Abstract
Horseshoe crabs are among the most studied invertebrates due to their unique, innate immune system and biological processes. The metabolomics study was conducted on lipopolysaccharide (LPS)-stimulated and non-stimulated hemocytes isolated from the Malaysian Tachypleus gigas and Carcinoscorpius rotundicauda. LC–TOF–MS, multivariate analyses, principal component analysis (PCA), and partial least squares-discriminant analysis (PLS-DA) were included in this study to profile the metabolites. A total of 37 metabolites were identified to be differentially abundant and were selected based on VIP > 1. However, of the 37 putative metabolites, only 23 were found to be significant with ANOVA at p < 0.05. The metabolites were identified using several databases, and the literature review of the metabolites was reported in the manuscript. Thus, this study has provided further insights into the putative metabolites' presence in the hemocytes of horseshoe crabs that are stimulated and non-stimulated with LPS and their abundance in each species. Several putative metabolites showed they have medicinal values from previous studies.
Similar content being viewed by others
Introduction
Metabolomics is the study of identifying and quantifying small molecules or metabolites in biological systems. Several technologies, such as nuclear magnetic resonance spectroscopy (NMR), gas chromatography-mass spectrometry (GC–MS), and liquid chromatography-mass spectrometry (LC–MS), are used to analyze the metabolites1,2. It is essential in clinical research, disease treatment, drug characterization, animal and plant research, agricultural research, and nutrition1. Metabolomics can be divided into targeted (analysis of known metabolites) and untargeted metabolomics (analysis of unknown metabolites)3. Metabolites are intermediate products of cellular metabolic reactions such as peptides, oligonucleotides, sugars, and amino acids.
Recently, horseshoe crab research has gained popularity among scientists due to its various benefits in the medical field. For example, its blood is widely used as an endotoxin tester in vaccines, drugs, and injectables, as it can clot in the presence of bacteria4. Furthermore, many discoveries in human eye research resulted from studies involving nerve pathways of the horseshoe crab eyes5. Aside from that, perivitelline fluid (PVF) from a fertilized horseshoe crab egg is rich in vital proteins and amino acids essential for embryogenesis4.
Horseshoe crabs belong to the phylum Arthropoda, subphylum Chelicerata, class Merostomata, order Xiphosura, and family Limulidae. Physically, it has a colourless to whitish hemolymph and a brown body consisting of a cephalothorax, abdomen, and swordtail6. The global distribution of horseshoe crabs is believed to be constrained by continental geomorphology, temperature barriers, tidal types, and benthic currents7,8. Limulus polyphemus, Tachypleus tridentatus, T. gigas, and C. rotundicauda are types of horseshoe crab species9,10.
Hemocytes are horseshoe crabs' primary regulators of innate immunity11. They are susceptible to gram-negative bacterial endotoxins, also known as lipopolysaccharides (LPS), in their outer cell wall. The hemocyte secretes transglutaminase (TGase) and several defence molecules, such as coagulation factors, lectins, antimicrobial peptides, and protein substrates, in response to stimulation by LPS12,13. The study of the effect of LPS on hemocytes has been considered one of the most important experimental tools to understand the horseshoe crab immune response. Previous studies by Sarmiento et al.11 and Adebayo et al.14 elucidated hemocytes' transcriptomic and proteomic data stimulated with LPS and non-stimulated hemocytes.
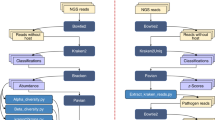
In this study, the metabolomics analysis of two species of horseshoe crab, T. gigas and C. rotundicauda was conducted. The study was conducted to profile the presence and abundance of the putative metabolites of horseshoe crabs with and without stimulation with LPS and variation among the two species. The study also is looking for metabolites that could have promising potential for medical applications. Analyses were conducted on isolated hemocytes stimulated and non-stimulated with lipopolysaccharides (LPS). LC-TOF–MS and several statistical analyses, such as principal component analysis (PCA), partial least squares discriminant analysis (PLS-DA), ANOVA, Tukey test and t-test, were applied in this study.
Materials and methods
Samples preparation, hemocyte culture, and LPS challenge
Three adult horseshoe crabs were collected from the Kuala Kemaman coastal region in Terengganu, Malaysia. Horseshoe crabs were caught during the day and acclimated for 24 h. Each horseshoe crab had 2 mL of hemolymph collected aseptically with pyrogen-free equipment. The hemolymph collection was performed in a biological safety cabinet (ESCO, USA) using patented techniques (MY-155541-A)11. The lipopolysaccharides (LPS) challenge on horseshoe crab hemolymph was carried out according to the protocol recently reported by Samiento et al.11. Briefly, the hemolymph was mixed with 25 ml 3% NaCl and plated into 6-well culture plates, in triplicates. After 15 min incubation at room temperature, 10–13 g/ml of LPS in 2 ml 3% NaCl was added to each well and incubated for 1 h at room temperature. The non-stimulated wells received only 3% NaCl.
Extraction of metabolite
The extraction was optimized based on Muelas et al.15. First, approximately 200 μl of cold extraction solvent [methanol/acetonitrile 50/50 (% v/v)] was added to 600 μl hemocytes stimulated and non-stimulated samples. The samples were mixed vigorously by vortex for approximately 30 s. After vigorously mixed, the samples were incubated at − 20 °C for 20 min. The samples were centrifuged at 13,000 rpm for 5 min at 4 °C. After being centrifuged, the supernatant was transferred and dried using a speed vacuum. The methanol extracts were stored at − 80 °C until the sample analysis.
Analysis of LC-TOF–MS
The mass spectrometry analysis was performed at the Institute of Systems Biology (INBIOSIS), Universiti Kebangsaan Malaysia. Three biological and six technical replicates from each group were analyzed using LC-TOF–MS. The analyses were performed using ultra-high-performance liquid chromatography (UHPLC) with a microTOF Q III mass spectrometer (MS) (Bruker Daltonics, Bremen, Germany) equipped with an electrospray source (ESI) and connected to an Ultimate 3000 UHPLC system (Dionex, Sunnyvale, CA, USA) equipped with an Acclaims Polar Advantage II, 3 × 150 mm, 3-μl particle size C18, reverse-phase column at the flow rate of 0.4 ml/min at 40 °C with a sample injection volume of 3 μl. The running buffers were (A) deionized distilled water with 0.1% formic acid and (B) 100% acetonitrile with a 22-min total run time. The gradient elution was carried out from 5 to 80% buffer B over 22 min as follows: 5% B (0–3 min), 80% B (3–10 min), 80% B (10–15 min), 5% B (15–22 min). MS was performed in ESI positive ionization mode with the capillary voltage at 4,500 V, nebulizer pressure at 1.2 bar, and drying gas at 8 L/min in 200 °C. The scan range was from 100 to 1000 m/z. Data processing was performed using the Data Analysis 4.0 and Profile Analysis (Bruker Daltonics) software. Lastly, the graphical representation was performed on WEGO for data analysis of the sample. The caffeic acid standard was used as a control to ensure data quality by providing the optimum condition of extraction and instrument.
Statistical analysis
Multivariate statistical evaluation of the preprocessed metabolic profiling data was performed with SIMCA-P + (version 12) (Umetrics, Umea, Sweden). Bucketing was generated using Data Analysis 4.0 and Profile Analysis (Bruker Daltonics). The differential metabolites obtained from the multivariate data analysis software were validated using ANOVA with post hoc Tukey's tests and t-tests. All metabolites contributing to group separation were significant at p < 0.05. The Permutation test was performed for validation.
Compound identification
Compound identification of metabolites was performed by comparing the accuracy of the m/z value < 10 ppm and MS/MS spectra with available online databases: Human Metabolome Database (HMDB)16, Metabolite and Chemical Entity Database (METLIN)17, KEGG18 and LIPIDMAPS19 and MassBank. KEGG pathway analysis was used to determine the putative metabolite pathway.
Pathway enrichment analysis
Pathway enrichment analysis was performed by analyzing data on putative metabolites, which were significant with ANOVA using MetaboAnalyst version 5.0.
Results and discussion
Chromatogram and multivariate analysis
Two forms of the horseshoe crab hemocyte metabolites were reported: the non-stimulated and stimulated with LPS. LPS is an essential outer membrane component of gram-negative bacteria, which consists of Lipid A, O-antigen, and hydrophilic core polysaccharide20. It is a primary factor in hemocyte activation and is regarded as one of the most important experimental tools for understanding the horseshoe crab immune response.
The metabolomics studies used a high throughput method (LC–TOF–MS) and multivariate analyses (PCA and PLS-DA). Based on the differences in the peak and area of the chromatogram observed following LC–TOF–MS analysis in Fig. 1, there are differences in the putative metabolite profile in both species stimulated and non-stimulated hemocytes. Multivariate analyses were then conducted to get a general overview and understanding of the spread of variability in the data.
This study used PCA and PLS-DA to analyze the preprocessed LC–TOF–MS datasets. The PCA model reveals the general metabolic information and visually eliminates abnormal sample data. It was also conducted to determine the global differences between the metabolic profiles of the groups. Based on the analyses, all samples from the two species appeared in the Hotelling T2 with 95% confidence, suggesting that all the samples can be used for further research. The parameters described the PCA model (R2X = 0.0918, Q2 = 0.246).
As depicted in Fig. 2, the PCA plot shows four different groups of the samples, which are C. rotundicauda stimulated with LPS (green), non-stimulated C. rotundicauda (blue), T. gigas stimulated with LPS (red) and non -stimulated T. gigas (yellow). Based on the plot, there are differences between both horseshoe crab species, which can be measured from the locations of the metabolite's distributions in the quadrant. The C. rotundicauda (green and blue) samples were clustered on the lower quadrant on the right side, while the T. gigas (yellow and red) samples were clustered on the left upper and lower quadrants.
Comparison between LPS-stimulated (red) and non-stimulated hemocytes (yellow) of T. gigas, reveals an apparent difference in the metabolites produced. In contrast, in C. rotundicauda, there is no evident difference in the metabolites produced by the hemocytes stimulated with LPS (green) and without stimulation with LPS (blue), as they were grouped within the same quadrant.
The PLS-DA model analysis further demonstrated distinct discrimination in the metabolomic changes between the two species (Fig. 3). The acceptable values for the intercepts R2 (cum), the goodness of fit, was 0.99, and Q2 (cum), predictability, was 0.78. Model cross-validation through permutation tests (100 permutations) and sevenfold cross-validation generated the intercept R2 and Q2 0.978 and 0.267, respectively. The results show that the PLS-DA model is not overfitting and is valid for this metabolomic profiling.
PLS-DA score plots for compounds or metabolites detected in each treatment: Hemocytes of C. rotundicauda and T. gigas non-stimulated and stimulated with LPS (C. rotundicauda stimulated with LPS (green), C. rotundicauda non-stimulated (blue), T. gigas stimulated with LPS (red), and T. gigas non-stimulated (yellow).
The data presented herein demonstrated a clear and significant separation between the two species of horseshoe crabs and significant differences in the putative metabolites of the stimulated and non-stimulated hemocytes of T. gigas by multivariate analyses using PCA and PLS-DA.
PLS-DA also allows for determining and discriminating metabolites using the variable importance on projection, known as VIP. The VIP score value indicates the contribution of a variable to the discrimination between all the classes of samples. Mathematically, these scores are calculated for each variable as a weighted sum of squares of PLS weights. The mean VIP value is 1, and VIP values over one are usually considered significant. A high score agrees with a solid discriminatory ability and thus constitutes a criterion for selecting biomarkers. The discriminating metabolites were obtained using a statistically significant threshold of VIP values obtained from the PLS-DA model on the normalized raw data at the univariate analysis level. The P value was calculated by one-way analysis of variance (ANOVA) for four groups analysis. Metabolites with VIP values greater than 1.0 and p-values less than 0.05 were considered statistically significant metabolites. Figure 4 depicts a PLS-DA loading plot showing the variables that contributed to separating the four groups.
Loading plots of PLS-DA for metabolites (masses) detected via untargeted LC–TOF–MS in different treatments. The green dots labelled with retention time represent the masses distributed w*c(1) and w*c(2) planes and the highest VIP (Variables Importance for the Projection) > 1. Blue dots represent the origin.
Based on Fig. 4, the farther the point is from the origin, the greater the weight value or the greater the effect of determining the grouping of the samples. In the non-stimulated C. rotundicauda hemocytes, the metabolite masses that distinguished it from the other three groups are 6.16 min:204.0492 m/z, 1.83 min:205.0693 m/z, 8.29 min:387.0849 m/z, 8.76 min:943.5543 m/z, 1.83 min: 387.1471 m/z. On the other hand, these metabolite masses; 1.90 min: 287.1969 m/z; 1.88 min: 147.0650 m/z; 12.39 min: 531.3464 m/z; 1.93 min: 309.1772 m/z; were unique, and highly abundant in LPS stimulated C. rotundicauda hemocytes. For the group of non-stimulated hemocytes of T. gigas, the metabolites that contributed to the separation were as follows: 12.26 min:911.5912; 13.24 min: 227.1270; 13.25 min: 339.2501 m/z; 12.45 min:713.4391 m/z; 12.19 min:999.6454 m/z and 12.39 min: 509.3332 m/z. Lastly, the group of metabolites from LPS-stimulated T. gigas hemocytes was separated from the other groups due to the following metabolite masses: 12.37 min:823.5371 m/z; 12.46 min: 779.510 m/z; 7.90 min: 364.0847 m/z; 14.12 min:807.5674 m/z; 11.41 min: 359.0756 m/z; 9.88 min: 313.0707 m/z; 2.45 min: 345.0350 m/z. All details can be found in Table 1.
Compound identification
Identification of the compounds was performed by comparing the accuracy of the m/z value (< 10 ppm) and MS/MS spectra with available online databases: Human Metabolome Database (HMDB)16, Metabolite and Chemical Entity Database (METLIN)17, KEGG18, LIPIDMAPS19 and MassBank. All the putatively identified metabolites are reported in Table 1.
Comparative metabolomics analyses
An untargeted metabolite profiling of two horseshoe crab species, C. rotundicauda, and T. gigas, was performed in this study. There were two conditions observed: stimulated and non-stimulated with LPS. Multivariate statistical analyses such as PCA, PLS-DA, ANOVA, Post Hoc Tukey test and t-test were conducted. Based on the PLS-DA analysis, 37 metabolites are in the VIP > 1 category. Of 37 metabolites, only 23 were statistically significant when ANOVA was performed at p-value < 0.05. All the putative metabolites were tabulated in Table 1. Further statistical analysis using Post Hoc Tukey was also conducted after performing ANOVA.
Of 23, 7, 8-Dihydroxanthopterin, Fig. 5a is one of the putative metabolites found to be significant in both tests. No research studies have reported on this metabolite in horseshoe crabs. However, several studies on this metabolite from other organisms have been found. For example, in Stizostedion lucioperca, this metabolite influences the eye colour and vision of the fish21. The guanine crystals form a reflective layer that produces the silvery colour present on the eye surface. Meanwhile, the block-shaped crystals backscatter light into the retina, which helps to increase the sensitivity to light.
Figure shows the representative ion intensities for m/z value (a) 7,8-Dihydroxanthopterin (287.196 m/z), (b) Harman (205.0693 m/z), (c) Phosphatidylglycerol PG (15:1(9Z)\/22:6(4Z,7Z,10Z,13Z,16Z19Z)) (779.5176 m/z), (d) Androstenedione (287.1969 m/z), (e) Diglyceride DG(18:2n6/0:0/20:4n6) (677.4942 m/z), (f) L-Iditol (183.0870 m/z), (g) Octaprenyl diphosphate (373.2712 m/z), (h) Valine (140.0685 m/z), (i) Anthranilate (138.0558 m/z), (j) N-acetyllactosamine (387.147 m/z). The blue colour indicates C. rotundicauda non-stimulated, yellow for C-rotundicauda stimulated, green for T. gigas non-stimulated, and orange for T. gigas stimulated.
In human studies, this putative metabolite was found in the urine of phenylketonuria and lethal hyperphenylalaninemia patients22. It was reported to play a role in the pathogenesis of neurological symptoms in both diseases. Figure 5a shows that this putative metabolite was reduced in both species' hemocytes after LPS stimulation. However, Tukey's multiple comparisons test shows that the reduction of metabolites after stimulation with LPS is not statistically significant in C. rotundicauda but statistically significant in T. gigas. A comparison between species using Tukey's also shows significant differences between both species, and this metabolite can be found more abundantly in C. rotundicauda than in T. gigas.
Next, Harman, Fig. 5b, a natural B-carboline alkaloid becoming interesting due to its anti-cancer properties23. It also was found to be decreased in C. rotundicauda and T. gigas after hemocytes were stimulated with LPS. However, despite reductions, Tukey's multiple comparisons test shows the reduction is insignificant in each species between stimulated and non-stimulated forms. Despite that, there are significant differences in the metabolite between both species in which this metabolite is abundant in C. rotundicauda. By knowing this, isolation of the metabolites can be suggested on C. rotundicauda instead of T. gigas. This metabolite is usually known to be derived from plants, and sesame seed oil was reported to have high levels of β-carbolines24. The fungi entomopathogen Conidiobolus coronatus25 can also secrete it. It is also considered a nonpolar heterocyclic aromatic amine with potential mutagenicity24. It is also a reversible competitive monoamine oxidase inhibitor, increasing serum serotonin concentrations in tissues25. Antibacterial activities of Harman analogues against four gram-positive and two gram-negative bacteria damaged bacterial cell membranes and walls and disrupted the function of type II topoisomerase26. These derivatives also have potential as new bactericides and antibiotics, as the in-vivo antibacterial assay shows a protective efficacy of 81%26. In insects, Harman also resulted in delayed pupation and adult eclosion and inhibited total monoamine oxidase activity25.
Phosphatidylglycerol, Fig. 5c, increased in hemocytes after stimulation with LPS in both species. However, despite having an increment, Tukey's multiple comparisons test shows the increment is insignificant in both species stimulated and non-stimulated groups. Despite that, there is a significant difference if comparing two species in which this metabolite can be found to be abundant in T. gigas. Phosphatidylglycerol (PG) is a naturally occurring phospholipid and is essential for the growth and photosynthesis of photosynthetic organisms27. It is the only major phospholipid in the thylakoid membrane of chloroplasts27,28. As it is crucial for photosynthesis, the loss of PG in Arabidopsis thaliana resulted in severe defects in the growth and development of chloroplast with decreased accumulation of chlorophyll, impaired thylakoid formation, and also downregulation of photosynthesis-associated genes encoded in nuclear and plastid genomes28. PG is also one of the components needed in daptomycin to exert its antibacterial effect29. PG and sulfoquinovosyldiacylglycerol (SQDG) have similar physicochemical properties, bilayer thickness, and bending rigidity30. However, the function of this metabolite in horseshoe crabs should be further elucidated.
Androstenedione, Fig. 5d increased after the stimulation of hemocytes with LPS in both species. However, the Tukey test shows that this increment is not statistically significant. Despite that, both species have a significant difference in metabolites. In C. rotundicauda hemocyte, this metabolite is abundant compared to in T. gigas. Thus, if Androstenedione isolation is yet to be performed, it can be isolated from C. rotundicauda. Several studies have reported on androstenedione, but no specific analysis of androstenedione of horseshoe crabs has previously been reported. Dong-Ma et al. reported that androstenedione and androgens androstenedione (ADD) are predominant steroid hormones in surface water or wastewater and can disrupt the endocrine system in fish31. Androstenedione is produced in male and female gonads and the adrenal glands and is known for its crucial role in producing estrogen and testosterone32. It is also a precursor for several steroid substances like testosterone, estradiol, ethinyl estradiol, testolactone, progesterone, cortisone, cortisol, prednisone, and prednisolone33. It is also sold as an oral supplement to increase testosterone levels32. The supplement can also lower triglycerides (TG) and high-density lipoprotein (HDL) cholesterol, increase oestradiol concentration, and is a natural alternative to an anabolic steroid32,34. Other uses of the metabolite include as an enhancer for athletic performance, building body muscles, reducing fats, increasing energy, maintaining healthy RBCs, and increasing sexual performance32. Androstenedione is also listed among performance-enhancing drugs (PEDs). However, it was banned by the World Anti-Doping Agency and International Olympic Committee32.
Diglycerides, Fig. 5e, were found to be increased after stimulation with LPS in C. rotundicauda; meanwhile, in T. Gigas, the stimulation of hemocytes with LPS decreased the metabolites abundantly. Statistical analysis using Tukey shows that the changes are significant in T. gigas but not C. rotundicauda. In a study conducted by Song et al.35, monoglyceride and diglyceride were shown to have antiviral and antibacterial properties and act as emulsifiers to increase the digestibility of dietary lipids. Its supplementation could also effectively reduce fat loss, decrease inflammatory factor levels, and control total cholesterol concentrations during lactation35. In brown adipose tissue, L-Carnitine helps increase TG and diglyceride levels and reduces glycerophospholipids and sphingolipids.
L-Iditol, Fig. 5f increased after stimulation with LPS in both species. Statistical analysis using Tukey shows that only changes in T. gigas are significant. Studies regarding L-iditol alone are underreported. Only two relevant studies have been reported regarding L-Iditol, the angiosperm- Yunnanopilia longistaminata, a new plant source for L-iditol and taxanes36. Secondly, a series of quaternary diammonium salt derivatives of 1,4:3,6-dianhydro-l-iditol were synthesized, and two quaternary ammonium salts (QAS) with octyl and decyl residues exhibited antimicrobial activity37.
Octaprenyl diphosphate Fig. 5g also was found to be decreased after stimulation with LPS in hemocytes of both species and is statistically significant in the Tukey test. This metabolite is essential for the normal growth of Escherichia coli 38. However, the relation between this metabolite and horseshoe crab is unknown.
Valine, Fig. 5h, was found to be increased in C. rotundicauda; meanwhile, in T. gigas, it was found to be decreased after stimulation with LPS. However, the changes were not statistically significant when a Tukey comparison was conducted. Despite that, this metabolite is abundant in T. gigas, which means it can be isolated in this species if needed. Valine has extensive industrial applications and is an intermediate for synthesizing agricultural pesticides and semisynthetic veterinary antibiotics39. Bacillus cereus could have a potential for industrial production of valine under optimized conditions39. Dietary L-Valine supplementation modulates the inflammatory response and microbial metabolites40.
Anthranilate Fig. 5i increased in C. rotundicauda and decreased in T. gigas after hemocyte stimulation with LPS. However, analysis using Tukey shows that the metabolite changes are not significant. Despite that, this metabolite was found to be abundant in T. gigas. This metabolite is widely used as a precursor in producing dyes, fragrances, plastics, and pharmaceutical compounds41,42. Microorganisms produce Anthranilate as an intermediate in the tryptophan biosynthetic pathway41. It has various biological activities, such as anti-inflammatory, antineoplastics, anti-malarial, and has α-glucosidase inhibitory properties43. Methyl anthranilate (2-aminobenzoic acid methyl ester) irritates birds' senses of taste and smell44, protecting sweet cherry orchards against birds. Anthranilate also increased the antibiotic susceptibility of other species of bacteria, such as Escherichia coli, Salmonella enterica, Bacillus subtilis, and Staphylococcus aureus45. Evaluating the antifungal activity in vitro of the active films containing methyl anthranilate showed great effectiveness against Penicillium expansum and Botrytis cinerea, demonstrating the potential applicability of the developed films for active food packaging. Evodileptin B (1) is a natural anthranilate derivative isolated from the ethanol extract of the aerial parts of Evodia lepta (Spreng.) Merr., a traditional medicinal plant of the family Rutaceae46. Evodileptin B has solid neuroprotective properties and may help treat Parkinson's Disease46. Linalyl anthranilate (LNA) generates reactive oxygen species, initiates lipid peroxidation, and damages the bacterial membrane, resulting in intracellular leakage and eventually killing Klebsiella pneumoniae47. Another study of Anthranilate is a novel anthranilate analogue (SI-W052) that inhibited LPS-induced tumour necrosis factor (TNF)-α and interleukin (IL)-6 on microglia48. However, further studies must be conducted to understand its relationship with horseshoes crab metabolites.
N-acetyllactosamine Fig. 5j was found to be decreased in C. rotundicauda, after being stimulated with LPS, but it increased in T. gigas. However, the Tukey test shows that the increment and reduction are not statistically significant. N-acetyllactosamine (LacNAc), specifically β-d-galactopyranosyl-1,4-N-acetyl-d-glucosamine, is a unique acyl-amino sugar and a critical structural unit in human milk oligosaccharides, an antigen component of many glycoproteins, and an active antiviral property for the development of effective drugs against viruses49. The 6-sulfo -N-acetyllactosamine was found to inhibit the binding of the SARS-CoV-2 spike protein S1 subunit with blood group A RBCs and reduce the interaction between the spike protein S1 subunit and Angiotensin-converting enzyme 2 (ACE2) in SARS-CoV-2 infection50.
We performed the t-test to analyze each species' pre- and post-LPS treatment metabolite changes. Only three metabolites exhibited a significant increase—specifically, the increments in L-iditol and Diacylglycerophospholipid in T. gigas. Meanwhile, dtMP deoxythymidylic acid was statistically significant in C. rotundicauda (Table S1).
Subsequent t-test analysis revealed that only three exhibited statistically significant decreases among eleven metabolites, showing a decrement pattern. These significant decrements were observed in 7,8-Dihydroxanthopterin, Phosphatidylinositol PI (18:3(6Z,9Z,12Z)/22:3(10Z,13Z,16Z)), and octaprenyl diphosphate in T. gigas, while in C. rotundicauda, the only metabolite with a significant decrement was octaprenyl diphosphate (Table S1).
Following LPS stimulation, a contrasting abundance pattern in 10 metabolites was observed in both species, with C. rotundicauda exhibiting an increase while T. gigas showed a decrease pattern. The metabolites were N-(1-Deoxy-1-fructosyl) tryptophan, 4-Hydroxy-5-(3″,5″-dihydroxyphenyl)-valeric acid-O-glucuronide, Contignasterol, Proline betaine (stachydrine), Diglyceride, Canavaninosuccinate, Stearoylglycerone phosphate, N-acetylactosamine, Valine, and Anthranilate. However, the t-test results indicated that only the change in Diglyceride in T. gigas was statistically significant (Table S1).
In another contrasting abundance pattern case, two metabolites, namely Phospholipid inositol PI (22:4(7Z,10Z,13Z,16Z)/16:1(9Z)) and Glycerophosphoinositol, exhibited a decrease in C. rotundicauda but an increase in T. gigas following LPS stimulation. However, t-test analysis indicated that none of these changes were statistically significant in either species (Table S1).
Another contrasting abundance pattern after LPS stimulation showed no changes in the metabolite's phospholipid inositol PI (21:0/20:2(11Z,14Z)) and PG (18:4(6Z,9Z,12Z,15Z)/14:1(9Z)) expression in C. rotundicauda, whereas T. gigas exhibited a decrease pattern in both metabolites. However, the t-test revealed that only the decreased metabolite phospholipid inositol PI (21:0/20:2(11Z,14Z)) in T. gigas showed a statistically significant. In the other cases, Mevaldate and Phosphatidylinositol PI(P-18:0V16:0) showed no change in expression in C. rotundicauda after LPS stimulation, but an increased pattern in T. gigas but none of these changes in T. gigas were significant (Table S1).
In summary, the significant changes observed in metabolites by the t-test, such as L-iditol, Diacylglycerophospholipid, and dtMP deoxythymidylic acid, following LPS stimulation in T. gigas and C. rotundicauda, suggest their involvement in crucial pathways; immune regulation, inflammation, and stress responses. These metabolites likely play roles in modulating immune cell function, membrane stability, or nucleotide metabolism, contributing to the immune response. The observed differences in metabolite abundance between the two species indicate unique strategies in response to the immune challenge posed by LPS. Species-specific responses, exemplified by T. gigas' significant decreases in 7,8-Dihydroxanthopterin, Phosphatidylinositol PI, and octaprenyl diphosphate, and C. rotundicauda's specific decrease in octaprenyl diphosphate, highlight distinct molecular adaptations which evolutionary history, environmental factors, and ecological niches might influence. These species-specific strategies reflect C. rotundicauda and T. gigas' adaptations to diverse environments and immune challenges.
Pathway enrichment analysis
MetaboAnalyst51 shows there are three significant pathways where the metabolites are found to be enriched, which are glycerophospholipid metabolism, valine, leucine, and isoleucine biosynthesis and glycosylphosphatidylinositol (GPI)-anchor biosynthesis (Fig. 6). Glycerophospholipids are the most abundant and dominant in cell membranes as they provide stability, fluidity, and permeability52. Moreover, they must function correctly as membrane proteins, receptors, and ion channels and as reservoirs for second messengers and their precursors. Thus, phosphatidylglycerol (12.46 min: 779.5176 m/z) in horseshoe crabs probably helps with cell membranes' excellent structure and signalling. On the other hand, the biosynthesis of valine, leucine, and isoleucine is crucial as they play critical roles in regulating energy homeostasis, nutrition metabolism, gut health, immunity, and disease in humans and animals53. Perhaps the existence of the metabolite valine (1.82 min: 140.0685 m/z) in horseshoe crabs also plays the same role as in other organisms. Glycosylphosphatidylinositol functions as an anchor to link cell membranes and proteins. These proteins act as enzymes, adhesion molecules, complement regulators, or co-receptors in signal transduction pathways54. The richness of this metabolite in this pathway is probably linked to the first hit, the glycerophospholipid metabolism, as it seems to work together. However, the metabolite name (1-Phosphatidyl-D-myo-inositol) that hit this pathway is not precisely the same as our putative metabolite Phosphatidylinositol (12.26 min: 911.5912 m/z). Despite that, as we refer to the KEGG database, it refers to the same metabolite but differs in name. The KEGG pathways involved are map00564 Glycerophospholipid metabolism, map00290 Valine, leucine and isoleucine biosynthesis and map00563 Glycosylphosphatidylinositol (GPI)-anchor biosynthesis55,56,57.
Transcriptomics, proteomics, and metabolomics of hemocytes after LPS stimulation
Previous studies had conducted transcriptomics and proteomisc using hemocytes of horseshoe crabs11,14. The transcriptomics analysis reported 1338 genes were significantly upregulated, and 215 genes were downregulated after hemocytes were stimulated with LPS. Meanwhile, proteomics analysis reported 154 proteins were identified in the stimulated and non-stimulated form of hemocytes. From 154 proteins, 54 were found to be unique in hemocytes stimulated with LPS, and 25 were unique in non-stimulated form. Thirty- seven proteins are found to be shared in both conditions. Tachylectin-2, coagulogen, c-reactive proteins, histones, hemocyanin, and DNA polymerase, all of which play essential roles in the organism's innate immunity, were found to be differentially expressed in hemocytes after the LPS challenge14.
Gene ontology enrichment analysis from both studies showed several differentially expressed genes and proteins predictively involved in several metabolic processes such as cellular metabolic process, protein metabolic process, macromolecule metabolic process, and organonitrogen compound metabolic process. Indeed, our study showed several putative metabolites such as Androstenedione, 7,8-Dihydroneopterin, and Phosphatidylglycerol involved in the metabolic pathways by KEGG pathway analysis. Metabolites such as 7,8-Dihydroxanthopterin and Harman involved in the KEGG biosynthesis pathway, which the pathway was also enriched in a study by Sarmiento et al.11 at the gene level.
Nevertheless, it requires extensive study to understand the metabolites pathway and its functions to correlate our findings with previous findings at the gene and protein levels. Despite that, several metabolites such as Harman, L-Iditol, contignasterol, valine, Anthranilate, N-acetylactosamine, and Glycerophosphoinositol could be validate in further studies as they have immense benefits.
Conclusions
Overall, this study successfully profiled the putative metabolites of horseshoe crab hemocytes stimulated with LPS and without stimulation with LPS. The result identified thirty-seven differentially abundant putative metabolites. Several putative metabolites, such as L-iditol, Diglyceride and Octaprenyl diphosphate, increased and decreased in abundance after LPS stimulation. Furthermore, metabolites such as Anthranilate and valine are more abundant in T. gigas than C. rotundicauda. Various medicinal values are reported from these metabolites. Examples of metabolites are Harman, L-Iditol, contignasterol, valine, Anthranilate, N-acetylactosamine, and Glycerophosphoinositol. Harman is a metabolite that has anti-cancer properties and bactericides. L-Iditol exhibited antimicrobial activity. Contignasterol acts as an anti-asthma agent. Anthranilate acts as an anti-inflammatory, antineoplastic, anti-malarial, and antifungal. Next, valine is an agricultural pesticide and a semisynthetic veterinary antibiotic. These findings emphasize a species-specific metabolic response, highlighting the complexity of host-specific reactions to LPS stimulation in these two species. Those metabolites merit further investigation for the validation study.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Yang, Q. et al. Metabolomics biotechnology, applications, and future trends: A systematic review. RSC Adv. 9, 37245–37257. https://doi.org/10.1039/C9RA06697G (2019).
Dayalan, S., Xia, J., Spicer, R. A., Salek, R. & Roessner, U. in Encyclopedia of Bioinformatics and Computational Biology (eds Shoba Ranganathan, Michael Gribskov, Kenta Nakai, & Christian Schönbach) 396–409 (Academic Press, 2019).
Pereira Braga, C. & Adamec, J. in Encyclopedia of Bioinformatics and Computational Biology (eds Shoba Ranganathan, Michael Gribskov, Kenta Nakai, & Christian Schönbach) 463–475 (Academic Press, 2019).
Thirumulu Ponnuraj, K., Ahmad, A., Mokhtar, K. & Ibrahim, N. Potential applications of horseshoe crab in biomedical research. J. Biomed. Sci. 4 (2020).
Vikash Kumar, S. R., A.K. Sahoo, B.K. Behera and A.P. Sharma. Horseshoe crab and its medicinal values. IJCMAS 4 (2015).
Luo, Z., Miao, F., Hu, M. & Wang, Y. Research development on horseshoe crab: A 30-year bibliometric analysis. Front. Mar. Sci. 7. https://doi.org/10.3389/fmars.2020.00041 (2020).
Sekiguchi, K. & Shuster, C. N. in Biology and Conservation of Horseshoe Crabs (eds John T. Tanacredi, Mark L. Botton, & David Smith) 5–24 (Springer US, 2009).
Smith, D. R. et al. Conservation status of the American horseshoe crab, (Limulus polyphemus): A regional assessment. Rev. Fish Biol. Fish. 27, 135–175. https://doi.org/10.1007/s11160-016-9461-y (2017).
Carmichael, R. H. & Brush, E. Three decades of horseshoe crab rearing: A review of conditions for captive growth and survival. Rev. Aquac. 4, 32–43. https://doi.org/10.1111/j.1753-5131.2012.01059.x (2012).
Mashar, A. et al. Biodiversity and distribution of horseshoe crabs in Northern coast of Java and Southern Coast of Madura. IOP Conf. Ser.: Earth Environ. Sci. 54, 012076. https://doi.org/10.1088/1755-1315/54/1/012076 (2017).
Sarmiento, M. E. et al. Comparative transcriptome profiling of horseshoe crab Tachypleus gigas hemocytes in response to lipopolysaccharides. Fish Shellfish Immunol. 117, 148–156. https://doi.org/10.1016/j.fsi.2021.08.001 (2021).
Nadabo, C. et al. Parasitic contamination of commonly consumed and locally cultivated leafy vegetables in Jos, North-Central Nigeria. J. Hum. Environ. Health Promot. 8, 1–9. https://doi.org/10.52547/jhehp.8.1.1 (2022).
S, K., Koshiba, T. & T, S. The lipopolysaccharideactivated innate immune response network of the horseshoe crab. Invertebr. Surviv. J. 6 (2009).
Adebayo, I. A. et al. Proteomic analysis of Malaysian Horseshoe crab (Tachypleus gigas) hemocytes gives insights into its innate immunity host defence system and other biological processes. PLoS ONE 17, e0272799. https://doi.org/10.1371/journal.pone.0272799 (2022).
Wright Muelas, M. et al. An untargeted metabolomics strategy to measure differences in metabolite uptake and excretion by mammalian cell lines. Metabolomics 16, 107. https://doi.org/10.1007/s11306-020-01725-8 (2020).
Wishart DS, T. D., Knox C, et al. HMDB: the Human Metabolome Database, <https://hmdb.ca/spectra/ms_ms/search> (2023).
Metabolite and Chemical Entity Database (METLIN), <https://hmdb.ca/> (2023).
Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes, <https://www.genome.jp/kegg/> (2000).
LIPIDMAPS, <https://www.lipidmaps.org/> (2023).
Farhana A, K. Y. Vol. 2022 (StatPearls Publishing, 21 April 2021).
Zhang, G. et al. Guanine and 7,8-dihydroxanthopterin reflecting crystals in the zander fish eye: Crystal locations, compositions, and structures. J. Am. Chem. Soc. 141, 19736–19745. https://doi.org/10.1021/jacs.9b08849 (2019).
Watson, B. M., Schlensinger, P. & Cotton, R. G. H. Dihydroxanthopterinuria in phenylketonuria and lethal hyperphenylalaninemia patients. Clin. Chim. 78, 417–423. https://doi.org/10.1016/0009-8981(77)90075-4 (1977).
Ferraz, C. A. A. et al. Complexation with β-cyclodextrin enhances apoptosis-mediated cytotoxic effect of harman in chemoresistant BRAF-mutated melanoma cells. Eur. J. Pharm. Sci. 150, 105353. https://doi.org/10.1016/j.ejps.2020.105353 (2020).
Liu, W., Yang, Z., Shi, L., Cui, Z. & Li, Y. Degradation of β-carbolines harman and norharman in edible oils during heating. Molecules 26 (2021).
Wrońska, A. K. & Boguś, M. I. Harman and norharman, metabolites of the entomopathogenic fungus Conidiobolus coronatus (Entomophthorales), affect the serotonin levels and phagocytic activity of hemocytes, insect immunocompetent cells, in Galleria mellonella (Lepidoptera). Cell Biosci. 9, 29. https://doi.org/10.1186/s13578-019-0291-1 (2019).
Dai, J., Dan, W., Ren, S., Shang, C. & Wang, J. Design, synthesis and biological evaluations of quaternization harman analogues as potential antibacterial agents. Eur. J. Med. Chem. 160, 23–36. https://doi.org/10.1016/j.ejmech.2018.10.012 (2018).
Endo, K. et al. Crucial importance of length of fatty-acyl chains bound to the sn-2 position of phosphatidylglycerol for growth and photosynthesis of Synechocystis sp. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1867, 159158. https://doi.org/10.1016/j.bbalip.2022.159158 (2022).
Fujii, S. et al. Impacts of phosphatidylglycerol on plastid gene expression and light induction of nuclear photosynthetic genes. J. Exp. Bot. 73, 2952–2970. https://doi.org/10.1093/jxb/erac034 (2022).
Moreira, R. & Taylor, S. D. Establishing the structure–activity relationship between phosphatidylglycerol and daptomycin. ACS Infect. Dis. 8, 1674–1686. https://doi.org/10.1021/acsinfecdis.2c00262 (2022).
Bolik, S., Albrieux, C., Schneck, E., Demé, B. & Jouhet, J. Sulfoquinovosyldiacylglycerol and phosphatidylglycerol bilayers share biophysical properties and are good mutual substitutes in photosynthetic membranes. Biochim. Biophys. Acta Biomembr 1864, 184037. https://doi.org/10.1016/j.bbamem.2022.184037 (2022).
Ma, D.-D. et al. Transgenerational effects of androstadienedione and androstenedione at environmentally relevant concentrations in zebrafish (Danio rerio). J. Hazard. Mater. 423, 127261. https://doi.org/10.1016/j.jhazmat.2021.127261 (2022).
Badawy, M. T., Sobeh, M., Xiao, J. & Farag, M. A. Androstenedione (a natural steroid and a drug supplement): A comprehensive review of its consumption, metabolism, health effects, and toxicity with sex differences. Molecules 26 (2021).
Nunes, V. O. et al. Biotransformation of phytosterols into androstenedione & mdash: A technological prospecting study. Molecules 27, 3164 (2022).
Pang, Q. et al. The effect of androstenedione supplementation on testosterone, estradiol, body composition, and lipid profile: A systematic review and meta-analysis of randomized controlled trials. Hormones https://doi.org/10.1007/s42000-022-00385-8 (2022).
Song, H. et al. Effects of dietary monoglyceride and diglyceride supplementation on the performance, milk composition, and immune status of sows during late gestation and lactation. Front. Vet. Sci. 8. https://doi.org/10.3389/fvets.2021.714068 (2021).
Xi-Kui, L. & Jian-Jun, L. New source for L-iditol and taxanes. Nat. Preced. https://doi.org/10.1038/npre.2008.1502.1 (2008).
Sikora, K. et al. Antimicrobial, cytotoxic and mutagenic activity of gemini QAS derivatives of 1,4:3,6-dianhydro-l-iditol. Molecules 27 (2022).
Okada, K. et al. The ispB gene encoding octaprenyl diphosphate synthase is essential for growth of Escherichia coli. J. Bacteriol 179, 3058–3060. https://doi.org/10.1128/jb.179.9.3058-3060.1997 (1997).
Aliyu, A., Nasiru, S., JU, M., BB, S. & Ibrahim, H. Optimization of valine production using Bacillus cereus isolated from soil. Sch. Int. J. Biochem. 5, 37–49. https://doi.org/10.36348/sijb.2022.v05i02.002 (2022).
Koo, B. & Nyachoti, M. Effect of dietary L-valine supplementation on growth performance, immune response, and microbial metabolites in weaned pigs raised in varying sanitary conditions. J. Anim. Sci. 99, 113–114. https://doi.org/10.1093/jas/skab235.207 (2021).
Kuivanen, J. et al. Engineering of Saccharomyces cerevisiae for anthranilate and methyl anthranilate production. Microb. Cell Fact. 20, 34. https://doi.org/10.1186/s12934-021-01532-3 (2021).
Fernández-Cabezón, L., Rosich i Bosch, B., Kozaeva, E., Gurdo, N. & Nikel, P. I. Dynamic flux regulation for high-titer anthranilate production by plasmid-free, conditionally-auxotrophic strains of Pseudomonas putida. Metab. Eng 73, 11–25. https://doi.org/10.1016/j.ymben.2022.05.008 (2022).
Nawaz, M. et al. Synthesis of metal anthranilate complexes: Catalytic and antipathogenic studies. BMC Chem. 16, 21. https://doi.org/10.1186/s13065-022-00817-x (2022).
Mikiciuk, G., Chełpiński, P., Mikiciuk, M., Możdżer, E. & Telesiński, A. The effect of methyl anthranilate-based repellent on chemical composition and selected physiological parameters of sweet cherry (Prunus avium L.). Agronomy 11 (2021).
Hwang, H.-J., Li, X.-H., Kim, S.-K. & Lee, J.-H. Anthranilate acts as a signal to modulate biofilm formation, virulence, and antibiotic tolerance of Pseudomonas aeruginosa and surrounding bacteria. Microbiol. Spectr 10, e01463-e11421. https://doi.org/10.1128/spectrum.01463-21 (2022).
Kim, S., Yang, W., Cha, D. S. & Han, Y. T. Synthesis of evodileptin B, a natural anthranilate derivative isolated from Evodia lepta, and evaluation of its therapeutic potential against parkinson’s disease. Chem. Biodivers. 19, e202100808. https://doi.org/10.1002/cbdv.202100808 (2022).
Yang, S.-K. et al. Antimicrobial activity and mode of action of terpene linalyl anthranilate against carbapenemase-producing Klebsiella pneumoniae. J. Pharm. Anal. 11, 210–219. https://doi.org/10.1016/j.jpha.2020.05.014 (2021).
Wang, Z., Huang, J., Yang, S.-P. & Weaver, D. F. Anti-inflammatory anthranilate analogue enhances autophagy through mTOR and promotes ER-turnover through TEX264 during alzheimer-associated neuroinflammation. ACS Chem. Neurosci. 13, 406–422. https://doi.org/10.1021/acschemneuro.1c00818 (2022).
Karimi Alavijeh, M., Meyer, A. S., Gras, S. L. & Kentish, S. E. Synthesis of N-acetyllactosamine and N-acetyllactosamine-based bioactives. J. Agric. Food Chem. 69, 7501–7525. https://doi.org/10.1021/acs.jafc.1c00384 (2021).
Lee, Y.-K. et al. Carbohydrate ligands for COVID-19 spike proteins. Viruses 14, 330 (2022).
MetaboAnalyst5.0. Enrichment Analysis, https://www.metaboanalyst.ca/ (2021).
Johnson, J. L. & Johnson, L. A. in Encyclopedia of Behavioral Neuroscience, 2nd edition (Second Edition) (ed Sergio Della Sala) 372–382 (Elsevier, 2022).
Nie, C., He, T., Zhang, W., Zhang, G. & Ma, X. Branched chain amino acids: Beyond nutrition metabolism. Int. J. Mol. Sci. 19, 1. https://doi.org/10.3390/ijms19040954 (2018).
Wu, T. et al. The glycosylphosphatidylinositol biosynthesis pathway in human diseases. Orphanet. J. Rare Dis. 15, 129. https://doi.org/10.1186/s13023-020-01401-z (2020).
Kanehisa, M., Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Research 28(1), 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28(11), 1947–1951. https://doi.org/10.1002/pro.3715 (2019).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M., Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51(D1), D587–D592. https://doi.org/10.1093/nar/gkac963 (2023).
Acknowledgements
To acknowledge Universiti Sains Malaysia for the facilities support.
Funding
This study is funded by Universiti Sains Malaysia grant no. 304/PPSP/602002.
Author information
Authors and Affiliations
Contributions
N.J. and N.O. wrote the manuscript. N.J., L.C.W., N.I. and N.O. performed the experiments and analysis. N.Y. obtained the funding. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jasni, N., Wee, C.L., Ismail, N. et al. Comparative putative metabolites profiling of Tachypleus gigas and Carcinoscorpius rotundicauda hemocytes stimulated with lipopolysaccharide. Sci Rep 14, 3968 (2024). https://doi.org/10.1038/s41598-024-54279-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54279-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.