Abstract
The World Health Organization/International Society of Urological Pathology (WHO/ISUP) grading of renal cell carcinoma (RCC) is classified from grade 1–4, regardless of subtype. The National Comprehensive Cancer Network (NCCN) guidelines (2022) state that if there is an adverse pathological feature, such as grade 3 or higher RCC in stage 1 patients, more rigorous follow-up imaging is recommended. However, the RCC guidelines do not provide specific treatment or follow-up policies by tumor grade. Therefore, this study attempted to find out whether tumor grade affects survival rates in patients with metastatic RCC. The Korean Renal Cancer Study Group (KRoCS) database includes 3108 patients diagnosed with metastatic RCC between September 1992 and February 2017, with treatment methods, progression, and survival data collected from 11 tertiary hospitals. To obtain information on survival rates or causes of death, we utilized the Korea National Statistical Office database and institutional medical records. Data were accessed for research purpose on June, 2023. We then reviewed these sources to gather comprehensive and reliable data on the outcomes of our study cohort. This database was retrospectively analyzed, and out of 3108 metastatic RCC patients, 911 had been identified as WHO/ISUP grade. Grades were classified into either a low-grade (WHO/ISUP grade 1–2) or a high-grade group (WHO/ISUP grade 3–4). The patients were then analyzed related to progression and overall survival (OS). In metastatic clear cell RCC patients, the 1-year OS rate was 69.4% and the median OS was 17.0 months (15.5–18.5) followed up to 203.6 months. When comparing the patient groups, 119 low-grade and 873 high-grade cases were identified. No baseline difference was observed between the two groups, except that the high-grade group had a higher ECOG 1 ratio of 50.4% compared with 34.5% for the low-grade group (p = 0.009). There was a significant difference in OS between high-grade and low-grade groups. OS was 16.0 months (14.6–17.4) in the high-grade group and 28.0 months (21.1–34.9) in the low-grade group (p < 0.001). However, there was no difference in progression-free survival (PFS) rates with 9.0 months (8.0–10.0) for the high-grade group and 10.0 months (6.8–13.2) for the low-grade group (p = 0.377) in first-line treatment. In multivariable analysis, WHO/ISUP grade was a risk factor (HR = 1.511[1.135–2.013], p = 0.005) that influenced the OS. In conclusion, WHO/ISUP grade is a major data source that can be used as a ubiquitous marker of metastatic RCC in pre-IO era. Depending on whether the RCC is high or low grade, the follow-up schedule will need to be tailored according to grade, with higher-grade patients needing more active treatment as it can not only affect the OS in the previously known localized/locoregional recurrence but also the metastatic RCC patient.
Similar content being viewed by others
Introduction
Renal cell carcinoma (RCC) is a common malignancy of the urinary tract. In the United States, 81,000 new cases of RCC were diagnosed in 2023, and there were 14,890 related deaths1.
RCC’s grading system has been used as a prognostic factor for nearly 100 years. Although there are many grading systems, the Fuhrman grade was first used in 1982 after it was first reported that there was a difference in prognosis depending on nuclear size and cell outline2. The Fuhrman system was later replaced by the World Health Organization/International Society of Urological Pathology (WHO/ISUP) grading system in 2016. RCC’s WHO/ISUP grade is classified from grade 1 to grade 4, regardless of subtype. The grade is determined mainly by the shape of nucleoli, with nuclear pleomorphism, tumor giant cells, and rhabdoid or sarcomatoid differentiation also present in grade 43.
With the development of advanced imaging techniques such as high-resolution CT/MRI, early detection of small RCC is increasing4. However, approximately 30% of patients with localized RCC eventually progress to disease recurrence or distant metastasis. Furthermore, 15–20% of RCC patients present with metastasis at the initial diagnosis5.
The National Comprehensive Cancer Network (NCCN) Guidelines (2022)6 state that if there is an adverse pathological feature such as a high grade of grade 3 or higher in stage 1 patients, more rigorous follow-up imaging is recommended. However, it does not include any specific information or treatment plan for metastatic RCC thus far. The importance of grading tumors is emphasized most clearly in the “follow-up after surgery” section of the American Urological Association guidelines7. For pT1 tumors, which include tumors up to 7 cm in size, the tumors are divided into low/intermediate risk based on grade 1–2 or 3–4. Depending on the risk level, different follow-up schedules are recommended. For low-risk tumors, follow-up after one year of surgery is recommended, while annual follow-ups are suggested recommended for all risk levels three years after surgery. No distinction is made based on the grade for tumors classified as pT2 or higher.
Metastatic RCC contains several subgroups that differ significantly in terms of clinical characteristics and prognoses8. However, if there is a prognosticator that helps predict prognosis, it can guide patient treatment. Therefore, this study attempted to investigate whether grade affects survival in patients with metastatic RCC in a large-volume database registry.
Materials and methods
The Korean Renal Cancer Study Group (KRoCS) was created in 2013 and comprises data from 11 university hospitals in Korea9. Since March 2014, a web-based metastatic kidney cancer database system for RCC has been established10. The database was named KRoCS database, and it contained the 3108 patients diagnosed with metastatic RCC from September 1992 to February 2017, along with the treatment methods, progression, and survival data collected from the 11 tertiary hospitals. It also contains data on what primary, secondary, and tertiary treatments the RCC patients received. Also, the survival status was updated in July 2018 with no patients enrolled from February 2017. All institutions were approved by their institutional review board committees before being enrolled in the database. Due to the retrospective nature of the database, Institutional Review Board of Seoul National University Bundang Hospital, and has been approved by all relevant institutions (B-1902-522-101), waived the need of obtaining informed consent. We have conducted an IRB review for this research topic, the Institutional Review Board of Chung-Ang University Gwangmyeoung Hospital approved this study (approval number: 2304-076-039). This study was conducted according to the ethical standards recommended by the 1964 Declaration of Helsinki and its later amendments.
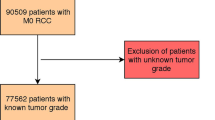
Data were accessed for research purpose on June, 2023, and we retrospectively reviewed 3,108 metastatic RCC patients, with 911 patients confirmed as having been given WHO/ISUP grade in this database. We excluded 2197 patients from the current study because they either lacked survival data or grade records. To obtain information on survival and cause of death, we utilized the Korea National Statistical Office database along with institutional medical records. We reviewed these sources to gather comprehensive and reliable data on the outcomes of our study cohort. Patients were classified into either a low-grade (grade 1–2) or a high grade (grade 3–4), then analyzed related to progression and overall survival (OS). Progression was defined according to radiographic criteria based on RECIST (Response Evaluation Criteria in Solid Tumors) ver 1.111.
In Tables 1, 2, and 3, the comparison between the two groups was conducted using Student’s t-test to compare means, and Fisher’s exact test was employed for the comparison of two categorical variables. For the comparison of overall survival and progression-free survival, Kaplan–Meier survival analysis and the log-rank test were employed. Multivariate Cox-regression model was used to identify overall and PFS predictors in Table 4 and 5. Statistical significance was set at p < 0.05. The SPSS software package (version 27.0; Statistical Package for Social Sciences, Chicago, IL, USA) and MedCalc (version 20; MedCalc Software, Ostend, Belgium) was used for all statistical analyses. All data used in the statistics has been provided in the supplementary material.
Results
Baseline characteristics and collected data are shown in Table 1. When comparing the patient groups, 119 low grades and 873 high grades were identified, with a median follow-up of 18.9 months (IQR 8.4–36.9). There was no baseline statistical difference between the two groups, except that the high-grade group had a higher ECOG 1 ratio of 50.4% compared with 34.5% (p = 0.009).
Detailed pathologic status is shown in Table 2. In both groups, the radical nephrectomy implementation rate was approximately 95% (low 95.8% vs. high 96.1%, p = 0.874), and in most cases was a clear cell type (93.2% vs. 88.4%, p = 0.0336). Additionally, the sarcomatoid ratio was significantly higher in the high grade (6.7% vs. 21.8%, p < 0.001).
Over a period of 25 years, various drugs such as cytokines, tyrosine kinase inhibitors (TKIs), and mTOR inhibitors have been used for treatment of metastatic RCC. TKI was mainly used as the first-line treatment (73.6% and 76.5%, p = 0.290), and there was no statistical difference in the treatment applied to the two groups (Table 3). In total, the 1-yr OS was 69.4% and the median OS was 17.0 months (15.5–18.5), with follow-up of up to 203.6 months (Fig. 1).
There was a significant difference in OS between the high-grade and low-grade groups (Fig. 2). The OS was 16.0 months (14.6–17.4) for the high-grade group and 28.0 months (21.1–34.9) for the low-grade group (p < 0.001). However, there was no significant difference in progression-free survival (PFS), with 9.0 months (8.0–10.0) for the high-grade group and 10.0 months (6.8–13.2) for the low-grade group (p = 0.377). In a multivariable analysis for OS (Table 4), WHO/ISUP grade (HR = 1.511[1.135–2.013], p = 0.005) influenced OS with patients who were ex-smokers (HR = 1.229, p = 0.045), with papillary RCC (HR = 1.586, p = 0.014), sarcomatoid component (HR = 1.617, p < 0.001) and margin status (HR = 1.828). According to the multivariable analysis related to progression-free survival (Table 5), papillary RCC (HR = 2.046, p < 0.001) and sarcomatoid component (HR = 1.446, p < 0.001) were both risk factors for cancer progression in first-line treatment.
Overall survival and progression-free survival of metastatic RCC patients by grade. (A) Overall survival graph of metastatic RCC patients with low (blue line) and high (green line) grades (total n = 911, p < 0.001). (B) Progression-free survival graph of metastatic RCC patients with low (blue line) and high (green line) grades in first-line treatment (total n = 911, p = 0.377).
Results excluding Chromophobe RCC are provided in Supplementary Material S2.
Furthermore, we investigated whether there is a difference in the effects of TKI and mTOR, representative treatments for metastatic RCC in pre-IO era, between high and low grades (Fig. 3). The results indicated a grade-dependent correlation, where TKI as a first-line treatment led to extended OS and PFS (all p < 0.05). Particularly in low-grade cases, the impact of TKI was more pronounced (all p < 0.01).
Lastly, to further stratify the impact of grade, we conducted OS analysis based on each T and N stage (Fig. 4). As a result, in T1, T3, and N0 stages, a statistically significant prolongation of OS was observed in the low-grade group (all p < 0.05). However, in T2, T4, and N1 stages, relatively higher stage, no significant difference was observed between the two groups.
Discussion
Our study revealed that there is approximately a one-year difference in OS depending on whether the RCC is high or low grade. This finding underscores the importance of considering tumor grade as a prognostic factor in the management of metastatic RCC. This result also indicates the potential value of considering grades in future follow-up schedules and observations.
Clear cell RCC is the predominant subtype of RCC, comprising approximately 80% of cases according to the World Health Organization (WHO) classification system. The other subtypes include papillary RCC, chromophobe RCC, collecting duct RCC, unclassified RCC, and Xp11.2 translocation RCC10. Metastatic RCC is a complex disease consisting of diverse subtypes, each with distinct morphological, genetic, clinical, and prognostic features12. These subgroups exhibit significant heterogeneity, making the accurate diagnosis and effective treatment of metastatic RCC challenging.
While there are relatively few studies that focus on the association between Fuhrman grades or WHO/ISUP grades and RCC, this is nonetheless a steadily emerging field. However, reports on this topic in metastatic RCC are scarce, and the value of tumor grading is not strongly emphasized in the guidelines of the National Comprehensive Cancer Network (NCCN), the European Association of Urology (EAU), or the AUA for the management of metastatic RCC.
A study similar to ours enrolled 266 patients with metastatic RCC who received treatment with TKIs13. They examined several serum biomarkers, including the neutrophil-to-lymphocyte ratio, and found that WHO/ISUP grade 3–4 increased the risk of metastatic RCC, with an HR of approximately 2.0. Their risk model revealed that there was a clear difference in OS based on the number of risk factors, with six risk factors indicating the highest risk.
In 2020, a nomogram study using the Surveillance, Epidemiology, and End Results (SEER) database was published14. The study enrolled 12,216 patients with metastatic RCC between 2010 and 2016 and used a training set of 1158 patients and a validation set of 1157 patients to develop the nomogram. Their multivariable analysis revealed that WHO/ISUP grade was a risk factor with the risk increasing with each grade. They assigned scores of 0 and 5 for WHO/ISUP grades 1–2, 20 for grade 3, and approximately 40 for grade 4 before calculating the total score to predict survival rates at one, three, and five years.
Generally, it is expected that low-grade tumors will have a better prognosis, while high-grade tumors may have a poorer prognosis. However, in our analyzed data, there are only cases of patients with low-grade tumors who developed metastasis. There is a possibility of selection bias within the analyzed patient group. Since our study includes only lower-grade cancer patients who have experienced metastasis, there is a higher likelihood of including a patient group with unfavorable conditions for metastasis, rather than representing the characteristics of the entire low-grade patient population. Furthermore, this could be a likely reason why the impact of grade appears relatively diminished in metastatic RCC. As a similar example, in the paper discussing late recurrence in patients with RCC, stating that T1a stage patients experience later recurrence more than T1b stage patients15. However, this phenomenon might not mean the actual truth that low-stage patients experience late recurrence more, but rather that high-stage patients experience more early recurrences, leading to a relatively lowerer proportion of high-stage patients in the late recurrence category.
A study on the risk factors for locoregional recurrence in patients who underwent radical nephrectomy16 focused on patients with T3–4 tumors in a non-metastatic setting. The results showed that locoregional recurrence was strongly associated with a sharp decline in five-year OS and that Fuhrman grade IV was a powerful risk factor for recurrence with an HR of 3.6 in multivariable analysis. This result indirectly suggests that Fuhrman grade IV may also impact OS.
Regarding grading of chromophobe RCC, there was no difference in OS among the three cell types (clear cell, papillary, chromophobe) for low-grade tumors (grades 1–2), but in high-grade tumors (grades 3–4), chromophobe RCC shows similar survival outcomes to low grade, while clear cell and papillary RCC have lower survival rates17. A recent study, therefore, has argued that the chromophobe tumor grade (CTG), consisting of three categories, should be used as a grading system18. Alternatively, Ohashi et al. have proposed a two-category grading system that only considers the presence of tumor necrosis or sarcomatoid component19. Regardless of RCC sub-classification, the sarcomatoid component has been identified as a prognostic factor for overall survival (HR = 1.617, p < 0.001) and PFS (HR = 1.446, p < 0.001). In our study, the high-grade cohort exhibited a 21.8% sarcomatoid component, while the low-grade cohort showed only 6.7%. Whether the sarcomatoid component and high grade are entirely independent factors is not fully understood, and sarcomatoid differentiation is also a characteristic of WHO/ISUP grade 4. Further research is necessary.
Although there is limited information on the mechanism of this grading phenomenon, a 2020 study suggested that as immunotherapy becomes more established as a standard treatment for RCC, the dysfunction of CD4 and CD8 T cells infiltrating tumor tissue is more pronounced in higher-grade tumors20. This indicates that immune responses may not function as effectively in high-grade RCC. The study examined 97 patients and found that in WHO/ISUP grades 3–4, CD4 and CD8 T cells were upregulated within cancer cells, while cytokine production was significantly lower. The results showed that while the cell ratio was higher in high-grade RCC, the proportion of granzyme B, which is associated with cytotoxic activity, was lower, indicating that effective immune responses did not occur.
Our study has some limitations that should be considered. First, the study cohort is highly heterogeneous due to the inclusion of patients with varying characteristics such as different first-line treatment agents, metastasis sites, and previous cytoreductive nephrectomy or metastasectomy statuses. However, we believe that grade could provide value as a ubiquitous marker for metastatic RCC in pre-IO era, further research on whether this holds true in the IO era would be highly valuable. Second, we did not perform a central pathology review, which may have resulted in some variability in the accuracy of our diagnosis. Third, our database includes the era of TKIs and does not include information on immune checkpoint inhibitors (IO) such as TKI + IO combinations, IO + IO combinations, and adjuvant IO, which are currently being actively studied. Our database is currently updated only until July 2018, and subsequent updates have been hindered by ongoing changes in IO treatment, compounded by restrictions on gatherings due to COVID-19. Therefore, new data on the impact of grades in the IO era is required and we are planning to update the database, including IO treatment. Fourth, this study has a retrospective design, there is a possibility of potential selection bias in our study. And while there were no deviations in data collection, the WHO/ISUP grade was only introduced in 2016 and was used interchangeably with the Fuhrman grade.
Despite the retrospective nature of this study, we believe that it holds significant value as it is based on long-term follow-up multicenter data obtained from a database21. The grading of RCC based only on cell morphology includes more than 11 categories according to the WHO classification3. Therefore, it may not be appropriate to uniformly classify the grade of RCC. However, even when considering RCC cell types without differentiation, as in our study, there was a significant difference in OS rates, suggesting that it has a meaningful role as a ubiquitous marker. Therefore, we consider our findings to be reliable and informative for future research in this field. It would also be valuable in comparison with the results of the IO era.
Conclusion
In conclusion, WHO/ISUP grade is a major data source that can be used as a ubiquitous marker of metastatic RCC in pre-IO era. Depending on whether the RCC is high or low grade, the follow-up schedule will need to be tailored according to grade, with higher-grade patients needing more active treatment as it can not only affect the OS in the previously known localized/locoregional recurrence but also the metastatic RCC patient.
Data availability
The authors declare that all data generated or analysed during this study are included in the Source Data file provided in the Supplementary Information files S1.
References
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73(1), 17–48. https://doi.org/10.3322/caac.21763 (2023).
Fuhrman, S. A., Lasky, L. C. & Limas, C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am. J. Surg. Pathol. 6(7), 655–663 (1982).
Moch, H., Cubilla, A. L., Humphrey, P. A., Reuter, V. E. & Ulbright, T. M. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: Renal, penile, and testicular tumours. Eur. Urol. 70(1), 93–105. https://doi.org/10.1016/j.eururo.2016.02.029 (2016).
Ha, S. C., Zlomke, H. A., Cost, N. & Wilson, S. The Past, present, and future in management of small renal masses. J. Oncol. 2015, 364807. https://doi.org/10.1155/2015/364807 (2015).
Logan, J. E. et al. Systemic therapy for metastatic renal cell carcinoma: A review and update. Rev. Urol. 14(3–4), 65–78 (2012).
Motzer, R. J. et al. Kidney cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 20(1), 71–90. https://doi.org/10.6004/jnccn.2022.0001 (2022).
Campbell, S. C. et al. Renal mass and localized renal cancer: Evaluation, management, and follow-up: AUA guideline: Part I. J. Urol. 206(2), 199–208. https://doi.org/10.1097/JU.0000000000001911 (2021).
Kim, J. K. et al. Survival and clinical prognostic factors in metastatic non-clear cell renal cell carcinoma treated with targeted therapy: A multi-institutional, retrospective study using the Korean metastatic renal cell carcinoma registry. Cancer Med. 8(7), 3401–3410. https://doi.org/10.1002/cam4.2222 (2019).
Choi, J. et al. Contemporary management of small renal masses by urologic oncologists: A 2022 Korean Renal Cancer Study Group Practice Pattern Survey. Korean J. Urol. Oncol. 21(1), 59–69. https://doi.org/10.22465/juo.234600120006 (2023).
Kim, J. K. et al. Application of the International Metastatic Renal Cell Carcinoma Database Consortium and Memorial Sloan Kettering Cancer Center risk models in patients with metastatic non-clear cell renal cell carcinoma: A multi-institutional retrospective study using the korean metastatic renal cell carcinoma registry. Cancer Res. Treat. 51(2), 758–768. https://doi.org/10.4143/crt.2018.421 (2019).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 45(2), 228–247. https://doi.org/10.1016/j.ejca.2008.10.026 (2009).
Park, J. Y. et al. Development of the clinical calculator for mortality of patients with metastatic clear cell type renal cell carcinoma: An analysis of patients from Korean Renal Cancer Study Group database. Investig. Clin. Urol. 61(3), 260–268. https://doi.org/10.4111/icu.2020.61.3.260 (2020).
Chrom, P. et al. Fuhrman grade and neutrophil-to-lymphocyte ratio influence on survival in patients with metastatic renal cell carcinoma treated with first-line tyrosine kinase inhibitors. Clin. Genitourin. Cancer. 14(5), 457–464. https://doi.org/10.1016/j.clgc.2016.02.005 (2016).
Zheng, W. et al. Development and validation of a nomogram to predict overall survival for patients with metastatic renal cell carcinoma. BMC Cancer 20(1), 1066. https://doi.org/10.1186/s12885-020-07586-7 (2020).
Ha, Y. S. et al. Predictive factors for late recurrence in patients with stage T1 clear cell renal cell carcinoma: A multiinstitutional study. Clin. Genitourin. Cancer 11(1), 51–55. https://doi.org/10.1016/j.clgc.2012.08.008 (2013).
Yoo, G. S. et al. Risk factors and patterns of locoregional recurrence after radical nephrectomy for locally advanced renal cell carcinoma. Cancer Res. Treat. 54(1), 218–225. https://doi.org/10.4143/crt.2020.1373 (2022).
Patard, J. J. et al. Prognostic value of histologic subtypes in renal cell carcinoma: A multicenter experience. J. Clin. Oncol. 23(12), 2763–2771. https://doi.org/10.1200/JCO.2005.07.055 (2005).
Paner, G. P. et al. A novel tumor grading scheme for chromophobe renal cell carcinoma: Prognostic utility and comparison with Fuhrman nuclear grade. Am. J. Surg. Pathol. 34(9), 1233–1240. https://doi.org/10.1097/PAS.0b013e3181e96f2a (2010).
Ohashi, R. et al. Multi-institutional re-evaluation of prognostic factors in chromophobe renal cell carcinoma: proposal of a novel two-tiered grading scheme. Virchows Arch. 476(3), 409–418. https://doi.org/10.1007/s00428-019-02710-w (2020).
Kawashima, A. et al. Tumour grade significantly correlates with total dysfunction of tumour tissue-infiltrating lymphocytes in renal cell carcinoma. Sci. Rep. 10(1), 6220. https://doi.org/10.1038/s41598-020-63060-1 (2020).
Shin, T. J. et al. Metastatic renal cell carcinoma to the pancreas: Clinical features and treatment outcome. J. Surg. Oncol. 123(1), 204–213. https://doi.org/10.1002/jso.26251 (2021).
Acknowledgements
The study was conducted using the Korean Renal Cancer Study Group (KRoCS) database, a kidney cancer research group under the Korean Urological Oncology Society.
Funding
This research was supported by the Korea Medical Device Development Fund grant funded by the Korean government (Ministry of Science and ICT, Ministry of Trade, Industry and Energy, Ministry of Health & Welfare, Republic of Korea, Ministry of Food and Drug Safety) (Project Number: KMDF_PR_20200901_0096) provided to S-H Hong, and also supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2021R1F1A1048198) provided to J. Choi.
Author information
Authors and Affiliations
Contributions
J.C. and S.B. wrote the main manuscript text. J.S. and C.I.C. prepared Figs. 1–2. W.S. and H.D.Y. prepared the tables and provided assistance in data curation. C.H.L., M.K., S.H.C., J.K.K., H.H.L, J.K.J., E.C.H., C.W.J., Y.H.K., J.Y.P., C.S., S.I.S., J.C., C.K., and S.H.H contributed to the formation of the database and the enrollment of patient. S.H.H was involved in the overall revision and supervision of the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choi, J., Bang, S., Suh, J. et al. Survival pattern of metastatic renal cell carcinoma patients according to WHO/ISUP grade: a long-term multi-institutional study. Sci Rep 14, 4740 (2024). https://doi.org/10.1038/s41598-024-54052-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54052-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.