Abstract
While the relationship between circulating osteoprotegerin (OPG) and cardiovascular events is well-established in the general population, its association with cardiovascular risks in chronic kidney disease (CKD) patients remains less robust. This study hypothesized that elevated circulating OPG levels might be associated with an increased risk of major adverse cardiac events (MACE) in CKD patients, a total of 2,109 patients with CKD stages 1 through pre-dialysis 5 from the KNOW-CKD cohort were categorized into quartiles based on serum OPG levels. The primary outcome of the study was 3-point MACE, defined as a composite of nonfatal myocardial infarction, nonfatal stroke, or cardiac death. The median follow-up duration was 7.9 years. The cumulative incidence of 3-point MACE significantly varied across serum OPG levels in Kaplan–Meier curve analysis (P < 0.001, log-rank test), with the highest incidence observed in the 4th quartile. Cox regression analysis indicated that, relative to the 1st quartile, the risk of 3-point MACE was significantly higher in the 3rd (adjusted hazard ratio 2.901, 95% confidence interval 1.009 to 8.341) and the 4th quartiles (adjusted hazard ratio 4.347, 95% confidence interval 1.410 to 13.395). In conclusion, elevated circulating OPG levels are associated with adverse cardiovascular outcomes in pre-dialysis CKD patients.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) remains a primary cause of mortality among patients with chronic kidney disease (CKD)1,2. Beyond the notable prevalence of coronary artery disease3,4, heart failure, stemming from either systolic or diastolic dysfunction, further exacerbates the adverse cardiovascular outcomes in this population5,6. Hence, the early stratification of CKD patients at heightened risk for cardiovascular events (CVEs) is crucial. Several biomarkers have been explored for their potential to predict forthcoming CVEs in the CKD cohort7,8,9; however, none has unequivocally outperformed the rest.
Osteoprotegerin (OPG), a secreted glycoprotein, is a member of the tumor necrosis factor receptor (TNF) superfamily10,11. While primarily known for its role in bone metabolism—it mediates osteoclastogenesis inhibition by binding to the receptor activator of nuclear factor-κB ligand (RANKL) and preventing the RANKL-RANK interaction10,11,12—circulating OPG’s clinical significance extends beyond bone health. Accumulating evidence underscores a robust correlation between elevated circulating OPG levels and heightened CVE risk13. Multiple studies have consistently linked increased circulating OPG concentrations with adverse CVE outcomes in patients diagnosed with CAD14,15,16. The predictive capacity of OPG in determining coronary artery calcification risk is further corroborated by meta-analyses17,18. Moreover, the CORONA trial’s post-hoc analysis affirmed the relationship between serum OPG concentrations and the exacerbation of heart failure19. Therefore, the prognostic utility of circulating OPG concerning CVEs in the broader population is well-established.
Despite the observed elevation in serum OPG levels among patients with CKD20, the evidence substantiating the association between circulating OPG and CVEs remains less robust than in the general population. Previous studies have proposed an association between circulating OPG levels and vascular calcification21, myocardial dysfunction22, cardiovascular mortality23,24, and all-cause mortality25. However, these studies are limited by their small sample sizes and relatively short follow-up durations. Furthermore, the evidence connecting serum OPG levels to overarching cardiovascular outcomes, particularly the risk of major adverse cardiac events (MACE), remains scant in the CKD patient cohort.
In the present study, utilizing a long-term prospective cohort of patients with CKD ranging from stage 1 to pre-dialysis stage 5, we sought to elucidate the association between circulating OPG levels and the risk of CVEs. Our hypothesis posits that elevated circulating OPG levels might correlate with a heightened risk of adverse CVEs in CKD patients.
Results
Baseline characteristics
Significant differences in serum OPG levels among participants were observed (Table 1). The shortest follow-up duration was noted in Q4. Additionally, the mean age was highest in Q4. There was a higher proportion of subjects with a Charlson comorbidity index ≥ 4 and those with DM in Q4. The frequency of diuretics, statins, and antiplatelet and/or anticoagulant usage was also higher in Q4. Waist-to-hip ratio (WHR) and blood pressure levels were notably elevated in Q4, whereas levels of hemoglobin, albumin, total cholesterol, high-density lipoprotein cholesterol (HDL-C), and estimated glomerular filtration rate (eGFR) were reduced. Conversely, high-sensitivity C-reactive protein (hs-CRP) and spot urine albumin-to-creatinine ratio (ACR) levels were increased in Q4. Echocardiographic data revealed an association between increased serum OPG levels and elevations in left ventricular mass index, E/e′ ratio, left atrium diameter, interventricular wall thickness, and posterior wall thickness (Supplementary Table S1). Moreover, the prevalence of regional wall motion abnormality and valvular calcification was higher in Q4. Conversely, left ventricular ejection fraction (LVEF) was diminished in Q4. In essence, adverse medical conditions frequently correlated with elevated serum OPG levels.
Association between serum OPG levels and the risk of MACEs in patients with CKD
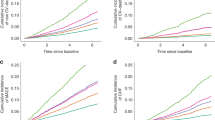
The study outcomes’ cumulative incidence was evaluated using the Kaplan–Meier curve. The risks associated with incident 3-point (Fig. 1), 4-point (Supplementary Fig. S1), and 6-point (Supplementary Fig. S2) MACEs varied significantly based on serum OPG levels, with the highest incidence observed in Q4 (all P < 0.001). Cox proportional hazard models were utilized to determine the independent association between serum OPG levels and MACE risks. Relative to Q1, there was a significant elevation in the risk of 3-point MACE in Q3 (adjusted HR 2.901, 95% CI 1.009–8.341) and Q4 (adjusted HR 4.347, 95% CI 1.410–13.395) as presented in Table 2. Additionally, the risks for 4-point (adjusted HR 2.571, 95% CI 1.006–6.570) and 6-point (adjusted HR 2.686, 95% CI 1.179–6.116) MACEs were notably higher in Q4 compared to Q1 (Supplementary Table S2). Spline curve analyses further illustrated a nearly linear, positive correlation between serum OPG levels and MACE risk (Fig. 2 and Supplementary Figs. S3 and S4).
Panelized spline curve of serum OPG level’s effect on 3-point MACE. The adjusted HR for serum OPG level, considered as a continuous variable for 3-point MACE, is depicted. This model was adjusted for factors including age, sex, Charlson comorbidity index, primary causes of CKD, smoking status, medications (ACEIs/ARBs, diuretics, statins, antiplatelets/anticoagulants), WHR, SBP, hemoglobin, albumin, total cholesterol, HDL-C, fasting glucose, 25(OH)D, hs-CRP, eGFR, spot urine ACR, and LVEF at baseline. HR hazard ratio, MACE major adverse cardiac event, OPG osteoprotegerin.
Sensitivity and subgroup analyses
Upon re-categorizing participants based on tertile and quintile divisions of serum OPG levels, as opposed to quartile divisions, a significant association between elevated serum OPG levels and an increased risk of 3-point MACE was observed. Specifically, the 3rd tertile versus the 1st tertile yielded an adjusted HR of 3.228 (95% CI 1.228–8.484), and the 5th quintile versus the 1st quintile resulted in an adjusted HR of 4.079 (95% CI 1.177–14.134) (Supplementary Table S3). When non-cardiac death events were censored prior to achieving the study outcome, the increased risk associated with 3-point MACE remained significant in the 4th quartile with an adjusted HR of 4.347 (95% CI 1.212–15.592) (Table 3). Further, after imputing missing values through multiple imputation techniques, the correlation between serum OPG levels and the 3-point MACE risk persisted (4th quartile vs. 1st quartile, adjusted HR 3.239, 95% CI 1.150–9.124) (Table 4). The subsequent subgroup analyses indicated that this association was not significantly influenced by variables such as age, sex, presence or absence of DM, BMI, eGFR, or albuminuria (Table 5).
Discussion
In the present study, we determined that elevated circulating levels of OPG correlate with an increased risk of MACE in pre-dialysis CKD patients. The robustness of this finding is underlined by sensitivity analyses, which encompassed both competing risk analysis and multiple imputation, confirming the association between serum OPG levels and MACE risk. Furthermore, subgroup analyses indicated that this correlation remained consistent irrespective of variables such as age, sex, the presence or absence of DM, BMI, eGFR, and albuminuria.
While previous studies have consistently highlighted the predictive capacity of circulating OPG in forecasting CVEs within the general population14,15,16,17,18, such a relationship has yet to be affirmed in patients exhibiting renal dysfunction. For instance, Scialla et al. reported that OPG may be risk factors for all-cause and cardiovascular mortality in patients initiating dialysis, but not in patients with pre-dialysis CKD, which analyzed 602 incident dialysis patients for median 3.4 years26. Marques et al. presented a data supporting the association between serum OPG levels and the risk of cardiovascular mortality in patients with CKD stages 3 to 5 (including those undergoing dialysis), while only a limited numbers of the patients were included (n = 145)24. Huang et al., in their meta-analysis, demonstrated that elevated OPG level are associated with an increased risk of cardiovascular death in patients with CKD27. In the meta-analysis, a total of 2120 patients from 10 studies included, while most of the patients (n = 1723) were receiving dialysis. Therefore, the evidence for the association between serum OPG levels and the risk of MACEs still remains weak especially in patient with pre-dialysis CKD (Supplementary Table S4). This gap in knowledge carries significant implications, especially considering that CVD is a predominant cause of mortality in the CKD population1,2. Notably, there remains a lack of specific biomarkers that outperform others in predicting CVD in this specific demographic7,8. Furthermore, it should be emphasized that patients with decreased eGFR tend to exhibit elevated serum OPG levels20. As such, the predictability of OPG based on findings from the general populace should be approached with caution. Our results, therefore, introduce pivotal evidence concerning the link between serum OPG levels and MACE risk in CKD patients.
Biochemically, OPG operates as a decoy receptor for RANKL and TNF-related apoptosis-inducing ligand (TRAIL)10,11,12. By interacting with RANKL and TRAIL, OPG impedes their subsequent attachment to RANK. It is a recognized phenomenon that the engagement of RANKL with RANK augments the calcification of vascular smooth muscle cells, predominantly through the activation of nuclear factor-κB28. Consequently, OPG’s inherent biological role is presumed to act as a defensive mechanism against vascular calcification. Supporting this assertion, one experimental study reported that deleting the gene responsible for encoding murine OPG, Tnsf11b, induced spontaneous vascular calcification in mice and accelerated atherosclerosis in Apoe-deficient mice29,30. This strongly suggests that the prevalent effect of circulating OPG on vascular tissue is fundamentally protective.
On the other hand, OPG treatment in isolated rodent endothelial cells has been shown to lead to a reduction in nitric oxide generation and an increase in reactive oxygen species production31. Furthermore, stimulation of these cells with inflammatory cytokines enhances OPG secretion, which subsequently amplifies the surface expression of adhesion molecules, notably ICAM-1 and VCAM-132. These findings suggest a potential role for OPG in early vascular inflammatory processes. Consequently, elevated circulating OPG levels might serve as indicators of vascular inflammation, potentially forecasting future CVEs.
Beyond vascular calcification and atherosclerosis, heart failure stands as a prominent cardiac manifestation correlated with CKD1,33. While there exists substantial evidence linking this manifestation to the general population34,35,36, the association between serum OPG levels and the risk of heart failure in CKD patients still requires further validation. In a recent study, a significant relationship was observed between serum OPG levels and risks associated with left ventricular hypertrophy, as well as systolic and diastolic dysfunction, in CKD patients ranging from stage 3 to pre-dialysis 522. However, the limited sample size (n = 101) poses a significant limitation to this study. Our present research does not negate the potential link between serum OPG levels and heart failure risk, especially considering that one of our secondary outcomes, the 6-point MACE, including hospitalization due to heart failure events. Comprehensive studies on a larger scale are imperative to elucidate the relationship between serum OPG levels and heart failure risk.
We acknowledge several limitations in our study. Firstly, given its observational design, this study cannot establish a causal relationship between serum OPG levels and the risk of MACEs. However, it is important to note that OPG serves as a potential biomarker and is not a therapeutic intervention. Conducting randomized controlled trials with serum OPG levels as a primary intervention is not feasible. Nevertheless, post-hoc analyses of clinical trials have shown an association between serum OPG levels and cardiovascular outcomes in the general population. Secondly, all variables, including serum OPG levels, were measured only at baseline. Despite this, many previous studies investigating the relationship between serum OPG levels and cardiovascular outcomes have employed a similar design and yielded results consistent with ours. This suggests a robust predictive value of serum OPG levels for long-term CVEs in patients with CKD. Lastly, the KNOW-CKD study comprised solely of ethnically Korean patients residing in South Korea. Therefore, extrapolating these findings to other populations should be approached with caution, even though studies from other countries have observed a comparable association between serum OPG levels and the risk of CVEs in the general population.
In conclusion, our findings indicate that elevated circulating OPG levels are associated with adverse cardiovascular outcomes in pre-dialysis CKD patients. Monitoring serum OPG levels could aid in the early detection of CKD patients at elevated risk for future CVEs.
Methods
Study population
The KNOW-CKD (KoreaN Cohort Study for Outcome in Patients With Chronic Kidney Disease)37 is a prospective cohort study focused on Korean patients with CKD stages ranging from 1 to pre-dialysis 5. This study was conducted in collaboration with nine tertiary hospitals in South Korea between 2011 and 2016. The study protocol adhered to the Declaration of Helsinki and received approval from the Institutional Review Board of each participating institution: Seoul National University Hospital (1104–089-359, May 25, 2011), Seoul National University Bundang Hospital (B-1106/129–008, August 24, 2011), Yonsei University Severance Hospital (4–2011-0163, June 2, 2011), Kangbuk Samsung Medical Center (2011–01-076, June 16, 2012), Seoul St. Mary’s Hospital (KC11OIMI0441, June 30, 2011), Gil Hospital (GIRBA2553, August 8, 2011), Eulji General Hospital (201105–01, June 10, 2011), Chonnam National University Hospital (CNUH-2011-092, July 5, 2011), and Busan Paik Hospital (11–091, July 26, 2011). Initially, a cohort of 2,238 subjects was established from individuals who provided informed consent. However, after excluding participants without baseline serum OPG measurements (n = 98) and those missing follow-up duration data (n = 31), a total of 2109 subjects remained eligible for further analyses (Fig. 3). Throughout the study, participants were closely monitored, with outcome events recorded by the clinical determination of the investigators at each center. To ensure accuracy, these events were cross-validated by investigators from other collaborating institutions. The median follow-up duration was 7.9 years.
Data collection from the participants
In accordance with the study protocol, demographic information, anthropometric measurements, and medical histories of the participants were obtained at the time of enrollment. Following an overnight fast, blood and urine samples were collected and subsequently analyzed at the central laboratory (Lab Genomics, Seongnam, Korea). The estimated glomerular filtration rate eGFR was determined using the Chronic Kidney Disease Epidemiology Collaboration equation with creatinine as a reference38. For the purpose of measuring the ACR, spot urine samples were collected, preferably as the second void. Echocardiographic evaluations were performed by cardiologists at participating hospitals. These cardiologists were blinded to the clinical data of the participants and carried out the assessments in alignment with the American Society of Echocardiography guidelines39.
Determination of serum OPG levels
The serum OPG concentrations were quantified utilizing an enzyme-linked immunosorbent assay kit (BioVendor R&D, Brno, Czech Republic)40,41. Results were reported using the mean values of the duplicate samples. For the samples (n = 3) that had values below the detection range (< 1.5 pmol/L), the value was standardized to 1.5 pmol/L for reporting purposes.
Exposure and study outcome
Serum OPG concentrations served as the primary exposure metric. Based on these levels, subjects were categorized into quartiles: Q1, Q2, Q3, and Q4 (Fig. 3). The primary study outcome was 3-point MACE, encompassing nonfatal myocardial infarction, nonfatal stroke, and cardiac death. Secondary study outcomes were categorized as 4-point MACE (nonfatal myocardial infarction, unstable angina, nonfatal stroke, and cardiac death) and 6-point MACE (nonfatal myocardial infarction, unstable angina, nonfatal stroke, hospitalization due to heart failure, symptomatic arrhythmia, or cardiac death).
Statistical analysis
Statistical comparisons of continuous and categorical baseline characteristics based on serum OPG levels were conducted using one-way analysis of variance (ANOVA) and the χ2 test, respectively. Kaplan–Meier survival curves were employed to visualize the cumulative incidences of study outcomes, which were subsequently compared using the log-rank test. For participants lost to follow-up, the date of the last visit was considered the censoring date. To determine the independent association between serum OPG levels and the risk of MACEs, Cox proportional hazard regression models were developed. Participants with any missing data were excluded from primary analyses. The models were adjusted as follows: Model 1 reported unadjusted hazard ratios (HRs); Model 2 adjusted for age and gender; Model 3 incorporated adjustments for medical history—specifically, Charlson comorbidity index, primary cause of CKD, smoking status, and medications (e.g., ACEIs/ARBs, diuretics, statins, and antiplatelet/anticoagulant agents)—as well as anthropometric data, namely WHR and SBP. Model 4 further adjusted for baseline laboratory parameters including hemoglobin, albumin, total cholesterol, HDL-C, fasting glucose, 25(OH)D, hs-CRP, eGFR, spot urine ACR, and LVEF. The outcomes of the Cox regression analyses were reported with 95% confidence intervals (CIs) accompanying the HRs. A penalized spline curve illustrated the linear relationship between serum OPG levels (treated as a continuous variable) and the risk of MACEs. To verify the robustness of our findings, we implemented several sensitivity analyses. Initially, participants were grouped by serum OPG levels into tertiles and quintiles instead of quartiles for Cox regression analyses. Next, for the primary study outcome, non-cardiac death events that occurred prior to reaching the study outcome were deemed as a competing risk and thus treated as censoring. Lastly, any missing values in the primary analyses were addressed using multiple imputation and the Cox regression analyses were repeated. Pre-defined subgroup analyses were crafted to explore if the relationship between serum OPG levels and the risk of MACEs differed across specific clinical settings. These subgroups included age (either < 60 or ≥ 60 years), sex (male or female), presence or absence of diabetes mellitus (DM), body mass index (BMI; either < 23 or ≥ 23 kg/m2), eGFR (either < 45 or ≥ 45 mL/min/1.73 m2), and spot urine ACR (either < 300 or ≥ 300 mg/g). Two-tailed p values below 0.05 were deemed statistically significant. All statistical analyses were executed using SPSS for Windows version 22.0 (IBM Corp., Armonk, NY) and R software (version 4.1.1; R Project for Statistical Computing, Vienna, Austria).
Data availability
The data collected for this study cannot be shared publicly because they contain information that could compromise the privacy of the research participants. The data are available from the corresponding author upon request.
References
Jankowski, J., Floege, J., Fliser, D., Böhm, M. & Marx, N. Cardiovascular disease in chronic kidney disease: Pathophysiological insights and therapeutic options. Circulation 143, 1157–1172. https://doi.org/10.1161/circulationaha.120.050686 (2021).
Go, A. S., Chertow, G. M., Fan, D., McCulloch, C. E. & Hsu, C. Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351, 1296–1305. https://doi.org/10.1056/NEJMoa041031 (2004).
Tonelli, M. et al. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: A population-level cohort study. Lancet 380, 807–814. https://doi.org/10.1016/s0140-6736(12)60572-8 (2012).
Vashistha, V., Lee, M., Wu, Y. L., Kaur, S. & Ovbiagele, B. Low glomerular filtration rate and risk of myocardial infarction: A systematic review and meta-analysis. Int. J. Cardiol. 223, 401–409. https://doi.org/10.1016/j.ijcard.2016.07.175 (2016).
Nichols, G. A., Amitay, E. L., Chatterjee, S. & Steubl, D. The bidirectional association of chronic kidney disease, type 2 diabetes, atherosclerotic cardiovascular disease, and heart failure: The cardio-renal-metabolic syndrome. Metab. Syndrome Relat. Disord. 21, 261–266. https://doi.org/10.1089/met.2023.0006 (2023).
Li, J. F. et al. Association between dosing of spironolactone and outcomes in heart failure with preserved ejection fraction patients combined with chronic kidney disease: Balance of efficacy and risk. Front. Pharmacol. 14, 1084442. https://doi.org/10.3389/fphar.2023.1084442 (2023).
Scheven, L. et al. High-sensitive troponin T and N-terminal pro-B type natriuretic peptide are associated with cardiovascular events despite the cross-sectional association with albuminuria and glomerular filtration rate. Eur. Heart J. 33, 2272–2281. https://doi.org/10.1093/eurheartj/ehs163 (2012).
Elewa, U. et al. Cardiovascular risk biomarkers in CKD: The inflammation link and the road less traveled. Int. Urol. Nephrol. 44, 1731–1744. https://doi.org/10.1007/s11255-012-0271-4 (2012).
Lidgard, B. et al. Cardiac biomarkers and risk of atherosclerotic cardiovascular disease in patients with CKD. Kidney 3, 859–871. https://doi.org/10.34067/kid.0006222021 (2022).
Simonet, W. S. et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 89, 309–319. https://doi.org/10.1016/s0092-8674(00)80209-3 (1997).
Yun, T. J. et al. OPG/FDCR-1, a TNF receptor family member, is expressed in lymphoid cells and is up-regulated by ligating CD40. J. Immunol. 161, 6113–6121 (1998).
Takahashi, N., Udagawa, N. & Suda, T. A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem. Biophys. Res. Commun. 256, 449–455. https://doi.org/10.1006/bbrc.1999.0252 (1999).
Browner, W. S., Lui, L. Y. & Cummings, S. R. Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J. Clin. Endocrinol. Metab. 86, 631–637. https://doi.org/10.1210/jcem.86.2.7192 (2001).
Jono, S. et al. Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation 106, 1192–1194. https://doi.org/10.1161/01.cir.0000031524.49139.29 (2002).
Jono, S. et al. Serum osteoprotegerin levels and long-term prognosis in subjects with stable coronary artery disease. J. Thromb. Haemost. 8, 1170–1175. https://doi.org/10.1111/j.1538-7836.2010.03833.x (2010).
Ma, T. et al. Plasma osteoprotegerin predicts adverse cardiovascular events in stable coronary artery disease: The PEACE trial. Front. Cardiovasc. Med. 10, 1178153. https://doi.org/10.3389/fcvm.2023.1178153 (2023).
Samadi, S. et al. Prognostic role of osteoprotegerin and risk of coronary artery calcification: A systematic review and meta-analysis. Biomark. Med. 17, 171–180. https://doi.org/10.2217/bmm-2022-0621 (2023).
Vazirian, F. et al. Correlation between osteoprotegerin and coronary artery calcification in diabetic subjects: A systematic review of observational studies. BMC Cardiovasc. Disord. 23, 96. https://doi.org/10.1186/s12872-023-03123-z (2023).
Ueland, T. et al. Osteoprotegerin predicts progression of chronic heart failure: Results from CORONA. Circ. Heart Fail. 4, 145–152. https://doi.org/10.1161/circheartfailure.110.957332 (2011).
Altinova, A. E. et al. Relationship between serum osteoprotegerin, glycemic control, renal function and markers of atherosclerosis in type 2 diabetes. Scand. J. Clin. Lab. Investig. 71, 340–343. https://doi.org/10.3109/00365513.2011.570868 (2011).
Spartalis, M. et al. Serum OPG and RANKL levels as risk factors for the development of cardiovascular calcifications in end-stage renal disease patients in hemodialysis. Life https://doi.org/10.3390/life13020454 (2023).
Romejko, K., Rymarz, A., Szamotulska, K., Bartoszewicz, Z. & Niemczyk, S. Serum osteoprotegerin is an independent marker of left ventricular hypertrophy, systolic and diastolic dysfunction of the left ventricle and the presence of pericardial fluid in chronic kidney disease patients. Nutrients https://doi.org/10.3390/nu14142893 (2022).
Kuźniewski, M. et al. Osteoprotegerin and osteoprotegerin/TRAIL ratio are associated with cardiovascular dysfunction and mortality among patients with renal failure. Adv. Med. Sci. 61, 269–275. https://doi.org/10.1016/j.advms.2016.03.003 (2016).
Marques, G. L. et al. Osteoprotegerin is a marker of cardiovascular mortality in patients with chronic kidney disease stages 3–5. Sci. Rep. 11, 2473. https://doi.org/10.1038/s41598-021-82072-z (2021).
Kamińska, J. et al. Circulating osteoprotegerin in chronic kidney disease and all-cause mortality. Int. J. Gen. Med. 14, 2413–2420. https://doi.org/10.2147/ijgm.S302251 (2021).
Scialla, J. J. et al. Biomarkers of vascular calcification and mortality in patients with ESRD. Clin. J. Am. Soc. Nephrol. 9, 745–755. https://doi.org/10.2215/CJN.05450513 (2014).
Huang, Q. X. et al. Elevated osteoprotegerin concentration predicts increased risk of cardiovascular mortality in patients with chronic kidney disease: A systematic review and meta-analysis. Kidney Blood Press. Res. 45, 565–575. https://doi.org/10.1159/000508978 (2020).
Panizo, S. et al. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ. Res. 104, 1041–1048. https://doi.org/10.1161/circresaha.108.189001 (2009).
Bennett, B. J. et al. Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE-/- mice. Arterioscler. Thromb. Vasc. Biol. 26, 2117–2124. https://doi.org/10.1161/01.ATV.0000236428.91125.e6 (2006).
Callegari, A., Coons, M. L., Ricks, J. L., Rosenfeld, M. E. & Scatena, M. Increased calcification in osteoprotegerin-deficient smooth muscle cells: Dependence on receptor activator of NF-κB ligand and interleukin 6. J. Vasc. Res. 51, 118–131. https://doi.org/10.1159/000358920 (2014).
Alves-Lopes, R. et al. Osteoprotegerin regulates vascular function through syndecan-1 and NADPH oxidase-derived reactive oxygen species. Clin. Sci. 135, 2429–2444. https://doi.org/10.1042/cs20210643 (2021).
Mangan, S. H., Van Campenhout, A., Rush, C. & Golledge, J. Osteoprotegerin upregulates endothelial cell adhesion molecule response to tumor necrosis factor-alpha associated with induction of angiopoietin-2. Cardiovasc. Res. 76, 494–505. https://doi.org/10.1016/j.cardiores.2007.07.017 (2007).
Schefold, J. C., Filippatos, G., Hasenfuss, G., Anker, S. D. & von Haehling, S. Heart failure and kidney dysfunction: Epidemiology, mechanisms and management. Nat. Revi. Nephrol. 12, 610–623. https://doi.org/10.1038/nrneph.2016.113 (2016).
Dutka, M. et al. Osteoprotegerin and RANKL-RANK-OPG-TRAIL signalling axis in heart failure and other cardiovascular diseases. Heart Fail. Rev. 27, 1395–1411. https://doi.org/10.1007/s10741-021-10153-2 (2022).
Chirinos, J. A. et al. Multiple plasma biomarkers for risk stratification in patients with heart failure and preserved ejection fraction. J. Am. Coll. Cardiol. 75, 1281–1295. https://doi.org/10.1016/j.jacc.2019.12.069 (2020).
di Giuseppe, R. et al. Plasma osteoprotegerin, its correlates, and risk of heart failure: A prospective cohort study. Eur. J. Epidemiol. 32, 113–123. https://doi.org/10.1007/s10654-016-0172-4 (2017).
Oh, K. H. et al. KNOW-CKD (KoreaN cohort study for outcome in patients with chronic kidney disease): Design and methods. BMC Nephrol, 15, 80. https://doi.org/10.1186/1471-2369-15-80 (2014).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006 (2009).
Lang, R. M. et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 18, 1440–1463. https://doi.org/10.1016/j.echo.2005.10.005 (2005).
Rochette, L. et al. The role of osteoprotegerin in the crosstalk between vessels and bone: Its potential utility as a marker of cardiometabolic diseases. Pharmacol. Ther. 182, 115–132. https://doi.org/10.1016/j.pharmthera.2017.08.015 (2018).
Chae, S. Y. et al. The correlation of serum osteoprotegerin with non-traditional cardiovascular risk factors and arterial stiffness in patients with pre-dialysis chronic kidney disease: Results from the KNOW-CKD study. J. Korean Med. Sci. 33, e322. https://doi.org/10.3346/jkms.2018.33.e322 (2018).
Acknowledgements
This work was supported by the Research Program funded by the Korea Disease Control and Prevention Agency (2011E3300300, 2012E3301100, 2013E3301600, 2013E3301601, 2013E3301602, 2016E3300200, 2016E3300201, 2016E3300202, 2019E320100, 2019E320101, 2019E320102, and 2022-11-007) and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2023-00217317 and RS-2023-00278258).
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualization: S.H.S. Methodology: S.H.S., T.R.O., H.S.C., C.S.K. Software: S.H.S., E.H.B., S.K.M., K.H.O. Validation: S.H.S., K.B.L., J.C.J., J.Y.J. Formal analysis: S.H.S., T.R.O., J.Y.J. Investigation: S.H.S., H.S.C., J.C.J. Data curation: S.H.S., C.S.K. Original draft preparation: S.H.S. Review and editing: S.H.S., T.R.O., H.S.C., C.S.K., E.H.B., S.K.M., K.H.O., K.B.L., J.C.J., J.Y.J., S.W.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suh, S.H., Oh, T.R., Choi, H.S. et al. Circulating osteoprotegerin levels and cardiovascular outcomes in patients with pre-dialysis chronic kidney disease: results from the KNOW-CKD study. Sci Rep 14, 4136 (2024). https://doi.org/10.1038/s41598-024-54335-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54335-y
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.