afromontane Biodiversity Hotspot, project #62584; Critical Ecosystem Partnership Fund-Eastern Afromontane Biodiversity Hotspot Small Grant S16-375-MOZ; National Geographic Society-Committee for Research and Exploration grants (# 9281-13 & 9934-16); the Global Challenges Research Fund pump-priming award (GCRF QR) administered by the University of York. Financial support was also provided by the Royal Botanic Gardens (RBG) Kew, Transglobe Expedition Trust (TET), Mulanje Mountain Conservation Trust (MMCT), African Butterfly Research Institute (ABRI), Biocensus, Fauna & Flora International (FFI), the Jonathan and Jennifer Oppenheimer Foundation, Stephen and Margaret Lansdown, BBC World Service and Natural History Unit (NHU), BirdLife International, South African National Biodiversity Institute (SANBI), Grain Media, Google Inc, Biofund, Rainforest Trust, World Wide Fund for Nature (WWF) Mozambique, Promove Biodiversity, European Union (EU), T. Colin E. Congdon, Monica and Allan Bayliss. The following people have contributed to the field work and logistical support in the establishment of SEAMA: Tereza Alves, Susana Baena, Ivan Bampton, Poppy Bayliss, Aurélio Banze, Jeff Barbee, Matthias De Beenhouwer, Carlos Bento, Christophe Bernier, Saheba Bhatnagar, Clemie Borgstein, Anton Bowring, Mike Brewin, Neil Burgess, Ana Gledis da Conceição, Katrina Cook, Matt Cooper, Madyo Couto, Ron Demey, Gimo M. Daniel, Tim Davenport, Francoise and Robert Dowsett-Lemaire, Ruben Foquet, Jorge Francisco, Hanlie Engelbrecht, Jonah Fisher, Tim Harris, Martin Hassan, Ben Hayes, Eric Herrmann, Tim Hounsome, Samuel Jones, Julian Kerbis, Mirjam Kopp, Dave Langerman, Bruce Liggitt, Jules Lines, Simon Loader, Kimma Long, Stephen Mphamba, Michele Menegon, Vanessa Muianga, David Nangoma, Bruno Nhancale, David Njagi, Hassam Patel, Mike Robertson, Lucas Sabão, Mike Scott, Khangela Safaris, Camila de Sousa, Malaika Sacranie, Margriet Sacranie, Reza Sacranie, Antonio Serra, Neil Shaw, Barnet Tippa, Roland van de Ven, Antonia Windsor, Richard Young, Sir David Attenborough (BBC NHU Africa series episode 4), and Sir Ranulph Fiennes. The article processing charge (APC) for this publication was kindly provided by Allan Bayliss and T. Colin E. Congdon, with support from Oxford Brookes University.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiment: J.B. & P.P. Data collection: All authors contributed to data collection. Data analysis: J.B., P.P., H.F. Writing of the manuscript: J.B.& P.P. led the writing of the manuscript, all authors contributed. Creation of Figures for the paper: J.S., J.B., P.P. Creation of spatial data layers: P.P., J.S., J.B. Creation of Tables for the paper: J.B., J.T., P.P., A.M., K.T., G.B.B., S.R.D., S.C. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bayliss, J., Bittencourt-Silva, G.B., Branch, W.R. et al. A biogeographical appraisal of the threatened South East Africa Montane Archipelago ecoregion. Sci Rep 14, 5971 (2024). https://doi.org/10.1038/s41598-024-54671-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54671-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.