Abstract
Autistic children often exhibit atypical brain lateralization of language processing, but it is unclear what aspects of language contribute to this phenomenon. This study employed functional near-infrared spectroscopy to measure hemispheric lateralization by estimating hemodynamic responses associated with processing linguistic and non-linguistic auditory stimuli. The study involved a group of autistic children (N = 20, mean age = 5.8 years) and a comparison group of nonautistic peers (N = 20, mean age = 6.5 years). The children were presented with stimuli with systematically decreasing linguistic relevance: naturalistic native speech, meaningless native speech with scrambled word order, nonnative speech, and music. The results revealed that both groups showed left lateralization in the temporal lobe when listening to naturalistic native speech. However, the distinction emerged between autism and nonautistic in terms of processing the linguistic hierarchy. Specifically, the nonautistic comparison group demonstrated a systematic reduction in left lateralization as linguistic relevance decreased. In contrast, the autism group displayed no such pattern and showed no lateralization when listening to scrambled native speech accompanied by enhanced response in the right hemisphere. These results provide evidence of atypical neural specialization for spoken language in preschool- and school-age autistic children and shed new light on the underlying linguistic correlates contributing to such atypicality at the sublexical level.
Similar content being viewed by others
Introduction
Individuals with autism spectrum disorders (henceforth autism) exhibit atypical language profile. According to the diagnostic criteria outlined in the DSM-51, autistic individuals universally manifest challenges in the social-pragmatic aspect of language. Despite the pronounced heterogeneity in language profiles associated with autism, a majority of children diagnosed with autism concurrently present language disorders, exhibiting delays and difficulties across various core language domains, including phonology, semantics, and syntax2,3. The prevalence of co-occurring language disorders is estimated to range from 50 to 60% within the spectrum4,5,6. Approximately 25~30% of autistic children remain nonverbal or minimally verbal7,8,9,10. One possible neurobehavioral mechanism underlying the language atypicalities in autism is reduced neural specialization for the processing of linguistic structures and vocal signals, as was found in earlier studies on autistic children11,12,13. Left-lateralization of speech processing is a hallmark of specialized language function in the brain14,15. This organization has been shown to signify language competence in a variety of populations, including children with typical and atypical language development and second language learners16,17,18,19,20. Because autistic children often show subtle or reversed hemispheric lateralization for language processing, understanding the underlying mechanisms is crucial for supporting their language development.
Language-related hemispheric dominance is well-documented in neurotypical infants and children. In neonates, natural speech predominantly activates the left fronto-temporal language regions21,22,23,24, which is considered to reflect a built-in bias for language learning in the left hemisphere25. Research has also established that as children age and their language skills progress, the lateralization of language processing becomes more pronounced14,26,27. Such developmental change is thought to be consequential from the growth of individual language experience. Specifically, as age increases and phonological knowledge consolidates, the processing functions of the left and right hemispheres differentiate for various linguistic features and components. Native phonological and linguistic information (e.g., phonemic and semantic processing) tends to be lateralized to the left hemisphere, and nonnative and paralinguistic information (e.g., intonational prosody) becomes specialized within the right hemisphere. Support for the experience-dependent language specialization also comes from adult studies, wherein the successful acquisition of a linguistic form, such as a nonnative language or sinewave speech, is associated with emergence of left lateralization16,19.
However, lateralization of the fronto-temporal language regions was found to be substantially deviated in infants and young children diagnosed with autism. Compared to age-matched typically developing (TD) peers, autistic children aged 1–4 years showed lower responsivity in the left superior temporal gyrus (STG) and higher responsivity in the right homologue region when listening to natural speech when asleep28,29. It was further demonstrated that this group difference became more prominent at 3- and 4-year of age29. Reversed lateralization has also been reported in electrophysiological studies examining cortical processing of words. Specifically, a link between reduced left lateralization in the ERP responses to word meaning and greater autism symptoms was found in 2-year-old autistic toddlers30,31.
Atypical lateralization is not a phenomenon confined to early development but also frequently seen in school-age children, adolescents and adults with autism during a variety of language-based tasks, including word production32, word detection33, sentence comprehension34,35,36, and communicative intent comprehension35. Others have found an association between individual language competence and hemispheric lateralization in this population. For example, left temporal lobe activation and language skills in 11- to 16-year-old autistic adolescents were positively correlated, although atypical right frontal activation was also found to be associated with better language abilities as well37. In another study, autistic adults who had a history of language delay showed no significant leftward asymmetry in the N400 component, a neurophysiological marker associated with semantic processing, unlike those without a history of language delay38. These findings suggest a long-lasting effect of atypical hemispheric specialization that could be an important neural marker for understanding autistic language12.
Notably, reduced leftward asymmetry in autism is often accompanied by increased involvement of the right hemisphere12,39. In neurotypical brain, the right hemisphere is considered responsible for the processing of paralinguistic and contextual information instead of phonological and lexical processing14,40,41. In autistic children who show reduced or reversed lateralization, the right-hemisphere network may compensate for the insufficient development of a language network in the left hemisphere12,29,37,42. Enhanced involvement of the right hemisphere in autism has been linked with auditory biases for computational properties of the right hemisphere, namely preferences for spectral information and slow-changing acoustic patterns13,43,44. The hyperactivity of the right hemisphere, whether due to compensation or auditory bias, could potentially suppress language functions related to the processing of paralinguistic cues and contextual information, which are typically handled by the right hemisphere. This possibility is in line with the clinical characterization of autism1 and lab-based studies that have identified marked difficulties with receptive and expressive prosody, pragmatic and contextual understanding in autistic individuals45,46,47,48,49,50.
Neuroimaging research on language lateralization in autism has mostly utilized higher-order tasks involving multiple linguistic aspects and skills beyond the language network in the brain (e.g., executive function), rendering it difficult to attribute the lateralization differences to specific language factors35. Studies with young autistic children using advanced imaging techniques (e.g., functional magnetic resonance imaging or fMRI) often struggle with testability51, and have mostly employed native speech without exploring the interaction between hemisphere and speech type28,29. Therefore, there remains a knowledge gap regarding the specific aspect within the linguistic hierarchy responsible for the altered lateralization and heightened right hemisphere activities in autism. Addressing this question can offer important information to the developmental mechanisms of neural specialization for language in autistic children with language difficulties.
Functional near-infrared spectroscopy (fNIRS) is a noninvasive neuroimaging technique that utilizes the absorption of near-infrared light to measure the fluctuation of hemoglobin concentrations in the cortex as a proxy for neuronal activity52,53. fNIRS has several advantages over other neuroimaging techniques including portability, ability to withstand head movement, affordability, and efficacy in natural experimental setting. fNIRS has been shown to be an effective technique for studying brain function in autism51,54 and language development55,56. Compared to the enclosed space typical of fMRI research, the bigger visible room with attenuated ambient noise in fNIRS testing provides a more comfortable setting for participants with special needs such as individuals with noise intolerance.
The present study aimed to identify the specific linguistic or phonological component that drives the atypical hemispheric lateralization in autistic children. fNIRS was utilized to examine cortical activation in the bilateral language-related areas when processing auditory signals that varied along the linguistic hierarchy. The linguistic hierarchy was created using four stimulus conditions with parametrically decreasing linguistic relevance or content: naturalistic native speech, native speech with scrambled word order, nonnative speech, and music. We hypothesized that leftward asymmetry in the nonautistic comparison children would decrease parametrically as a function of linguistic relevance of the stimuli. In contrast, autistic children would exhibit atypical patterns of lateralization. The level of linguistic relevance at which the atypical lateralization became evident would provide insight into the specific language component that drives atypical auditory-linguistic processing in autism.
Methods
Participants
Thirty autistic children and 25 nonautistic comparison children aged 3–10 years old participated in the study. The children were recruited through advertisements requesting study volunteers in the pediatric department of a local hospital. One child's data were excluded due to left-handedness57. Two children's data were excluded due to incompletion of the fNIRS experiment. After preprocessing of fNIRS data, 9 autistic children and three nonautistic children were excluded from final analysis due to channel noise or poor data quality (see fNIRS data processing). Participant attrition details, aligning with reporting guidelines to address phenotypic bias in neuroscientific research58,59,60, are provided in Supplementary Material Table S1. The final dataset consisted of 20 autistic children (1 girl and 19 boys, M = 5.8, SD = 2.2, 3.7–10.8 years) and 20 nonautistic children (10 girls and 10 boys, M = 6.5, SD = 1.7, 3.8–9.9 years). There was no significant difference in age between the two groups (t(38) = − 1.12, p = 0.269; Table 1).
The children in the autism group received best-estimate diagnosis of autism by pediatricians with at least 10 years of expertise in diagnosing autism. Because the Autism Diagnostic Observation Schedule (ADOS) and the Autism Diagnostic Interview-Revised (ADI-R) have not been validated or widely adopted in China61,62,63, the autistic participants’ diagnoses were supplemented by Childhood Autism Rating Scale (CARS)64 and Autism Behavior Checklist (ABC)65. Both are widely used diagnostic instruments in China62,66. All 20 children in the autism group had CARS scores at or above the cutoff of 30 for autism (M = 33.5, SD = 3.4, range = 30–44), and 17 had ABC scores at or above the cutoff of 53 for autism (M = 63.4, SD = 18.4, range = 15–96). Additionally, Social Response Scale (SRS)67 scores were available for 16 autistic children (M = 70.4, SD = 22.6, range = 36–112) and 12 nonautistic children (M = 37.3, SD = 17.7, range = 14–83). The autistic children scored significantly higher than the nonautistic children on SRS (t(26) = 4.19, p < 0.001), indicating greater social communication atypicalities. None of the children had any known genetic condition, psychiatric condition, or neurodevelopmental disorder other than autism. None were regularly taking medications at the time of the study. All the participants spoke Mandarin Chinese as their native language.
Chinese version of the Peabody Picture Vocabulary Test-Revised (PPVT-R)68,69 was administered by a trained experimenter to measure the children’s receptive vocabulary. PPVT scores were available for 12 children in the autism group, with 8 children either not meeting the minimal scoring requirement or discontinuing the test due to agitation. The autism group's average PPVT-R score was significantly lower than that of the comparison group (t(30) = − 7.23, p < 0.001), indicating lower receptive language level.
Stimuli
There were four stimulus conditions with decreasing linguistic relevance: natural native speech, native speech with scrambled word order, nonnative speech, and music. In the natural native speech condition (Native), a children’s story “The Mower and the Wolf” was read by a female speaker in Mandarin Chinese, and was expected to produce canonical leftward asymmetry of brain activation in native listeners. In the scrambled native speech condition (Native-scrambled), words within each sentence of the native story were shuffled such that the sentence preserved the phonological and prosodic features of the native speech but eliminated the lexico-semantic content. The nonnative speech (Nonnative) was the original story read in Russian, which allowed testing of language-general phonetic processes. The speech stimuli were recorded by the same female speaker who was a Chinese-Russian bilingual. The music condition (Mozart’s Piano Sonata No. 4 in E flat major) was used as a non-verbal auditory control. Music shares similar acoustic and structural complexity with speech but requires specific tonal pitch processing, which is known to engage distinct brain networks with a rightward asymmetry70,71.
Each audio recording was divided into nine 15-s segments (trials; M = 15.3 s, SD = 0.6 s), resulting in a total of 36 trials. Each speech trial contained complete sentence(s) and each music trial contained entire musical phrases with natural beginnings and endings. A block design was used based on previous studies23,72. Each block presented four trials from each of the four conditions in a random order (Fig. 1). There was a 30-s silence before the presentation of stimuli and a 20-s silence between each trial to allow the hemodynamic activity to reset to baseline55,73. There were 9 blocks with a total of 21 min.
Apparatus and procedure
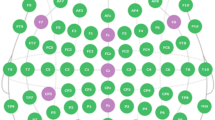
FNIRS data were collected using a 52-channel continuous wave system (ETG-4000, Hitachi Medical Corporation, Tokyo, Japan) at a sampling rate of 10 Hz (wavelengths: 695 and 830 nm). A 3 × 11 probe set including 17 light sources and 16 detectors was adjusted with elastic straps over the participant's head. The positioning of the optodes was based on the international 10–20 system. To cover the regions of interest, the patch was placed 3–5 cm vertically from the eyebrow centering the middle probe of the most inferior row over Fpz, and the left and right sides were respectively centered at T7 and T8, so that measurement channels covered large areas of temporal and frontal parietal lobe cortex (Fig. 2).
Probeset distribution and channel clusters with significant activation in response to natural native speech (Native). Red circles are the sources, blue circles are the detectors, and thin lines represent channels. The yellow color (5 channels per hemisphere) represent the ROIs in the autism group, and the pink color (3 channels per hemisphere) represent the ROIs in the comparison group.
The children were accompanied by their caregivers in a quiet room and sat in a comfortable chair in front of a mobile phone screen used to play cartoons of their own choice. A research assistant positioned the fNIRS headband over designated regions of interest, and made adjustment to ensure the participant’s comfort while maintaining adequate contact between the optodes and the participant’s scalp. The children wore inserted earphones that played the sounds at a comfortable level (70 dB SPL). The children were instructed to watch cartoons while ignoring any sounds from their earphones. They were encouraged to remain still while seated. The child and their parent could withdraw at any time whenever the child expressed discomfort or a desire to stop participation.
The experimental protocol was approved by the Research Ethics Committee of South China Normal University, and all experiments were performed in accordance with relevant guidelines and regulations. The informed consent was obtained from parents or guardians of all participating children in the study.
fNIRS data processing
The analysis of the fNIRS data was performed using the HOMER2 package74 together with custom scripts. Data from the initial 52 participants after preliminary screening (see Participants section) were preprocessed using methods recommended for infant and child data75,76. First, the measured light intensity was converted to optical density. Then, channels with optical intensity that were stronger or weaker than the set standard range [mean (d) < 0.001 or > 7] were discarded from the analyses. Next, motion artifacts were defined as signal changes exceeding an amplitude of 0.5 mmol × mm threshold and/or a standard deviation of 50 within a one-second timeframe, to reject the remaining uncorrected motion artifacts of the optical intensity data77,78. Wavelet filtering (iqr = 0.8) was performed for de-noising and waveform smoothing75,79, and PCA (nSV = 0.85) was performed on detected motion artifacts 75,80. The optical density signals were then band-pass filtered to attenuate low-frequency drift and cardiac oscillations with cut-off frequencies of 0.01–0.1 Hz. The signals were converted to estimated changes in the concentration of HbO and HbR through application of the modified Beer-Lambert Law74. The hemodynamic responses of trials from a time interval of − 5.0 s to 25.0 s were extracted and averaged for each stimulus condition. After preprocessing, we used plotting and visual inspection to remove noisy channels and trials that were not identified in the previous steps81,82,83,84. In this step, children were included in the analysis if they had less than or equal to 20 out of 52 channels detected as noisy and less than or equal to 4 out of 36 trials rejected. By applying these criteria, data were available for 20 autistic children and 20 nonautistic children. The average number of trials was 35 per participant.
Statistical analyses
Nonparametric cluster-based permutation tests (CBPT)85 were performed to identify channel clusters showing significant responses in each condition. The signal from multiple channels and time points were subjected to 1000 permutations to reduce the error rate. The HbO and HbR concentration values within 5–25 s post-stimulus were compared with zero baseline. The zero baseline was established by linearly fitting the 5 s preceding trial onset. Significant HbO clusters identified by the CBPT in the Native condition were used as regions of interest (ROI) in each hemisphere. These clusters represent the canonical language region that offers a unified criterion to examine how sounds at different linguistic levels activate this region. Because oxyhemoglobin (HbO) has been reported to show higher signal-to-noise ratio than deoxyhemoglobin (HbR) in children86,87 and that no significant HbR clusters were identified for the Native condition in either group, we used HbO for further statistical analysis and HbR was only reported in Table 2 and Fig. 3 for descriptive purposes.
Group-averaged hemodynamic response function (HRF). Red and blue colors represent oxyhemoglobin (HbO) and deoxyhemoglobin (HbR), respectively. Shaded areas indicate standard error values (± 1SE). The significant channel clusters in the left temporal lobe was selected as the left ROI, and the significant channel clusters in the right temporal lobe (symmetrical to the left cluster) was used as the right ROI. The average hemoglobin concentration levels of the channels in ROIs was calculated to obtain the HRF for the autism group and the comparison group in the four stimulus conditions: natural native speech condition (Native), scrambled native speech condition (Native-scrambled), nonnative speech (Nonnative), and music (Music). The x axis represented the time from 5 s pre-stimulation to 25 s post-stimulation.
Linear mixed modeling (LMM) implemented by the R package lme488 was used to assess the effects and interactions of the factors of interest on HbO. First, we evaluate whether the groups differed in responses in the two hemispheres: model1 = lmer (meanOxyhemoglobin ~ Age + Group*Hemisphere + (1|subject)). In this model, age was first entered as a controlled variable; group (autistic or nonautistic), hemisphere (left or right), and their interactions were fixed factors, while subject was entered as a random effect to control for individual variance. Additionally, the effects of Hemisphere × Condition were examined for autism and nonautistic separately: model2 = lmer (meanOxyhemoglobin ~ Age + Condition*Hemisphere + (1|subject)). Finally, Pearson correlation analysis was performed to examine potential brain-behavior relationships between neural responses to different sound classes and clinical measures.
Results
Selection of regions of interest
Table 2 shows the significant channel clusters identified by the cluster-based permutation tests for each stimulus condition in reference to baseline.
According to the permutation test results, the autism and comparison groups had overlapping channels showing significant activation in the Native condition (Table 2, Fig. 2). Therefore, in both groups, the set of significant channel clusters in the left temporal lobe was selected as the left ROI, and the set of significant channel clusters in the right temporal lobe (symmetrical to the left cluster) was used as the right ROI (Fig. 2). The mean HbO concentration for each participant within each hemisphere was computed for further statistical analysis. Figure 3 shows the group-averaged hemodynamic response function (HRF) in each hemisphere in each stimulus condition.
Lateralization patterns in the autism and comparison groups
In the LME regression combining both groups, there was a significant two-way interaction of Group × Hemisphere (F = 5.01, p < 0.05, η2p = 0.01; Fig. 4). Post hoc comparisons indicated that HbO concentration in the left ROI was significantly greater than that in the right ROI in the comparison group (t(278) = 4.61, p < 0.001, Cohen’s d = 0.73, 95% CI = [0.41, 1.05]) but not in the autism group (t(278) = 1.45, p = 0.150, Cohen’s d = 0.23, 95% CI = [− 0.08, 0.54]); The comparison group had a greater response than the autism group in the left ROI (t(76.7) = − 2.60, p < 0.05, Cohen’s d = − 0.41, 95% CI = [− 0.72, − 0.09]) but response amplitude was comparable between the two groups in the right ROI (t(76.7) = − 0.06, p = 0.948, Cohen’s d = − 0.01, 95% CI = [− 0.32, 0.30]).
We then analyzed the effects of Condition × Hemisphere for each group separately. In the autism group, the Condition × Hemisphere interaction was significant (F = 4.87, p < 0.01, η2p = 0.09). Further analysis revealed that left hemisphere activation decreased as linguistic relevance decreased (Native > Native-scramble > Nonnative > Music), and that the right hemisphere was the most responsive for the Native-scrambled speech. In addition, left lateralization was evident in the Native and Nonnative conditions (Native: t(133) = 3.02, p < 0.01, Cohen’s d = 0.96, 95% CI = [0.29, 1.61]; Nonnative: t(133) = 2.35, p = 0.020, Cohen’s d = 0.74, 95% CI = [0.10, 1.38]). In the Music condition, there was a trend towards right lateralization (t(133) = − 1.68, p = 0.096, Cohen’s d = − 0.53, 95% CI = [− 1.16, 0.10]). The only condition lacking hemispheric difference was the Native-scramble condition (t(133) = − 0.27, p = 0.785, Cohen’s d = − 0.09, 95% CI = [− 0.70, 0.54]; Fig. 5).
In the comparison group, no significant Condition × Hemisphere interaction was found. However, exploratory analysis revealed that the effect size of hemispheric difference decreased as a function of linguistic relevance of the stimuli, suggesting decreasing left lateralization (Native: t(133) = 3.66, p < 0.001, Cohen’s d = 1.16, 95% CI = [0.48, 1.82]; Native-scramble: t(133) = 2.60, p = 0.010, Cohen’s d = 0.82, 95% CI = [0.17, 1.46]; Nonnative: t(133) = 1.73, p = 0.086, Cohen’s d = 0.55, 95% CI = [− 0.09, 1.18]; Music: t(133) = 0.54, p = 0.593, Cohen’s d = 0.17, 95% CI = [− 0.45, 0.79]; Fig. 5).
Brain-behavior correlations
In the comparison group, age was negatively correlated with the response to native and nonnative speech in the left hemisphere (r native = − 0.51, r nonnative = − 0.46, ps < 0.05, Fig. 6), displaying a developmental decrease of left hemisphere activity to native language.
In the autism group, several significant correlations were identified (Fig. 6). Specifically, response to nonnative speech in the right hemisphere increased with age (r = 0.71, p < 0.001). The SRS score was positively correlated right hemisphere response to native and nonnative speech (r native = 0.61, r nonnative = 0.63, ps < 0.05), suggesting a link between greater social symptoms and higher right hemisphere responsivity to speech. The ABC score was negatively correlated with the response of right hemisphere to music (r = − 0.49, p < 0.05).
No other brain-behavior correlations were found (for a summary of the statistical results, see Supplementary Material Tables S3).
Discussion
In this fNIRS study, we examined the activation patterns of frontal–temporal language regions during the processing of auditory stimuli with varying linguistic relevance in autistic children and nonautistic children. As hypothesized, the comparison group demonstrated an increasing left lateralization as the linguistic hierarchy of the auditory stimuli became more pronounced. In contrast, the autism group in the current study displayed a distinct lateralization pattern, showing heightened activity in the right hemisphere specifically when listening to scrambled native speech. These findings suggest that atypical language specialization in autism is associated with the phonological aspects of speech processing, and the right hemisphere involvement in autistic children may be influenced by sublexical information.
Reduced neural specialization for language processing in autistic children
One main finding was the absence of overall lateralization for sound processing in the autism group as compared with the comparison group. Specifically, the comparison group showed a clear leftward asymmetry in the temporal lobe, whereas no such pattern was found in the autism group. Further analysis confirmed that the leftward asymmetry in the comparison group was unique to speech processing, in particular native speech. The robust left lateralization for native speech in the comparison group aligns well with a previous report of reliable left lateralization for language in the temporal network by the age of 7 years26.
Rather than finding focal cortical activity in terms of left lateralization, we observed more broadly activated cortical areas for sound processing in the autistic children compared to the nonautistic children. The significant cluster in response to normal native speech in the autism group was larger than that in the comparison group. In addition, the significant cluster in the autism group were located at supramarginal gyrus in the left hemisphere, whereas the comparison group’s significant cluster was concentrated in the Wernicke's area. This observation aligns with a recent meta-analysis of 11 studies utilizing fMRI89. The results of the meta-analysis showed that autistic individuals recruited additional areas in the middle temporal gyrus (MTG) and superior temporal gyrus (STG) during semantic processing, which were not activated in the TD brain. These findings, combined with the current results, suggest that these autistic children might need more widely distributed cortical resources for speech processing when compared to nonautistic children.
Moreover, we observed a positive association between right hemisphere response to nonnative speech and age in the autism group. This finding coincides with existing developmental data from autistic toddlers aged 12–48 months obtained using fMRI, which demonstrated an age-dependent increment of right lateralization for speech processing29,42. However, in the current study, age-related change in right hemisphere response was evident for nonnative speech only, but not for native speech and music. Because the nonnative speech is phonetic in nature but lacking linguistic relevance, we could imply that the developmental change was related to language-independent auditory function. Given that brain response to nonnative speech is considered an indicator of atypical neural commitment for language90,91, we have reasons to argue that an over-responsive right hemisphere for auditory processing might negatively impact neural commitment or specialization for linguistic processing in autistic children.
Atypical right hemisphere involvement for sublexical speech processing in autistic children
The comparison group demonstrated a clear pattern of left lateralization in response to native speech, regardless of its semantic integrity. However, the left lateralization was only weakly present in the nonnative speech condition and was absent for music. This parametrically decreasing left-lateralization as a function of linguistic relevance fits well with our hypothesis about the nonautistic children. Notably, the lateralization measure but not the unilateral activation measure was uniquely reflective of linguistic processing. This result could signify a neurobehavioral outcome of language acquisition in which the brain becomes specialized for auditory signals of linguistic relevance. However, the systematic pattern of brain lateralization was absent in the autism group.
Another main finding was the lack of increasing left lateralization as a function of linguistic relevance in the autism group. As in the comparison group, the autism group showed leftward asymmetry when the speech was native and naturally presented; however, the asymmetry disappeared for the scrambled native speech associated with elevated responsivity in the right hemisphere. Lack of hemispheric asymmetry for speech processing is a highly replicated phenomenon in autism research11,12,13,44,92. Yet little is known about the specific component of language that could drive such differential lateralization in autistic children. The current findings provide important evidence of underlying correlates in this regard.
First, we consider psychoacoustic factors associated with the hyper-responsivity of the right hemisphere. Autistic individuals show an auditory bias for right-hemisphere functions such as the bias for spectral information13,44,93. This bias could be responsible for an atypical functional specialization for speech characterized by prominent pitch variations. In the current study, this type of speech was presented as scrambled Mandarin Chinese. The Chinese language is a typical tonal language with syllable-level pitch variations, a quality not present in the Nonnative Russian speech. Moreover, the Chinese speech in the Native-scramble condition did not have the grammatical markers that are present in naturally produced speech with regular word order. Thus, this scrambled speech might have appeared extra prosodic compared to normal Chinese. The right hemisphere of the Chinese autistic children might overly respond to these stimuli due to their spectrally prominent prosodic variations33,43. In line with this interpretation, our data demonstrated a trend of right lateralization in the autism group when listening to music, consistent with a right-hemisphere bias for melodic information.
Evidence of a second factor potentially responsible for the atypical lateralization concerns the “holistic” nature of processes carried out by the right hemisphere. A recent ERP study with school-age autistic children identified an atypical engagement of the right hemisphere in response to speech differing in linguistic relevance, namely, native versus nonnative pseudowords43. This finding suggests a sublexical component driving atypical cerebral specialization for language that is consistent with the current observation. This study did not support a pure auditory explanation because there was no difference between groups in lateralization for nonspeech sounds (native vs. nonnative prosodic acoustics). Instead, the researchers proposed a neurolinguistic explanation. Specifically, the right hemisphere is predominantly responsible for processing slow-changing holistic speech patterns such as supra-segmental word forms and envelope modulations15,94,95,96. Thus, it is conceivable that the atypical right hemisphere effect in autism could reflect overly holistic processing of speech. In other words, neurotypical children might hear the scrambled Chinese as combinations of fine-grained phonological units or potential words, whereas some autistic children might hear it as holistic speech forms97. Over-processing of such information might hinder their mastery of the correct phonological rules and grammatical structures. The over-responsivity of the right hemisphere might also inhibit paralinguistic and contextual processing functions that typically rely on the right hemisphere29,40.
A third factor that potentially contributes to the observed differential lateralization in autism could be restricted language experience. Although school-age autistic children have received ample language exposure, exposure is not equivalent to vocabulary and language experience. According to models of language neurodevelopment, brain functional change has its dynamic interplay with individual language experience14,98,99,100. Specifically, it was proposed that cerebral specialization for language is not only age-dependent but also synergistic with the mastery of the native phonology that facilitates efficient speech processing and learning14. In the current study, the autistic children displayed a bilateral activation pattern unique to the sublexical, phonological level of native speech processing associated with elevated right-hemisphere response, which could reflect an unreliable phonological representation insufficient to support a specialized language network. In line with this notion is the limited but converging evidence showing increased functional connectivity within homologue right-hemisphere regions of the core language network throughout development in autism101. An insufficiently specialized neural network could, in turn, impede efficient word use and acquisition, further limiting the child’s experience with language14,100.
Limitations
First, we did not have standardized assessments for high-order receptive language such as syntactic ability or expressive language, which limits our ability to further examine the relationship between brain lateralization and language development in autism. For instance, previous work demonstrated a link between lateralization (as indexed by handedness) and expressive language, but not receptive language in autism102. PPVT as a measure of receptive vocabulary also has its apparent limitations in revealing structural language differences in children103. Second, the age range of our participants spanned across preschool to school age. We statistically controlled for age effects in the regression models, but it will also be useful to test the hypotheses in different age groups. Nonetheless, fNIRS data on language lateralization in preschoolers, and in children on the autism spectrum is extremely rare104. Finally, our speech materials were recorded by human voices rather than computer-generated speech. This method provides high ecological validity but lacks strict control over some acoustic parameters such as envelope, amplitude and frequency. However, the psychoacoustic differences across conditions were inherent in presenting stimuli that varied in linguistic relevance, and thus it might be impossible to fully eliminate the effects of these differences. Future investigations of fine-grained auditory and linguistic components are needed to better understand the underlying correlates of brain lateralization in autism.
Conclusion
This study demonstrated the atypical brain lateralization in autistic children during speech processing. The nonautistic children showed an overall left-lateralization for sound processing, whereas the autism group displayed a bilateral activation pattern. Moreover, the nonautistic children's degree of left lateralization systematically decreased with decreasing linguistic relevance of the auditory stimuli. In contrast, the autism group’s brain lateralization did not follow the linguistic hierarchy. Instead, autistic children showed a lack of neural specialization for language at the sublexical level, driven by an over-responsivity of the right hemisphere. The findings underscore the potential auditory and neurolinguistic correlates of autism-related biases for right-hemisphere functions, and the role of language experiences in strengthening specialized language networks in the brain. However, it is essential to apply caution in interpreting these conclusions and acknowledge the limitations of generalizability across the entire spectrum, given the marked heterogeneity present in the language profiles of autistic individuals2,3. The ongoing debate surrounding the nature of language disorder as either a dimensional feature intrinsic to autism or a distinct condition additive to the autistic phenotype further underscores the complexity of the heterogeneity issue2,3,105,106,107. Future research may incorporate these considerations into study designs to advance our understanding of the underlying language mechanisms in autism.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.) (American Psychiatric Association, 2013).
Tager-Flusberg, H. Defining language impairments in a subgroup of children with autism spectrum disorder. Sci. China Life Sci. 58, 1044–1052. https://doi.org/10.1007/s11427-012-4297-8 (2015).
Schaeffer, J. et al. Language in autism: domains, profiles and co-occurring conditions. J. Neural. Transm. 130, 433–457. https://doi.org/10.1007/s00702-023-02592-y (2023).
Levy, S. E. et al. Autism spectrum disorder and co-occurring developmental, psychiatric, and medical conditions among children in multiple populations of the United States. J. Devel. Behav. Pediatr. 31, 267–275 (2010).
Loucas, T. et al. Autistic symptomatology and language ability in autism spectrum disorder and specific language impairment. J. Child Psychol. Psychiatry 49, 1184–1192. https://doi.org/10.1111/j.1469-7610.2008.01951.x (2008).
Kjelgaard, M. M. & Tager-Flusberg, H. An investigation of language impairment in autism: Implications for genetic subgroups. Lang. Cogn. Process. 16, 287 (2001).
Tager-Flusberg, H. & Kasari, C. Minimally verbal school-aged children with autism spectrum disorder: the neglected end of the spectrum. Autism Res. 6, 468–478. https://doi.org/10.1002/aur.1329 (2013).
Norrelgen, F. et al. Children with autism spectrum disorders who do not develop phrase speech in the preschool years. Autism 19, 934–943. https://doi.org/10.1177/1362361314556782 (2015).
Anderson, D. K. et al. Patterns of growth in verbal abilities among children with autism spectrum disorder. J. Consult. Clin. Psychol. 75, 594–604. https://doi.org/10.1037/0022-006X.75.4.594 (2007).
Rose, V. The proportion of minimally verbal children with autism spectrum disorder in a community-based early intervention programme. J. Intellect. Disabil. Res. 60, 464–477 (2016).
Sperdin, H. F. & Schaer, M. Aberrant Development of Speech Processing in Young Children with Autism: New Insights from Neuroimaging Biomarkers. Front. Neurosci. 10, 393. https://doi.org/10.3389/fnins.2016.00393 (2016).
Lindell, A. K. & Hudry, K. Atypicalities in cortical structure, handedness, and functional lateralization for language in autism spectrum disorders. Neuropsychol. Rev. 23, 257–270. https://doi.org/10.1007/s11065-013-9234-5 (2013).
Yu, L. & Wang, S. Aberrant auditory system and its developmental implications for autism. Sci. China Life Sci. 64, 861–878. https://doi.org/10.1007/s11427-020-1863-6 (2021).
Minagawa-Kawai, Y., Cristia, A. & Dupoux, E. Cerebral lateralization and early speech acquisition: a developmental scenario. Dev. Cogn. Neurosci. 1, 217–232. https://doi.org/10.1016/j.dcn.2011.03.005 (2011).
Hickok, G. & Poeppel, D. The cortical organization of speech processing. Nat. Rev. Neurosci. 8, 393–402. https://doi.org/10.1038/nrn2113 (2007).
Möttönen, R. et al. Perceiving identical sounds as speech or non-speech modulates activity in the left posterior superior temporal sulcus. Neuroimage 30, 563–569. https://doi.org/10.1016/j.neuroimage.2005.10.002 (2006).
Xu, Y. et al. Activation of the left planum temporale in pitch processing is shaped by language experience. Hum. Brain Mapp. 27, 173–183. https://doi.org/10.1002/hbm.20176 (2006).
Zatorre, R. J. & Gandour, J. T. Neural specializations for speech and pitch: moving beyond the dichotomies. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 1087–1104. https://doi.org/10.1098/rstb.2007.2161 (2008).
Wong, P. C. M., Perrachione, T. K. & Parrish, T. B. Neural characteristics of successful and less successful speech and word learning in adults. Hum. Brain Mapp. 28, 995–1006. https://doi.org/10.1002/hbm.20330 (2007).
Bishop, D. V. Cerebral asymmetry and language development: cause, correlate, or consequence?. Science 340, 1230531. https://doi.org/10.1126/science.1230531 (2013).
Sato, H. et al. Cerebral hemodynamics in newborn infants exposed to speech sounds: a whole-head optical topography study. Hum. Brain Mapp. 33, 2092–2103. https://doi.org/10.1002/hbm.21350 (2012).
Dehaene-Lambertz, G., Dehaene, S. & Hertz-Pannier, L. Functional Neuroimaging of Speech Perception in Infants. Science 298, 2013–2015 (2002).
Vannasing, P. et al. Distinct hemispheric specializations for native and non-native languages in one-day-old newborns identified by fNIRS. Neuropsychologia 84, 63–69. https://doi.org/10.1016/j.neuropsychologia.2016.01.038 (2016).
Dehaene-Lambertz, G. et al. Functional organization of perisylvian activation during presentation of sentences in preverbal infants. Proc. Natl. Acad. Sci. U. S. A. 103, 14240–14245. https://doi.org/10.1073/pnas.0606302103 (2006).
Dehaene-Lambertz, G. The human infant brain: A neural architecture able to learn language. Psychon Bull Rev 24, 48–55. https://doi.org/10.3758/s13423-016-1156-9 (2017).
Berl, M. M. et al. Regional differences in the developmental trajectory of lateralization of the language network. Hum. Brain Mapp. 35, 270–284. https://doi.org/10.1002/hbm.22179 (2014).
Olulade, O. A. et al. The neural basis of language development: Changes in lateralization over age. Proc. Natl. Acad. Sci. U. S. A. 117, 23477–23483. https://doi.org/10.1073/pnas.1905590117 (2020).
Redcay, E. & Courchesne, E. Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2–3-year-old children with autism spectrum disorder. Biol. Psychiatry 64, 589–598. https://doi.org/10.1016/j.biopsych.2008.05.020 (2008).
Eyler, L. T., Pierce, K. & Courchesne, E. A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain 135, 949–960. https://doi.org/10.1093/brain/awr364 (2012).
Coffey-Corina, S., Padden, D. & Kuhl, P. K. ERPs to words correlate with behavioral measures in children with Autism Spectrum Disorder. J. Acous. Soc. Am. 123, 3742 (2008).
Kuhl, P. K. et al. Brain responses to words in 2-year-olds with autism predict developmental outcomes at age 6. PLoS One 8, e64967. https://doi.org/10.1371/journal.pone.0064967 (2013).
Kleinhans, N. M., Muller, R. A., Cohen, D. N. & Courchesne, E. Atypical functional lateralization of language in autism spectrum disorders. Brain Res. 1221, 115–125. https://doi.org/10.1016/j.brainres.2008.04.080 (2008).
Minagawa-Kawai, Y. et al. Cerebral laterality for phonemic and prosodic cue decoding in children with autism. Neuroreport 20, 1219–1224. https://doi.org/10.1097/WNR.0b013e32832fa65f (2009).
Wang, A. T., Lee, S. S., Sigman, M. & Dapretto, M. Neural basis of irony comprehension in children with autism: The role of prosody and context. Brain 129, 932–943. https://doi.org/10.1093/brain/awl032 (2006).
Jouravlev, O. et al. Reduced language lateralization in autism and the broader autism phenotype as assessed with robust individual-subjects analyses. Autism Res. 13, 1746–1761. https://doi.org/10.1002/aur.2393 (2020).
Anderson, J. S. & Lange, N. Decreased left posterior insular activity during auditory language in autism. Am. J. Neuroradiol. https://doi.org/10.3174/ajnr.A1789 (2010).
Knaus, T. A., Burns, C., Kamps, J. & Foundas, A. L. Atypical activation of action-semantic network in adolescents with autism spectrum disorder. Brain Cogn. 117, 57–64. https://doi.org/10.1016/j.bandc.2017.06.004 (2017).
Ahtam, B., Braeutigam, S. & Bailey, A. Semantic processing in autism spectrum disorders is associated with the timing of language acquisition: A magnetoencephalographic study. Front. Hum. Neurosci. 14, 267. https://doi.org/10.3389/fnhum.2020.00267 (2020).
Herringshaw, A. J., Ammons, C. J., DeRamus, T. P. & Kana, R. K. Hemispheric differences in language processing in autism spectrum disorders: A meta-analysis of neuroimaging studies. Autism Res. 9, 1046–1057. https://doi.org/10.1002/aur.1599 (2016).
Lindell, A. K. In your right mind: right hemisphere contributions to language processing and production. Neuropsychol. Rev. 16, 131–148. https://doi.org/10.1007/s11065-006-9011-9 (2006).
Vigneau, M. et al. What is right-hemisphere contribution to phonological, lexico-semantic, and sentence processing? Insights from a meta-analysis. Neuroimage 54, 577–593. https://doi.org/10.1016/j.neuroimage.2010.07.036 (2011).
Flagg, E. J., Cardy, J. E., Roberts, W. & Roberts, T. P. Language lateralization development in children with autism: Insights from the late field magnetoencephalogram. Neurosci. Lett. 386, 82–87. https://doi.org/10.1016/j.neulet.2005.05.037 (2005).
Yu, L., Huang, D., Wang, S. & Zhang, Y. Reduced neural specialization for word-level linguistic prosody in children with autism. J. Autism Dev. Disord. 53, 4351–4367. https://doi.org/10.1007/s10803-022-05720-x (2023).
Haesen, B., Boets, B. & Wagemans, J. A review of behavioural and electrophysiological studies on auditory processing and speech perception in autism spectrum disorders. Res. Autism. Spect. Dis. 5, 701–714. https://doi.org/10.1016/j.rasd.2010.11.006 (2011).
Järvinen-Pasley, A., Peppe, S., King-Smith, G. & Heaton, P. The relationship between form and function level receptive prosodic abilities in autism. J. Autism Dev. Disord. 38, 1328–1340. https://doi.org/10.1007/s10803-007-0520-z (2008).
Jiang, J., Liu, F., Wan, X. & Jiang, C. Perception of melodic contour and intonation in autism spectrum disorder: Evidence from Mandarin speakers. J. Autism Dev. Disord. 45, 2067–2075. https://doi.org/10.1007/s10803-015-2370-4 (2015).
McCann, J., Peppe, S., Gibbon, F. E., O’Hare, A. & Rutherford, M. Prosody and its relationship to language in school-aged children with high-functioning autism. Int. J. Lang. Commun. Disord. 42, 682–702. https://doi.org/10.1080/13682820601170102 (2007).
Peppe, S., McCann, J., Gibbon, F., O’Hare, A. & Rutherford, M. Receptive and expressive prosodic ability in children with high-functioning autism. J. Speech. Lang. Hear. Res. 50, 1015–1028 (2007).
Eigsti, I. M., Schuh, J., Mencl, E., Schultz, R. T. & Paul, R. The neural underpinnings of prosody in autism. Child Neuropsychol. 18, 600–617. https://doi.org/10.1080/09297049.2011.639757 (2012).
Tesink, C. et al. Neural correlates of language comprehension in autism spectrum disorders: When language conflicts with world knowledge. Neuropsychologia 49, 1095–1104. https://doi.org/10.1016/j.neuropsychologia.2011.01.018 (2011).
Butler, L. K., Kiran, S. & Tager-Flusberg, H. Functional near-infrared spectroscopy in the study of speech and language impairment across the life span: A systematic review. Am. J. Speech Lang. Pathol. 29, 1674–1701. https://doi.org/10.1044/2020_ajslp-19-00050 (2020).
Scholkmann, F. et al. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 85(Pt 1), 6–27. https://doi.org/10.1016/j.neuroimage.2013.05.004 (2014).
Boas, D. A., Elwell, C. E., Ferrari, M. & Taga, G. Vol. 85 1–5 (Elsevier, 2014).
Zhang, F. & Roeyers, H. Exploring brain functions in autism spectrum disorder: A systematic review on functional near-infrared spectroscopy (fNIRS) studies. Int. J. Psychophysiol. 137, 41–53. https://doi.org/10.1016/j.ijpsycho.2019.01.003 (2019).
Gervain, J., Macagno, F., Cogoi, S., Pena, M. & Mehler, J. The neonate brain detects speech structure. Proc. Natl. Acad. Sci. U. S. A. 105, 14222–14227. https://doi.org/10.1073/pnas.0806530105 (2008).
Quaresima, V., Bisconti, S. & Ferrari, M. A brief review on the use of functional near-infrared spectroscopy (fNIRS) for language imaging studies in human newborns and adults. Brain Lang. 121, 79–89. https://doi.org/10.1016/j.bandl.2011.03.009 (2012).
Oldfield, R. C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9, 97–113 (1971).
Girolamo, T., Butler, L., Canale, R., Aslin, R. N. & Eigsti, I. M. fNIRS studies of individuals with speech and language impairment underreport sociodemographics: A systematic review. Neuropsychol. Rev. https://doi.org/10.1007/s11065-023-09618-y (2023).
Webb, E. K., Etter, J. A. & Kwasa, J. A. Addressing racial and phenotypic bias in human neuroscience methods. Nat. Neurosci. 25, 410–414. https://doi.org/10.1038/s41593-022-01046-0 (2022).
Kwasa, J. et al. Demographic reporting and phenotypic exclusion in fNIRS. Front. Neurosci. 17, 1086208. https://doi.org/10.3389/fnins.2023.1086208 (2023).
Sun, X. et al. Prevalence of autism in mainland China, Hong Kong and Taiwan: A systematic review and meta-analysis. Mol. Autism 4, 7. https://doi.org/10.1186/2040-2392-4-7 (2013).
Pang, Y. et al. Challenges of case identification and diagnosis of Autism Spectrum Disorders in China: A critical review of procedures, assessment, and diagnostic criteria. Res. Autism Spectr. Disord. 53, 53–66. https://doi.org/10.1016/j.rasd.2018.06.003 (2018).
Yang, T. et al. China multi-center preschool autism project (CMPAP): Design and methodologies to identify clinical symptom features and biomarkers of autism spectrum disorders. Front. Psychiatry 11, 613519. https://doi.org/10.3389/fpsyt.2020.613519 (2020).
Schopler, E., Reichler, R. J., DeVellis, R. F. & Daly, K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). J. Autism Dev. Disord. (1980).
Krug, D. A., Arick, J. & Almond, P. Behavior checklist for identifying severely handicapped individuals with high levels of autistic behavior. Child Psychology & Psychiatry & Allied Disciplines (1980).
Sun, X. et al. Autism prevalence in China is comparable to Western prevalence. Mol. Autism 10, 7. https://doi.org/10.1186/s13229-018-0246-0 (2019).
Constantino, J. N. & Gruber, C. P. Social responsiveness scale (Western Psychological Services, 2009).
Dunn, L. M. & Dunn, L. M. Manual for the peabody picture vocabulary test-revised (American Guidance Service, 1981).
Sang, B. & Liao, X. The revision of trail norm of peabody picture vocabulary test revised (PPVT-R) In Shanghai proper. Psychol. Sci. 5, 003 (1990).
Rosenthal, M. A. Hemispheric asymmetry in the hierarchical perception of music and speech. Psychol. Bull. 142, 1165–1178. https://doi.org/10.1037/bul0000076 (2016).
Zatorre, R. J., Belin, P. & Penhune, V. B. Structure and function of auditory cortex: Music and speech. Trends Cogn. Sci. 6, 37–46 (2002).
May, L., Byers-Heinlein, K., Gervain, J. & Werker, J. F. Language and the newborn brain: Does prenatal language experience shape the neonate neural response to speech?. Front. Psychol. 2, 222. https://doi.org/10.3389/fpsyg.2011.00222 (2011).
Peña, M. et al. Sounds and silence: An optical topography study oflanguage recognition at birth. Proc. Natl. Acad. Sci. 100, 11702–11705 (2003).
Huppert, T. J., Diamond, S. G., Franceschini, M. A. & Boas, D. A. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl Opt 48, D280-298. https://doi.org/10.1364/ao.48.00d280 (2009).
Di Lorenzo, R. et al. Recommendations for motion correction of infant fNIRS data applicable to multiple data sets and acquisition systems. Neuroimage 200, 511–527. https://doi.org/10.1016/j.neuroimage.2019.06.056 (2019).
Kruppa, J. A. et al. Brain and motor synchrony in children and adolescents with ASD-a fNIRS hyperscanning study. Soc. Cogn. Affect. Neurosci. 16, 103–116. https://doi.org/10.1093/scan/nsaa092 (2021).
Arredondo, M. M., Aslin, R. N. & Werker, J. F. Bilingualism alters infants’ cortical organization for attentional orienting mechanisms. Dev Sci 25, e13172. https://doi.org/10.1111/desc.13172 (2022).
Cholemkery, H., Kitzerow, J., Rohrmann, S. & Freitag, C. M. Validity of the social responsiveness scale to differentiate between autism spectrum disorders and disruptive behaviour disorders. Eur. Child Adolesc. Psychiatry 23, 81–93. https://doi.org/10.1007/s00787-013-0427-5 (2014).
Sato, H. et al. Wavelet analysis for detecting body-movement artifacts in optical topography signals. Neuroimage 33, 580–587. https://doi.org/10.1016/j.neuroimage.2006.06.028 (2006).
Bortfeld, H., Wruck, E. & Boas, D. A. Assessing infants’ cortical response to speech using near-infrared spectroscopy. Neuroimage 34, 407–415. https://doi.org/10.1016/j.neuroimage.2006.08.010 (2007).
Hocke, L. M. et al. Automated processing of fNIRS data-a visual guide to the pitfalls and consequences. Algorithms https://doi.org/10.3390/a11050067 (2018).
Kelly, R. E. Jr. et al. Visual inspection of independent components: defining a procedure for artifact removal from fMRI data. J. Neurosci. Methods 189, 233–245. https://doi.org/10.1016/j.jneumeth.2010.03.028 (2010).
Minagawa-Kawai, Y. et al. Optical brain imaging reveals general auditory and language-specific processing in early infant development. Cereb. Cortex 21, 254–261. https://doi.org/10.1093/cercor/bhq082 (2011).
Sutoko, S. et al. Adaptive algorithm utilizing acceptance rate for eliminating noisy epochs in block-design functional near-infrared spectroscopy data: application to study in attention deficit/hyperactivity disorder children. Neurophotonics 5, 045001. https://doi.org/10.1117/1.NPh.5.4.045001 (2018).
Maris, E. & Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164, 177–190. https://doi.org/10.1016/j.jneumeth.2007.03.024 (2007).
Gervain, J. et al. Near-infrared spectroscopy: A report from the McDonnell infant methodology consortium. Dev. Cogn. Neurosci. 1, 22–46. https://doi.org/10.1016/j.dcn.2010.07.004 (2011).
Su, W. C., Culotta, M., Tsuzuki, D. & Bhat, A. Cortical activation during cooperative joint actions and competition in children with and without an autism spectrum condition (ASC): An fNIRS study. Sci. Rep. 12, 5177. https://doi.org/10.1038/s41598-022-08689-w (2022).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:1406.5823 (2014).
Phan, L., Tariq, A., Lam, G., Pang, E. W. & Alain, C. The neurobiology of semantic processing in autism spectrum disorder: An activation likelihood estimation analysis. J. Autism Dev. Disord. 51, 3266–3279. https://doi.org/10.1007/s10803-020-04794-9 (2021).
Zhang, Y., Kuhl, P. K., Imada, T., Kotani, M. & Tohkura, Y. Effects of language experience: Neural commitment to language-specific auditory patterns. Neuroimage 26, 703–720. https://doi.org/10.1016/j.neuroimage.2005.02.040 (2005).
Kuhl, P. K. Brain mechanisms in early language acquisition. Neuron 67, 713–727. https://doi.org/10.1016/j.neuron.2010.08.038 (2010).
Pearson, A. & Hodgetts, S. Can cerebral lateralisation explain heterogeneity in language and increased non-right handedness in autism?. A literature review. Res. Dev. Disabil. 105, 103738. https://doi.org/10.1016/j.ridd.2020.103738 (2020).
Groen, W. B. et al. Intact spectral but abnormal temporal processing of auditory stimuli in autism. J. Autism Dev. Disord. 39, 742–750. https://doi.org/10.1007/s10803-008-0682-3 (2009).
Abrams, D. A., Nicol, T., Zecker, S. & Kraus, N. Right-hemisphere auditory cortex is dominant for coding syllable patterns in speech. J. Neurosci. 28, 3958–3965. https://doi.org/10.1523/JNEUROSCI.0187-08.2008 (2008).
Bradshaw, J. L. & Nettleton, N. C. The nature of hemispheric specialization in man. Behav. Brain Sci. 4, 51–63 (1981).
Nora, A. et al. Children show right-lateralized effects of spoken word-form learning. PLoS One 12, e0171034. https://doi.org/10.1371/journal.pone.0171034 (2017).
Prizant, B. M. Language acquisition and communicative behavior in autism: Toward an understanding of the “whole” of it. J. Speech Hear. Disord. 48, 296–307. https://doi.org/10.1044/jshd.4803.296 (1983).
Sanchez-Alonso, S. & Aslin, R. N. Towards a model of language neurobiology in early development. Brain Lang. 224, 105047. https://doi.org/10.1016/j.bandl.2021.105047 (2022).
Mills, D. L., Plunkett, K., Prat, C. & Schafer, G. Watching the infant brain learn words: Effects of vocabulary size and experience. Cogn. Dev. 20, 19–31. https://doi.org/10.1016/j.cogdev.2004.07.001 (2005).
Mills, D. L., Conboy, B. T. & Paton, C. Symbol use and symbolic representation 123–153 (2005).
Larson, C., Thomas, H. R., Crutcher, J., Stevens, M. C. & Eigsti, I.-M. Language networks in autism spectrum disorder: a systematic review of connectivity-based fMRI studies. Rev. J. Autism Devel. Disord. https://doi.org/10.1007/s40489-023-00382-6 (2023).
Knaus, T. A., Kamps, J. & Foundas, A. L. Handedness in children with autism spectrum disorder. Percept. Mot. Skills 122, 542–559. https://doi.org/10.1177/0031512516637021 (2016).
Calder, S. D., Brennan-Jones, C. G., Robinson, M., Whitehouse, A. & Hill, E. How we measure language skills of children at scale: A call to move beyond domain-specific tests as a proxy for language. Int. J. Speech Lang. Pathol. 25, 440–448. https://doi.org/10.1080/17549507.2023.2171488 (2023).
Conti, E. et al. Looking for “fNIRS Signature” in Autism Spectrum: A Systematic Review Starting From Preschoolers. Front. Neurosci. 16, 785993. https://doi.org/10.3389/fnins.2022.785993 (2022).
Rice, M. L. Specific language impairment, nonverbal IQ, attention-deficit/hyperactivity disorder, autism spectrum disorder, cochlear implants, bilingualism, and dialectal variants: Defining the boundaries, clarifying clinical conditions, and sorting out causes. J. Speech. Lang. Hear. Res. 59, 122–132. https://doi.org/10.1044/2015_JSLHR-L-15-0255 (2016).
Bishop, D. V. Overlaps between autism and language impairment: Phenomimicry or shared etiology?. Behav. Genet. 40, 618–629. https://doi.org/10.1007/s10519-010-9381-x (2010).
Kover, S. T. & Abbeduto, L. Toward equity in research on intellectual and developmental disabilities. Am. J. Intell. Dev Disab 128, 350–370. https://doi.org/10.1352/1944-7558-128.5.350 (2023).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31900775), Medical Science and Technology Research Fund of Guangdong Province (A2021363), and Research Fund of Guangdong TCM Bureau (20221372), Guangdong Basic and Applied Basic Research Foundation (2020A1515111034). We thank our participants and their families, as well as the staff who assisted with data acquisition.
Author information
Authors and Affiliations
Contributions
B.L., A.Y., F.Z., and L.Y. contributed to conception and design of the study. B.L., A.Y., S.W., J.X., and S.L. contributed to data acquisition. B.L. performed analysis and prepared first draft of the manuscript. All contributed to data interpretation and review, and approved the submitted work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lai, B., Yi, A., Zhang, F. et al. Atypical brain lateralization for speech processing at the sublexical level in autistic children revealed by fNIRS. Sci Rep 14, 2776 (2024). https://doi.org/10.1038/s41598-024-53128-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53128-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.