Abstract
Coronary artery calcification (CAC) is associated with cardiovascular disease (CVD). CAC might contain calcium oxalate, and a high serum oxalate (SOx) concentration is associated with cardiovascular mortality in dialysis patients. We assessed the associations between SOx and CAC or CVD events in Japanese hemodialysis patients. This cross-sectional and retrospective cohort study was done in 2011. Seventy-seven hemodialysis patients’ Agatston CAC score was measured, and serum samples were collected. SOx concentrations were measured in 2021 by using frozen samples. Also, new-onset CVD events in 2011–2021 were retrospectively recorded. The association between SOx concentration and CAC score ≥ 1000, and new-onset CVD events were examined. Median SOx concentration and CAC score were 266.9 (229.5–318.5) µmol/L and 912.5 (123.7–2944), respectively. CAC score ≥ 1000 was associated with SOx [adjusted odds ratio (OR) 1.01, 95% confidence interval (CI), 1.00–1.02]. The number of new-onset CVD events was significantly higher in patients with SOx ≥ median value [hazard ratio (HR) 2.71, 95% CI 1.26–6.16]. By Cox proportional hazard models, new-onset CVD events was associated with SOx ≥ median value (adjusted HR 2.10, 95% CI 0.90–4.91). SOx was associated with CAC score ≥ 1000 and new-onset CVD events in Japanese hemodialysis patients.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) is a major complication of end-stage renal disease (ESRD)1. Vascular calcification, especially coronary artery calcification (CAC), is associated with CVD in ESRD patients2,3. Especially, “very high” CAC individuals, defined as Agatston CAC score4 ≥ 1000, are increasingly recognized as higher risk for CVD events and mortality5,6,7. A large body of evidence suggests that dysregulation of calcium and phosphate homeostasis, which are factors related to mineral and bone disorder in chronic kidney disease patients, has direct effects on vascular smooth muscle cells and promotes vascular calcification8. Indeed, vascular calcification in ESRD patients is formed from hydroxyapatite and calcium phosphate, both of which contain phosphate9,10. Phosphate binders and other treatments are available for the control of mineral and bone disorders11; however, even after these treatments, the prevalence of vascular calcification is still higher in ESRD patients than in the population with normal kidney function.
Studies in non-ESRD patients12,13 and ESRD patients14 indicate that CAC contains calcium oxalate crystals. Oxalate is an organic acid abundant in plants such as spinach, where it acts as a controller of calcium concentration15. However, in humans, it is a waste product16. Urine is the major excretion pathway of oxalate17, meaning that patients with decreased kidney function also show elevated serum oxalate (SOx) concentrations, which can be 20–100 times normal in ESRD patients18. SOx is a small molecule, and its serum concentration can be reduced by approximately 90% per hemodialysis session, although it can easily rebound to its pre-dialysis level; for example, at only 2 h after hemodialysis treatment, SOx can already be back at its mid-dialysis level19. Excess SOx combines with calcium to form calcium oxalate crystals that are deposited in various tissues; for example, myocardium, renal tubules, and interstitium20,21. A recent study has reported a relationship between high SOx concentration and CVD mortality, especially in dialysis patients with high SOx concentrations22. This suggests that controlling SOx could be a novel strategy to reduce the risk of CVD in ESRD patients. However, the mechanism of how oxalate affects CVD is currently unknown, limiting the rational development of an effective therapeutic approach.
Here, we conducted analyses to examine the association between SOx and CAC and the association between SOx and CVD events in Japanese hemodialysis patients. First, we conducted a cross-sectional analysis to understand more about the relationship between high SOx concentration and CAC ≥ 10005,6,7. Then, we conducted a retrospective cohort study to understand more about the relationship between SOx and CVD events.
Results
Baseline patient characteristics
Of the 77 patients that underwent the voluntary atherosclerosis checkup, 17 had normal SOx concentrations and were excluded from the study. There were no missing values except for ankle-brachial index of two cases. Of the remaining 60 patients, 41 (68.3%) were male, mean age was 63.1 ± 11.9 years, and median dialysis duration was 87.6 (44.1–152.2) months. Twenty-one patients (35%) had diabetes mellitus and 10 (16.7%) had CVD. Median SOx, Agatston CAC score, and major artery calcification volume were 266.9 (229.5–318.5) µmol/L, 912.5 (123.7–2943.8), and 7.0 (3.0–18.4) cm3, respectively; these data were right skewed. When the subjects were divided into two groups according to CAC score ≥ 1000, baseline CVD, serum phosphate, low-density lipoprotein (LDL) cholesterol, and major artery calcification volume were significantly higher in the CAC ≥ 1000 group. Table 1 provides the baseline patient characteristics.
Cross-sectional analysis of the association between SOx and vascular calcification
To examine the association between SOx and vascular calcification, we performed a logistic regression analysis. The patients were stratified into those with CAC score < 1000 and those with CAC score ≥ 1000. All of the patients with CVD (n = 10) were in the CAC score ≥ 1000 group; those patients were excluded from this analysis, leaving 50 patients in the analysis group.
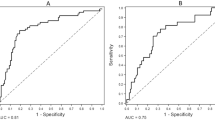
In a univariate analysis examining associations with CAC score ≥ 1000, variables with P value < 0.2 were SOx concentration, male, body mass index (BMI), serum phosphate, intact parathyroid hormone, triglyceride, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, uric acid, and blood urea nitrogen (Table 2). In a multivariate analysis using these selected variables, SOx [odds ratio (OR) 1.01, 95% confidence interval (CI) 1.00–1.02, P = 0.168], male (OR 6.90, 95% CI 1.10–77.5, P = 0.065), serum phosphate (OR 2.82, 95% CI 1.17–8.94, P = 0.033), intact parathyroid hormone (OR 1.01, 95% CI 1.00–1.01, P = 0.058), and LDL cholesterol (OR 1.04, 95% CI 1.00–1.10, P = 0.055) were selected as factors associated with CAC score ≥ 1000 (Table 2). In a risk prediction model predicting CAC score ≥ 1000, we used the five selected factors (i.e., SOx, male, serum phosphate, intact parathyroid hormone, and LDL cholesterol); the area under the receiver operating characteristic (ROC) curve was 0.87 (95% CI 0.76–0.98), and sensitivity and specificity were 81.8% and 76.5%, respectively (Fig. 1).
Receiving operating curve for predicting Agatston coronary artery calcification score ≥ 1000. In a risk prediction model for predicting CAC score ≥ 1000, we used five factors obtained from an earlier variable selection (i.e., SOx, male, serum phosphate, intact parathyroid hormone, and LDL cholesterol). The area under the receiver operating characteristic curve was 0.87 (95%CI 0.76–0.98), and sensitivity and specificity were 81.8% and 76.5%, respectively. AUC area under the curve, CAC coronary artery calcification, CI confidence interval, LDL low-density lipoprotein, SOx serum oxalate concentration.
In a univariate analysis examining associations with major artery calcification volume, variables with a P value < 0.2 were SOx, age, male, albumin-adjusted calcium, HDL cholesterol, beta-2 microglobulin, serum albumin, and calcium carbonate intake (Table 3). In a multivariate analysis using these selected variables after variable selection, SOx (β = 0.03, 95% CI 0.00–0.06, P = 0.084), age (β = 0.26, 95% CI 0.05–0.47, P = 0.018), and beta-2 microglobulin (β = 0.55, 95% CI 0.00–1.09, P = 0.048) were selected as factors associated with major artery calcification volume (Table 3).
Retrospective cohort analysis of the relationship between SOx and new-onset CVD events
During the 10-year observation period, seven patients (11.7%) were censored due to transfer to another dialysis center, three patients (5.0%) were censored due to kidney transplantation, and nine patients (15.0%) died before experiencing a CVD event. A total of 29 (48.3%) patients experienced new-onset CVD events. We divided the subjects into two groups using the median SOx value of 266.9 µmol/L. New-onset CVD events occurred in 19/30 (63.3%) patients with SOx greater than or equal to the median value, whereas new-onset CVD events occurred in only 10/30 (33.3%) patients with SOx lower than the median value; the risk of new-onset CVD events was greater in the patients with SOx greater than or equal to the median value than in those with SOx lower than the median value [hazard ratio (HR) 2.71, 95% CI 1.26–6.16, P = 0.008; Fig. 2a]. By restricting the analysis to patients with CAC score < 1000 and without history of CVD (n = 33), CVD events occurred in 10/13 (76.9%) patients with SOx greater than or equal to the median value and in 7/20 (35.0%) patients with SOx lower than the median value, respectively. The number of events was significantly higher in the patients with SOx greater than or equal to the median value than in the patients with lower than the median value (HR 2.94, 95% CI 1.10–7.85, P = 0.020; Fig. 2b).
Kaplan–Meier analysis of the relationship between SOx and new-onset cardiovascular disease events. Subjects were divided into two groups by median SOx concentration (SOx < 266.9 µmol/L and SOx ≥ 266.9 µmol/L). New-onset cardiovascular events occurred in 29/60 (48.3%) patients: in 19/30 (63.3%) patients with SOx greater than or equal to the median value and in 10/30 (33.3%) patients with SOx less than the median value. The number of events was significantly higher in the patients with SOx ≥ 266.9 µmol/L than in those with SOx < 266.9 µmol/L (a), even after limiting the analysis only to patients with CAC score < 1000 and without history of cardiovascular disease (n = 33, b). CAC coronary artery calcification, CI confidence interval, HR hazard ratio, SOx serum oxalate.
We performed Cox proportional hazard analyses to examine the relationship between SOx and new-onset CVD events. In a univariate analysis, variables with a P value < 0.2 were SOx greater than or equal to the median value, age, male, history of CVD, alkaline phosphatase, HDL cholesterol, and hemoglobin (Table 4). In a multivariable analysis using these variables after variable selection, SOx (HR 2.10, 95% CI 0.90–4.91, P = 0.086), history of CVD (HR 3.84, 95% CI 1.44–10.2, P = 0.007), and HDL cholesterol (HR 0.97, 95% CI 0.94–1.00, P = 0.062) were selected as factors associated with new-onset CVD events (Table 4).
Discussion
In this study, higher SOx concentration was associated with vascular calcification in both the coronary artery and other major arteries, and with new-onset CVD events in Japanese dialysis patients. Several previous studies have concluded that CAC contains calcium oxalate12,13,14; however, no studies have examined SOx concentration and vascular calcification in dialysis patients. This was the first report revealed relationship between SOx and vascular calcification in dialysis patients as far as we know. Previous studies20,21 have revealed that excess SOx combines with calcium to form calcium oxalate crystals that are deposited in various tissues; for example, myocardium, renal tubules, and interstitium. In uremic atherosclerosis mice, excess SOx has been shown to alter intracellular calcium to increase in endothelial cells, promote oxidative stress, severely inhibit proliferation and migration of human endothelial cells, and induce endothelial injury23. Excess SOx is also correlated with aortic calcification containing a major oxalate component in the aortic wall in uremic mice24. Although the mechanism underlying how excess oxalate promotes vascular calcification is unknown, these previous findings support our present results.
In this retrospective study, we found that SOx concentration was associated with CVD events in dialysis patients. Recently, Pfau et al.22 reported that SOx concentration was associated with CVD mortality in 1108 dialysis patients over a 2.5-year observation period. Our findings are similar to those of Pfau et al.; however, in our study we adjusted for factors for mineral and bone disorder factors as covariates, whereas Pfau et al. did not. We think it is important to adjust for mineral and bone disorder factors because they are currently considered some of the most powerful factors that promote vascular calcification in ESRD patients1. Our present findings are the first to reveal an association between SOx and CAC or vascular calcification. These findings are supported by our analysis selecting factors that are already known risk factors for vascular calcification or CVD events, for example, mineral and bone disorder factors or LDL cholesterol. In addition, our study also revealed a relationship between SOx and CVD events, which is consistent with the findings from other study22.
Although we found that higher SOx concentration was associated with vascular calcification and CVD events, it is important to note that many factors are involved in these outcomes. In other words, traditional factors, for example those related to mineral and bone disorder8 or elevated LDL-cholesterol25, are also important to prevent vascular calcification or CVD events in dialysis patients. Some gut microbes, for example, Oxalobacter formigenes, Lactobacillus, and Bifidobacterium produce specific enzymes that help in the degradation of oxalate salts; humans produce no enzymes for oxalate biotransformation26. The use of probiotics might be a logical treatment for lowering SOx. Indeed, Oxalobacter formigenes intervention lowered SOx concentrations in uremic atherosclerosis mice, however, it did not significantly improve vascular calcification24. This result indicated that vascular calcification in ESRD patients is the result of many interrelated factors, not only oxalate, multiple target therapies will likely be needed for optimal prevention for vascular calcification or CVD events.
There are several limitations to the present study. The first is that we measured SOx concentrations retrospectively by using serum that had been in storage for around 10 years. As ascorbate converts nonenzymatically to oxalate at pH > 4, it is recommended that samples are immediately cooled and acidified to lower pH to halt this biochemical process27,28. However, a recent study has revealed that immediate freezing without acidic conditions and maintaining the samples at − 80 °C has been shown provide accurate and stable SOx assessments for up to 21 months28. The serum samples used in the present study were frozen immediately upon collected and stored at − 80 °C until analysis with strict thermal management. The second limitation is that this was a retrospective study in which confounding factors were not fully considered. For example, smoking and C-reactive protein are also associated with CVD events in ESRD patients29, but we were unable to add them as covariates because many of the patient records were missing these values. Moreover, the study’s sample size was relatively small. The final limitation is that this study might contain sampling bias. The study participants voluntarily underwent the initial atherosclerosis checkup, meaning they may comprise a group of particularly health-conscious individuals. Although, the study population was similar to the whole Japanese dialysis population reported in 201230 in terms of age, gender, dialysis duration, and diabetes mellitus, the estimated 5-year mortality of the present study population was 82.3% (95%CI, 72.9–92.9%), whereas that of the whole Japanese dialysis population was 60.0%31. It should be noted that the prognosis of even health-conscious ESRD patients is poorer than that of the general population, meaning that strategies to lower SOx concentrations may bring about desirable outcomes even in well-managed ESRD patients.
High SOx concentration was associated with Agatston CAC score ≥ 1000, major artery calcification volume, and new-onset CVD events in Japanese ESRD patients. A novel strategy for SOx control may bring a better prognosis to ESRD patients.
Methods
Study design and population
A total of 540 ESRD patients undergoing maintenance hemodialysis at a single hemodialysis center (Heisei Hidaka Clinic, Gunma, Japan) were enrolled in the study (Fig. 3). All subjects were receiving hemodialysis administered in 3.5–4.5 h sessions three times weekly using a polysulfone hollow-fiber dialyzer (APS-SA, Asahi Kasei Medical, Tokyo, Japan or NV-U, Toray Industries, Tokyo, Japan) and a membrane area of 0.8–2.5 m2. Blood and dialysate flows were 180–250 mL/min and 500 mL/min, respectively, with a constant ultrafiltration rate. The dialysate bath comprised 140 mmol/L sodium, 2.0 mmol/L potassium, 2.5 mmol/L calcium, 1.0 mmol/L magnesium, 8.0 mmol/L acetate, 25.0 mmol/L bicarbonate, and 150 mg/dL glucose (Kindaly 3D; Fuso, Osaka, Japan). From April 2011 to March 2012, 77 subjects voluntarily received an atherosclerosis checkup in which the patients were examined by multidetector spiral computed tomography (MDCT), and Agatston CAC score4 and major artery calcification volume were determined by a skilled radiologist; further details are provided in the section titled “MDCT and Measurement of CAC and Major Artery Calcification Volume”. We did not calculate the sample size. We included all the participants who voluntarily received an atherosclerosis checkup from April 2011 to March 2012 with consent (N = 77) to this study. Patients without consent were excluded. All of the study participants provided written informed consent at the time of the atherosclerosis checkup in 2011, and we afforded them the opportunity to opt-out from this secondary analysis in 2021. The data were collected by using the hospital chart from January 2021 to May 2021. The study complies with the Declaration of Helsinki, and the study was approved by the Ethics Committee on Human Research at Heisei Hidaka Clinic (Gunma, Japan; No. 46) and the Ethics Committee on Clinical Research at Teikyo University (Tokyo, Japan; No. 22-058). Confirms that all experiments were performed in accordance with relevant named guidelines and regulations.
Cross-sectional analysis of the association between SOx and vascular calcification
In January 2021, SOx concentration was measured retrospectively by a laboratorian not directly involved in the planning of the study by using frozen serum collected around the atherosclerosis checkup date and stored at − 80 °C. Because our study targeted subjects with higher SOx concentrations, we excluded patients with SOx concentrations in the normal range (< 181 µmol/L) at the time of analysis. Detailed methods of SOx measurement were provided in the “Sample handling and measurement of SOx” section.
To examine the association between SOx and vascular calcification, we conducted a cross-sectional analysis using data collected at the atherosclerosis checkup (age, gender, hemodialysis duration, dialysis prescriptions, past medical history, medications, BMI, ankle-brachial index, CAC score, and major artery calcification volume). Laboratory data were also collected from medical records at the start of the dialysis session with the longest interdialytic interval around the atherosclerosis checkup date. We divided subjects into two groups: those with CAC score < 1000 and those with CAC score ≥ 1000 to define “very high” CAC5,6,7 group. The predictor was SOx, and outcome was Agatston CAC score ≥ 1000. A logistic regression analysis was performed for the association between SOx and CAC score ≥ 1000. We further analyzed the relationship between SOx and major artery calcification volume in addition to the relationship between SOx and CAC. Potential confounders8,25,29,32,33,34,35,36 were age, gender, hemodialysis duration, history of CVD, diabetes mellitus, BMI, ankle-brachial index, albumin-adjusted calcium, serum phosphate, intact parathyroid hormone, alkaline phosphatase, serum magnesium, triglyceride, LDL cholesterol, HDL cholesterol, uric acid, beta-2 microglobulin, blood urea nitrogen, hemoglobin, serum albumin, vitamin D medication, calcium carbonate intake, and statin intake.
Retrospective cohort analysis of the relationship between SOx and new-onset CVD events
To examine the relationship between SOx and new-onset CVD events, we conducted a retrospective cohort analysis. New-onset CVD events during the 10-year period from serum sample collection date to observation end date of May 15th, 2021 were recorded by medical staff blinded to the SOx results to avoid information bias. “New-onset CVD event” was defined as any admission due to non-fatal myocardial infarction, coronary artery disease, or heart failure during the observation period. We divided subjects into two groups by SOx median and compared new-onset CVD events between two groups. The explanatory variable was SOx lower or grater than median, and outcome was new-onset CVD event. Potential confounders were the same as listed on the “Cross-sectional Analysis of the Association Between SOx and Vascular Calcification” section.
Sample handling and measurement of SOx
Blood samples were collected at the start of the dialysis session with the longest interdialytic interval around the atherosclerosis checkup date. The collected samples were mixed immediately with 5 mg edetic acid, centrifuged at 3000 rpm for 5 min to separate the serum, and stored immediately at − 80 °C with strict thermal management until analysis. SOx concentration was measured in January 2021 by using an Oxalate Assay Kit (Colorimetric) (ab196990; Abcam, Cambridge, MA, USA). With this assay kit, the normal range of human SOx is < 181 µmol/L. All serum samples were analyzed in duplicate.
MDCT and measurement of CAC and major artery calcification volume
Multi-slice computed tomography (CT) scans were performed with an Aquilion TSX-101A (Toshiba, Tokyo, Japan). Slices of 1.25-mm thickness were acquired eight at a time under the following conditions: 120–140 kVp, 85–150 mA, 500 ms exposure, and 0.5 s gantry rotation time. The entire heart was covered in a single breath-hold (20–30 s). The CT images were transferred to a Ziosoft M900 QUADRA workstation (AMIN, Inc, Tokyo, Japan) and CAC score according to the algorithm suggested by Agatston et al.4 (area × cofactor; 1: 130–199H; 2: 200–299H; 3: 300–399H; 4: > 400H), along with the volume (area × slice increment), mass (area × slice increment × mean CT density/250), and density (mass/volume) of the CAC were determined by a single radiologist. Using the electrocardiogram tracing, the workstation software automatically selected a reduced set of diastolic images from each cardiac cycle. All pixels with density > 130H were automatically highlighted on the images. The radiologist assigned one of four locations to each calcified plaque: left main, left anterior descending, circumflex, or right coronary artery. The score for each plaque equaled the plaque area × weighting factor × increment/slice width. The score for the entire specimen equaled the sum of the scores for each plaque. The mean intra-reader variability for CAC was 1.8%.
Quantification of major artery calcification volume was performed by three-dimensional calcified lesion reconstitution from multiple slices of the aorta from the top of the arch to the abdominal artery just before the bifurcation of the iliac artery along the longitudinal axis by using the Ziosoft M900 QUADRA workstation.
Statistical analysis
Continuous variables are reported as mean ± standard deviation for normally distributed data, or median (inter-quartile range) for non-normally distributed data. Discrete variables are expressed as numeral (percentage). Differences between subjects with CAC score ≥ 1000 and those with CAC score < 1000 were tested for statistical significance by using Welch’s t-test for normally distributed data, the Wilcoxon rank sum test for non-normally distributed data, and Fisher’s exact test for discrete variables. A two-tailed P value of < 0.05 was considered to indicate statistical significance. In univariate analyses, we did available-case analyses, whereas in multivariable analyses, we did complete-case analyses. No imputation methods for sensitivity analysis were used in our study.
In the analysis of an association between SOx concentration and CAC score, we divided subjects into two groups: those with CAC score < 1000 and those with CAC score ≥ 1000. This cutoff value was selected from previous studies indicating that CAC score ≥ 1000 is associated with cumulative incidence of cardiovascular events5,6,7. A logistic regression analysis was performed for the association between SOx and CAC score ≥ 1000, and a linear regression analysis was performed for the association between SOx and major artery calcification volume. Moreover, we performed risk prediction modeling to predict CAC score ≥ 1000 for validation. We present a ROC curve for predicting CAC score ≥ 1000 by using variables selected from multivariable logistic regression with variable selection.
In the analysis of an association between SOx and new-onset CVD events, we divided subjects into two groups by SOx median and performed a Kaplan–Meier analysis and log-rank test to compare the survival functions of the two groups and to determine whether SOx is associated with CVD events. We repeated the analysis, restricting it to patients with CAC score < 1000 and without history of CVD. We also conducted a Cox proportional hazard analysis using the SOx cut-off value to determine which factors were associated with CVD events.
In each analysis, the explanatory variable was SOx and the covariates were factors that are reported to be associated with vascular calcification8,25,32,33 or CVD29,34,35,36; specifically, age, gender, hemodialysis duration, history of CVD, diabetes mellitus, BMI, ankle-brachial index, albumin-adjusted calcium, serum phosphate, intact parathyroid hormone, alkaline phosphatase, serum magnesium, triglyceride, LDL cholesterol, HDL cholesterol, uric acid, beta-2 microglobulin, blood urea nitrogen, hemoglobin, and serum albumin, vitamin D medication, calcium carbonate intake, and statin intake were used as covariates. A univariable analysis was performed first, and factors with a two-tailed P value < 0.2 were used for a multivariable analysis. For variable selection, we used Akaike’s information criteria with stepwise backward elimination. All statistical analyses were performed using R software, version 4.1.3.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Cozzolino, M. et al. Cardiovascular disease in dialysis patients. Nephrol. Dial. Transplant 33, iii28–iii34. https://doi.org/10.1093/ndt/gfy174 (2018).
Raggi, P. et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease?. J. Am. Coll. Cardiol. 39, 695–701. https://doi.org/10.1016/s0735-1097(01)01781-8 (2002).
Wang, X. R., Zhang, J. J., Xu, X. X. & Wu, Y. G. Prevalence of coronary artery calcification and its association with mortality, cardiovascular events in patients with chronic kidney disease: A systematic review and meta-analysis. Renal Fail. 41, 244–256. https://doi.org/10.1080/0886022X.2019.1595646 (2019).
Agatston, A. S. et al. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 15, 827–832. https://doi.org/10.1016/0735-1097(90)90282-t (1990).
Yamamoto, H. et al. Coronary calcium score as a predictor for coronary artery disease and cardiac events in Japanese high-risk patients. Circ. J. 75, 2424–2431. https://doi.org/10.1253/circj.cj-11-0087 (2011).
Peng, A. W. et al. Very high coronary artery calcium (≥1000) and association with cardiovascular disease events, non-cardiovascular disease outcomes, and mortality: results from MESA. Circulation 143, 1571–1583. https://doi.org/10.1161/CIRCULATIONAHA.120.050545 (2021).
Obisesan, O. H., Osei, A. D., Uddin, S. M. I., Dzaye, O. & Blaha, M. J. An update on coronary artery calcium interpretation at chest and cardiac CT. Radiol. Cardiothorac. Imaging 3, e200484. https://doi.org/10.1148/ryct.2021200484 (2021).
Shanahan, C. M., Crouthamel, M. H., Kapustin, A. & Giachelli, C. M. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ. Res. 109, 697–711. https://doi.org/10.1161/CIRCRESAHA.110.234914 (2011).
Contiguglia, S. R., Alfrey, A. C., Miller, N. L., Runnells, D. E. & Le Geros, R. Z. Nature of soft tissue calcification in uremia. Kidney Int. 4, 229–235. https://doi.org/10.1038/ki.1973.104 (1973).
Schwarz, U. et al. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol. Dial. Transplant 15, 218–223. https://doi.org/10.1093/ndt/15.2.218 (2000).
Wang, A. Y. et al. Kidney disease: Improving global outcomes (KDIGO) chronic kidney disease-mineral and bone disorder (CKD-MBD) guideline update implementation: Asia summit conference report. Kidney Int. Rep. 4, 1523–1537. https://doi.org/10.1016/j.ekir.2019.09.007 (2017).
Fishbein, G. A., Micheletti, R. G., Currier, J. S., Singer, E. & Fishbein, M. C. Atherosclerotic oxalosis in coronary arteries. Cardiovasc. Pathol. 17, 117–123. https://doi.org/10.1016/j.carpath.2007.07.002 (2018).
Matsui, K. et al. Analysis of coronary arterial calcification components with coronary CT angiography using single-source dual-energy CT with fast tube voltage switching. Int. J. Cardiovasc. Imaging 31, 639–647. https://doi.org/10.1007/s10554-014-0574-x (2015).
Nishizawa, Y. et al. Compositional analysis of coronary artery calcification in dialysis patients in vivo by dual-energy computed tomography angiography. Ther. Apher. Dial. 22, 365–370. https://doi.org/10.1111/1744-9987.12662 (2018).
Khan, S.R. Referencing. In Calcium Oxalate in Biological Systems (ed. Khan, S.R.) (CRC Press, 1995).
Hagler, L. & Herman, R. H. Oxalate metabolism. Am. J. Clin. Nutr. 26, 758–765. https://doi.org/10.1093/ajcn/26.6.758 (1973).
Elder, T. D. & Wyngaarden, J. B. The biosynthesis and turnover of oxalate in normal and hyperoxaluric subjects. J. Clin. Invest. 39, 1337–1344. https://doi.org/10.1172/JCI104151 (1960).
Prenen, J. A., Dorhout Mees, E. J. & Boer, P. Plasma oxalate concentration and oxalate distribution volume in patients with normal and decreased renal function. Eur. J. Clin. Invest. 15, 45–49. https://doi.org/10.1111/j.1365-2362.1985.tb00142.x (1985).
Ermer, T. et al. Impact of regular or extended hemodialysis and hemodialfiltration on plasma oxalate concentrations in patients with end-stage renal disease. Kidney Int. Rep. 2, 1050–1058. https://doi.org/10.1016/j.ekir.2017.06.002 (2017).
Salyer, W. R. & Keren, D. Oxalosis as a complication of chronic renal failure. Kidney Int. 4, 61–66. https://doi.org/10.1038/ki.1973.80 (1973).
Ogawa, Y. et al. Calcium oxalate saturation in dialysis patients with and without primary hyperoxaluria. Urol. Res. 34, 12–16. https://doi.org/10.1007/s00240-005-0004-6 (2006).
Pfau, A. et al. High oxalate concentrations correlate with increased risk for sudden cardiac death in dialysis patients. J. Am. Soc. Nephrol. 32, 2375–2385. https://doi.org/10.1681/ASN.2020121793 (2021).
Recht, P. A. et al. Oxalic acid alters intracellular calcium in endothelial cells. Atherosclerosis 173, 321–328. https://doi.org/10.1016/j.atherosclerosis.2003.11.023 (2004).
Sun, K. et al. Hyperoxalemia leads to oxidative stress in endothelial cells and mice with chronic kidney disease. Kidney Blood Press. Res. 46, 377–386. https://doi.org/10.1159/000516013 (2021).
McCullough, P. A., Sandberg, K. R., Dumler, F. & Yanez, J. E. Determinants of coronary vascular calcification in patients with chronic kidney disease and end-stage renal disease: A systematic review. J. Nephrol. 17, 205–215 (2004).
Sadaf, H., Raza, S. I. & Hassan, S. W. Role of gut microbiota against calcium oxalate. Microb. Pathog. 109, 287–291. https://doi.org/10.1016/j.micpath.2017.06.009 (2017).
Ladwig, P. M., Liedtke, R. R., Larson, T. S. & Lieske, J. C. Sensitive spectrophotometric assay for plasma oxalate. Clin. Chem. 51, 2377–2380. https://doi.org/10.1373/clinchem.2005.054353 (2005).
Pfau, A. et al. Assessment of plasma oxalate concentration in patients with CKD. Kidney Int. Rep. 5, 2013–2020. https://doi.org/10.1016/j.ekir.2020.08.029 (2020).
Ma, L. & Zhao, S. Risk factors for mortality in patients undergoing hemodialysis: A systematic review and meta-analysis. Int. J. Cardiol. 238, 151–158. https://doi.org/10.1016/j.ijcard.2017.02.095 (2017).
Nakai, S. et al. An overview of regular dialysis treatment in Japan (as of December 31, 2012). J. Jpn. Soc. Dial. Ther. 47, 1–56. https://doi.org/10.4009/jsdt.47.1.[InJapanese] (2014).
Masakane, I. et al. An overview of regular dialysis treatment in Japan (as of December 31, 2012). J. Jpn. Soc. Dial. Ther. 50, 1–62. https://doi.org/10.4009/jsdt.50.1.[InJapanese] (2017).
Shantouf, R. et al. Association of serum alkaline phosphatase with coronary artery calcification in maintenance hemodialysis patients. Clin. J. Am. Soc. Nephrol. 4, 1106–1114. https://doi.org/10.2215/CJN.06091108 (2009).
Jono, S., Nishizawa, Y., Shioi, A. & Morii, H. 1,25-Dihydroxyvitamin D3 increases in vitro vascular calcification by modulating secretion of endogenous parathyroid hormone-related peptide. Circulation 98, 1302–1306. https://doi.org/10.1161/01.cir.98.13.1302 (1998).
Jamal, S. A. et al. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: An updated systematic review and meta-analysis. Lancet 382, 1268–1277. https://doi.org/10.1016/S0140-6736(13)60897-1 (2013).
Huang, C. Y. et al. Association between hypomagnesemia and mortality among dialysis patients: A systematic review and meta-analysis. PeerJ. 10, e14203. https://doi.org/10.7717/peerj.14203 (2022).
Liabeuf, S. et al. Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int. 82, 1297–1303. https://doi.org/10.1038/ki.2012.301 (2012).
Acknowledgements
The authors thank all participating patients, medical staff of the dialysis center, and the laboratorian at the Heisei-Hidaka Clinic for collecting the medical records.
Author information
Authors and Affiliations
Contributions
Y.N. planed the study; S.M. conducted statistical analyses; M.T., E.H., and K.O. drafted the manuscript; Y.H. and A.H. conducted the study; K.I. collected data; N.N. supervised the study; J.H. and T.O. reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nishizawa, Y., Miyata, S., Tosaka, M. et al. Serum oxalate concentration is associated with coronary artery calcification and cardiovascular events in Japanese dialysis patients. Sci Rep 13, 18558 (2023). https://doi.org/10.1038/s41598-023-45903-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-45903-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.