Abstract
The article summarizes the activity concentrations data of 226Ra and the sum of uranium isotopes (∑U) in samples of drinking underground water for different regions of Ukraine studied during 1998–2023 in the radiation monitoring laboratory of the State Institution "O.M. Marzieiev Institute of Public Health National Academy of Medical Sciences of Ukraine. Arithmetic mean and standard deviations, minimum and maximum values for 226Ra and ∑U activity concentrations are presented for the entire 1240 sample set and for each region separately. Collected data show that the established state permissible level for drinking water of 1.0 Bq/l is exceeded for 226Ra in 1.1% of the studied samples, and for ∑U—in 3.9% correspondingly. The detected high levels of 226Ra and ∑U activity concentrations correspond to certain regions belonging to the Ukrainian crystalline shield territory. A comparison of the current data with the data of previous studies held during of 1989–1991 indicates a significant difference: for the previous studies the average and standard deviations are much higher. We attribute this to the fact that the centralized sampling of previous studies was random, and it was related exclusively to communal water supply systems. At the same time, the current sample set covers a much larger number of regions, different water consumers; the data set includes the results of repeated studies for a large number of sources, in particular, sources with purified water. Hypothetical exposure doses caused by consumption of 226Ra and ∑U in water for the current sample set were estimated for different age groups for each sample studied, as is, without taking into account the pattern of water consumption. The corresponding dose exceeds the WHO recommended value of 0.1 mSv per year for children under the age of one year for 220 cases (17.7%). This dose limit excess for other age groups corresponds—for children: aged 12–17 years—13.1%, aged 1–2 years—7.4%, 7–12 years old—5.6%, 2–7 years old—3.9% and for adults—4.1%.
Similar content being viewed by others
Introduction
Protection of underground water is commonly much higher than for surface water with regard to chemical and microbiological pollutants, which sets the priority of its use as drinking water source1. At the same time, underground water contains natural radionuclides of the U and Th series. The radioactivity concentration in water is formed by the radioactive composition of the rocks, the level of fissuring of the rocks, the chemical properties of the water and the contact time of the water with the rocks2. Author shoved scatted plot of uranium versus 226Ra in random Australian ground water illustrating the lack of correlation between parent and decay product. The introduction of natural radionuclides into water occurs due to diffusion of gas (222Rn) or leaching (U, Ra) from the surface of rocks. The chemical composition of water defines the intensity and priority of certain radionuclides leaching.
High levels of natural radionuclides in drinking water are observed primarily for artesian wells with a depth of several tens to hundreds of meters, although in some places favorable conditions for the entry of natural radionuclides into water present even for aquifers with a depth of only a few meters or even for surface streams3. In addition, high levels of natural radionuclides in surface water can form discharges from mining and processing industrial enterprises, mines.
Approaches to the national monitoring programs development for radioactivity of potable water and the selection of mandatory components have been formed historically (NRBU-97, EPA, Chen et al. 2018, Vesterbacka et al. 2005)4,5,6,7. Recently worldwide, in particular in Ukraine8, measurement of total alpha and total beta activity has been introduced as an essential element of mass screening of water radioactivity, as it may simplify research, shorten time and save money. At the same time, such studies reveal a special role—a "false alarm" of high alpha activity caused by 224Ra, which is not accompanied by 228Ra9 and the necessity to determine 40 K to exclude its contribution when the total beta activity exceed permissible level.
Drinking water is an important route of natural radionuclides intake into the human body, forming natural exposure, which is difficult to avoid5. The most significant contributors of the water radioactivity are 238U and 234U, 226Ra, 228Ra, 222Rn, 210Pb and 210Po. In most cases, exposure due to the water radioactivity is a combination where main contributors include 222Rn. Natural radionuclides of the uranium series are, as believed, making the main contribution to exposure due to water consumption. 222Rn forms a significant inhalation component of exposure due to its entry into the air of residential premises. Harm to human health from water consumption is caused its radioactivity, in particular, uranium and radium isotopes. At the same time chemical toxicity of uranium, primarily 238U, can wrongly be estimated like in our case, when known is only the ∑U activity concentration without knowing 234U/238U activities ratio10.
Assessments of human exposure to radionuclides contained in drinking water have long be receiving considerable attention worldwide (EPA, Council Directive, 1998, UNSCEAR, 2000, 2013/51/EURATOM, 2013, WHO, 2018)5,11,12,13,14. Relevant studies cover certain geographical areas. They take into account natural landscape features and geology anomalies and cover role of industrial impact on water sources contamination. Study were held to evaluate the radioactivity of water as a commercial raw material15,16,17,18,19,20 or as a market end-product sold in a retail network21,22,23,24. Practically all modern water radioactivity studies include estimation of the irradiation dose to the population caused by this water consumption, focusing in particular on the use of age-dependent dose factors (Council Directive 96/29/Euratom)25.
Regulations of water radioactivity in Ukraine
National radiation safety standard was created in 19974 and it covers natural radioactivity of water: 222Rn—100 Bq/l, 226Ra—1.0 Bq/l, ∑U—1.0 Bq/l. Corresponding water sources to be studied supposed to be wells or groups of wells operating together. Later in 2010, sanitary regulations corresponding to the radioactivity of water were updated as Ref.8, requiring measurement of total alpha- and total beta- activity (permissible levels 0.1 and 1.0 Bq/l, respectively), and only if there is an excess, to do measurement of uranium and radium. 222Rn test requires anyway for every water sample tested in lab. The preparing to release of a number of Ukrainian agricultural and food products to the EU market caused the application of the requirements of the Council Directive 98/83/EC11, and more recently the Council Directive 2013/51/EURATOM13 to the relevant Ukrainian agricultural and processing producers. Thus, the need arose to conduct local relevant studies of the water radioactivity. So in all above requirement options4,8,11,13, we had worked with water radioactivity and accumulated the data of 226Ra and ∑U isotopes activity in drinking water made to order and finally consider them now.
Studies of natural water radioactivity in Ukraine
In Ukraine, the research of natural radionuclides in water for the purpose of geological exploration of minerals was carried out since the 40 s of the last century. Later some geological features of 222Rn and 226Ra formation and distribution in the ground water were studied and described by Ref.4.
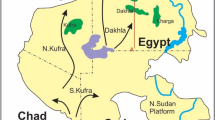
Our previous studies of 222Rn in drinking water show us significant dose forming role, especially when water supplied into residential premises, which made it possible for us understand and determine the water-to-air transfer coefficient26. The results of comprehensive hygienic studies of natural radionuclides radioactivity in a statistically weighted sample set of drinking water samples (> 1400 samples) in Ukraine conducted in the early 1990s were presented by Ref.1. These studies covered five regions within the Ukrainian Crystalline Shield27, see Fig. 1, and three regions outside of it, for comparison. The obtained data proved the extreme heterogeneity of the natural radionuclides concentration in underground waters of different regions of Ukraine. The maximum activity of 222Rn for the territories belonging to the Ukrainian shield reached 2660 Bq/l compared to 195 Bq/l outside the shield. For 226Ra, the maximum values on the shield’s territory were 5.3 Bq/l compared to 0.24 Bq/l outside the shield, and for uranium, the maximum values reached 21.3 Bq/l on shield, compared to 0.77 Bq/l outside the shield (Zelenskyi et al., 1993)2. So, authors showed that the activity of natural radionuclides in water is significantly higher within the Ukrainian Crystalline Shield than outside of it.
Tectonic structure of Ukraine: 1. Ukrainian Shield; 2. Kovel salient; 3. Volyn-Podilla plate; 4. Carpathian fold and thrust belt; 5. West-European platform; 6. Dnieper-Donets rift; 7. Voronezh massif; 8. Donets fold belt; 9. Black Sea depression; 10. Scythian plate; 11. Crimean fold and thrust belt27.
These data1 actualized the existing problem, encouraged us to consider natural radioactivity of artesian water as an important factor in shaping the exposure of the population of Ukraine and became the basis for the development of national radiation standards for drinking water4,8. The data and the given estimates of expected exposure doses attracted attention even during their presentation at the conference1, and they became the background for the discussion of the following results presented later28,29,30,31.
Among sample set of1, about 100 was investigated for Th and U using inductively coupled mass spectrometry10. Corresponding data confirmed the data1 on the uranium isotopes concentration and, in addition, drew attention to the high 234U/238U activity ratio, which sometimes reached here level of 10–20 and higher.
Recently, we summarized the radon concentration data in water studied for the period 1998–202232, where estimates were made for the entire sample set and for its parts corresponding to different regions of Ukraine and, in addition, obtained for separate consecutive time intervals.
In addition, we had recently received interesting signal results33 in the course of water research accompanying the installation of individual water purification systems in private homes in the city of Zhytomyr and suburb, an area that belongs to the Ukrainian Crystalline Shield27. According to the results of the study of 20 samples taken with the consent of the owners, in 17 cases (85%) the level of 222Rn exceeds 100 Bq/l, when uranium only in 2 cases (10%), and 226Ra only in 1 case (5%) exceed the permissible level of 1.0 Bq/l4,8.
Methods
Water sample set
Here we have been analyzing the data of natural radionuclide activity concentration in drinking water from the territory of several regions of Ukraine, which have been studied out since 1998 at the request of the owners regarding water compliance with the requirements of the standards4,8, as well as lately European legislation11,13, around 150 samples.
Part of the samples of the general sample set was studied according to indicators of total alpha and beta activity31,34, however, in the vast majority of samples we studied 226Ra and ∑U. So, in this work, we consider the activity concentration of 226Ra and the ∑U in 1240 water samples.
A subset of 62 water samples data was also presented here, which were recently studied to support the implementation of individual water purification systems in the Zhytomyr region as a development of the previously presented research33.
Methods of determining activity concentration
To determine the activity concentration of 222Rn, 226Ra and the sum of uranium isotopes, we had used liquid scintillation spectrometer Quantulus 1220™ and extractive sample preparation methods. Our scintillation "cocktail" was based on PPO (4 g/l) and POPOP (0.1 g/l) dissolved in toluene or o-xylene.
For 222Rn measurement we initially place 10 ml of our scintillation "cocktail" in 20 ml glass vial, and then we placed there 10 ml of the water sample. We cover and close vial tightly and shake it for a minute. Sample is ready for measurement after 10 min of equilibration of two-phase sample, we always used the part of the alpha spectrum corresponding to 222Rn + 218Po. In 2019, we participated in comparative tests for radon measurements in water (Jobbagy et al. 2019, Lab code #27)35. We obtained acceptable results, both for water miscible and immiscible scintillator used especially considering that the water sample arrived at the laboratory with a delay of more than 7 days.
When calculating the 222Rn activity of the samples (see Formula (1)), we took into account the actual water sample volume (V = 10 ml) and the 222Rn decay for the time interval since sampling to the moment of measurement.
ARn—222Rn activity concentration; CPM—Sample count rate (count per minute); BG—Background sample count rate (count per minute); ΔT—Sample delay time interval, since sampling to counting; 0.693 = log(2); E—Counting efficiency, for counting window corresponding to 222Rn + 218Po (~ 200%); V—Corresponding water sample volume, L.
To determine 226Ra activity concentration, we used 100–200 ml of water, which we evaporate to dryness in heat resistant glass vessel. Evaporation was performed with heat above middle of electric plate or using of sand bath. The residue we dissolve in 4 ml (2 ml) of a 1 M HNO3 solution, and then put it into a Teflon vial (20 ml or 7 ml). Finally we rinsed the glass vessel again with 4 ml (2 ml) of distilled water, which was also introduced into the same Teflon vial where then we placed at the end 12 ml (4 ml) of mentioned above scintillation "cocktail", which is immiscible with water. The Teflon vial was closed with a tight cap, and the measurement of 222Rn accumulated in the solvent with 226Ra was carried out in two phase sample no earlier than after 7–10 days29.
When calculating the 226Ra activity of the samples (see Formula (2)), we took into account the actual volume of the water sample used and the 222Rn/226Ra equilibrium coefficient achieved at the time of measurement, which, for the above mentioned time interval, was 72–84%.
ARa—226Ra activity concentration; CPM—Sample count rate (count per minute); BG—Background sample count rate (count per minute); ΔT—Sample delay time before counting; 0.693 = log(2); E—Counting efficiency which depends on width of counting window corresponding to 222Rn + 218Po (~ 200%) or 222Rn + 218Po + 214Po (~ 300%); V—Corresponding water sample volume, L.
Periodically, we check the quality of sample material washing 226Ra from the walls of the glass beaker in the process of sample preparation after evaporation of water to dryness. For this, we use two identical washings of the sample from the beaker (see above), performed one after the other. The recently performed test showed that when the sample is concentrated from 100 ml of mineralized water, which has a dry residue of 1.12 g per liter, 3.3% ± 0.9% of the activity of the sample remains in the second consecutive sample (we had used 3 repeats). Such a high amount of dry residue is more characteristic for mineral water, but sometimes it is also occur in the studied drinking water. We used a similar control procedure while the participation in laboratory intercomparison tests on measurements of total alpha- and total beta- activity of two water samples (dry residue of 0.37 and 0.96 g/l) organized by the JRC EC, our Lab code #1753736.
It should be added that for certainty in the results of measurements of 226Ra in water, it is very important to test Teflon vials for their tightness. For this we determine the individual loss of the organic phase mass of the sample during the time before its measurement (7–10 days), or in a separate check of the set of vials, without water phase, when the entire volume is occupied by the organic phase, and the waiting period is 3–5 weeks.
To study the activity concentration of ∑U, we used the method described earlier29. It includes the water sample pre-concentration from 1 L, for which to the water sample in heat-resistant beaker we added 5 ml of FeCl3 solution (~ 10 mg/ml on iron) and 5 ml of HNO3 and brought it to a boil. Next, we added 10 ml of ammonia solution (25%) to the beaker for co-precipitation of uranium with iron hydroxide. We cooled the water at air, and after about 1 h, the precipitate was first vacuum filtered on a paper filter, and then it was dissolved in 40 ml of 6 M HNO3 solution.
Next, uranium was extracted (3 min) in 10 ml of a 20% solution of tributyl phosphate in toluene or o-xylene. The obtained organic phase was washed twice (3 min each) from acid residues with 20 ml of 6 M NH4OH solution, and finally bubbled with argon (3 min). For measurements, the obtained sample was placed in a 20 ml plastic vial, where 10 ml of the scintillation "cocktail" mentioned above was added. The average chemical yield of uranium was 65 ± 3%. For each sample, we monitored losses of extracting solution by weighing the organic phase after the preparing was complete. We calculated the activity of the samples according to Formula (3).
AU—activity concentration for ∑U; CPM—sample count rate (count per minute); BG—background count rate (count per minute); Cy—base extraction empirical parameter (65%) defined by 20% TBP in toluene extraction solution and water (HNO3) to organic phases ratio 40: 10; mei—initial mass of extraction solution added; me—final mass of extraction solution extracted; E—counting efficiency (~ 100%); V—Water sample volume, L.
The extraction method we use for uranium measurements is implemented under the condition of highly selective co-precipitation of uranium with iron hydroxide at pH 9, which gives a moderate extraction efficiency of about 65%. For quality control and determining the efficiency of the method, a calibrated solution of 232U (1.0 Bq/ml) and/or a laboratory solution of uranyl nitrate are used. Freshly extracted sample, 232U gives a single high-resolution alpha peak (without daughter products), which we use to determine the efficiency of extraction, and later the spectrum changes with the appearance and accumulation of daughter products. Under using uranyl nitrate, we have the opportunity to determine its activity in a water solution sample, when the extracted sample shows, in particular, how selectively uranium is extracted in relation to 234Th and 234 Pa (both beta emitters) and we also use it to determine the efficiency of extraction. This is the depleted uranium calibration sample we use, and it has a ratio of 234U/238U < 1.0, which is different from what we observe for groundwater samples.
When using the above specified sample preparation scenarios and taking into account a measurement time of 180 min, the minimum detected activity (MDA) is equal to 0.004 Bq/l for uranium and 0.005 Bq/l for 226Ra, according to formulae Formula (4)37. MDA for 222Rn measurement calculated for 20 min was 0.5 Bq/l.
MDA—minimum detected activity; BG—background count rate, CPM (0.15 for ∑U and 0.01 for 226Ra); T—counting time, min; M—sample volume, L; Error—uncertainty, percent (30%); 60—time units conversion factor, sec/min; Eff—counting efficiency.
Usually, while Quantulus 1220™ measurements, we use the parameter of Pulse Shape Analyzer (PSA) equal 60 for 222Rn and 226Ra measurements in a two-phase sample (Teflon vial) and PSA = 30 for measurements of uranium (plastic vial), but in some cases when samples were quenched, measurements had to be repeated under other PSA conditions. We should note that in recent years it was mainly used o-xylene as a solvent.
Methodology of dose estimation
Annual dose Dx for age group x is calculated from the expression, see Formula (5):
where: Cx is the annual water consumption for an age group x (L/a), Ai is the activity concentration of nuclide i (Bq/L), (DCF)ix is the dose conversion factor (dose coefficient) for nuclide i and age group x (mSv/Bq).
The lifetime average annual dose associated with a water resource is calculated from the expression, see Formula (6):
where: D is the lifetime average annual dose (mSv/a), Ai is the activity concentration of nuclide i (Bq/L), Fi is a proportionality constant for nuclide i with units of (mSv/a) per (Bq/L).
The proportionality constant Fi for nuclide i was determined from the following relationship, see Formula (7):
where: Cx is the annual water consumption for an age group x (L/a), Wx is a weighting factor for age group x.
The weighting factor for each age group was determined by dividing the number of years in the age group by the average life expectancy, taken to be 70 years. For example, the weighting factor for the 7–12 years age group was, see Formula (8):
The results and discussion
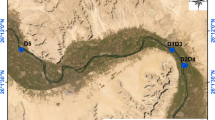
Figure 2 shows how water sample set covers territory of Ukraine. It is clearly seeing that most representative are three regions, closest to laboratory location: Kyiv, Zhytomyr and Vinnytsia, which are located on Ukrainian Shield27.
Water sample set by regions on map of Ukraine, where we use map38 as a blank.
Radioactivity of water samples
In Table 1 are given generalized data (number, sampling period, average, standard deviation, median, interquartile range, minimum and maximum) for 226Ra and ∑U activity concentration in total set and by region, taking into account the corresponding sample number for each region (descending order). Maximum values exceeding the permissible level of 1.0 Bq/l according to national radiation safety standard (NRBU-97 and SanPiN-2010)4,8 are highlighted in bold.
Support of installation of water purification systems in Zhytomyr city and suburb
Zhytomyr region generalized data (average, standard deviation, maximum), according to the Table 1, were among highest both for 226Ra and ∑U activity concentration. Earlier, we mentioned how impressive are our data presented recently33 obtained when implementing water purification systems (Zhytomyr and suburb). Here we consider separately an extended set of 62 samples, see Table 1, Figs. 3 and 4. It is noteworthy that the subset has average and standard deviation close to the general Zhytomyr region set for the entire observation period.
Individual sample activity concentrations pairs plot for 226Ra and ∑U (Bq/l). Vertical and horizontal lines set frame of 1.0 Bq/l permissible level (SanPiN)8.
When evaluating this data subset, we sorted the obtained results of the activity concentration of 222Rn, 226Ra, ∑U, as well as the sum of 226Ra + ∑U (each separately, ascending order) and placed them on the Fig. 3. For ease of evaluation, we built horizontal lines corresponding to the permissible levels of (SanPiN-2010)8: 100 Bq/l—for 222Rn, 1.0 Bq/l—for 226Ra and for the ∑U and 0,1 Bq/l—for the calculated sum 226Ra + ∑U.
According to the data shown on Fig. 3 priority of this particular research is 222Rn, as far as the 222Rn activity concentration exceeds the permissible level of 100 Bq/l for the vast majority of samples (71%), when permissible level for 226Ra is exceeded in 3 cases (< 5%), and for ∑U—in 6 cases (< 10%). At the same time, Fig. 3 show that sum 226Ra + ∑U in only 15 cases (< 25%) is below the level of 0.1 Bq/l, which indicates that it is impractical to investigate the total alpha activity for such a set of research.
Figure 4 shows 226Ra and ∑U activity pairs for each of the 62 samples, with the horizontal and vertical lines limiting the permissible level of 1 Bq/l. Individual pairs of 226Ra and ∑U activity show no correlation between parent and daughter radionuclides, similar to mentioned above2.
Dose estimation for water samples
We consider hypothetical exposure dose and for this, each sample corresponds to separate water source. For the dose assessment we use age-dependent dose coefficients for ∑U and for 226Ra for six age groups of the population (Council Directive 96/29/EURATOM)25, see Table 2. Table 3 shows generalized dose data for each age group, when at a Fig. 5 are shown dose distributions (ascending order). Each curve correspond to one age group, from top to bottom: children under 1 year, adolescents 12–17 years old, children aged 1–2 years, children aged 7–12 years, adults. The last two practically coincide visually.
Distribution (ascending order) of irradiation doses, which were calculated for individual water samples and for representatives of different age groups. Curves correspond to age groups, from top to bottom: children under 1 year, adolescents 12–17 years, children aged 1–2 years, children aged 7–12 years, adults and children 2–7 years old.
According to Table 3 dose excess of WHO recommended level of 0.1 mSv per year is varying from 3.9% to 17.7% of total sample set depending on age group.
It should be mentioned that this data set includes 150 tests that were carried out for industrial customers to check compliance water radioactivity with the requirements of the Council Directive 98/83/EC. Here some other radionuclides were also investigated. For this we calculated corresponding indicative dose for adults, it showed no any excess of the WHO recommended 0.1 mSv per year.
Data comparison
At first we compare our current 226Ra and ∑U data with a previous study1 held in early 1990st—now 1240 compared to 520 samples then. Total sets data are comparable by maxima and maxima for some of the regions belonging to Ukrainian shield: Kyiv, Ghytomyr, Vinnytsia27. Different minima caused by presence in current set purified water, different (wider) geography of sampling and application of more sensitive and precise methods in our current study.
When comparing our data to data of other locations, we consider at first national wide studies. Thus, Sweden according39, have many geological oddities including environmental radioactivity, and there radioactivity accompanying drinking water as well. Water radioactivity is relatively low in dug wells, when drilled wells give moderate activities of 222Rn—203 ± 86 Bq/l, 226Ra—0.09 ± 0.01 Bq/l and Uranium—14.3 ± 4 μg/L as average in normal Precambrian rocks. Only uranium-rich granites and pegmatites give high 222Rn—300–4000 or 18,000–55,000 Bq/l, 226Ra—0.05–0.8 or 0.35–2.5 Bq/l and U—up to 268 μg/L.
Similarly in Finland according to Asikainen40 average water radioactivity at waterworks is relatively low—25 Bq/l of 222Rn and 0.004 Bq/l of 226Ra in average. Higher radioactivity of drilled wells—630 Bq/l of 222Rn and 0.11 Bq/l of 226Ra in average, when considering drilled wells they may contain abnormally high U up to 21 mg/l or even higher. Later Vesterbacka41 estimate the mean annual effective dose from natural radionuclides for users of drilled wells to be 0.41 mSv, for users of wells dug in the ground 0.05 mSv and for people using water from waterworks 0.02 mSv. Main role as dose forming factor in wells water play 222Rn forming up to 60–75% of irradiation. Most of dose by long-lived radionuclides cause 210Pb and 210Po, when 226Ra and 228Ra role is minor.
In Estonia42 activity concentration of 226Ra and 228Ra considered as highest contributors to the total indicative dose (TID) for waters of Cambrian-Vendian (Cm-V) aquifer. In study report43, it was concluded, that about 91% of Cm-V aquifer consumers (20% of the Estonian population) obtain high doses, i.e. TID exceeding 0.1 mSv/y.
The activity concentrations of 238U, 234U and 210Po in drinking water from certain sources in South-Central Bulgaria reported recently by Ref.44. The results obtained varied from 79 to 826 mBq/l for 238U, 130 to 1623 mBq/l for 234U, < 0.5 to 25.5 mBq/l for 210Po. Corresponding annual effective dose varied from 8.9 to 62.5 μSv/y with a mean of 30.1 μSv/y. It is below the individual dose criterion of 100 μSv/y. The highest contribution to the annual effective dose was found to be 234U.
In Ireland during of 2007–2011 243 public water supply sources was tested45 and in all total beta fit requirement 1.0 Bq/l, actual max 0.55 Bq/l results of total alpha in 175 cases (86%) comply with limit 0,1 Bq/l. Among 14% samples highest of alpha is 0.24 Bq/l. 222Rn in all samples is below 500 Bq/l—national recommended level, while highest is 345 Bq/l. no correlation observed between uranium or 222Rn with alpha activity of samples exceeding 0.1 Bq/l.
In Poland drinking water radioactivity and corresponding irradiation is warring wide from low for Warsaw46, where considered 210Po, 234U and 238U isotopes as for surface 0.12, 3.91 and 2.75 mBq/l and for deep-water intakes were 0.25, 0.24 and 0.20 mBq/l, respectively. The annual dose absorbed because of the consumption of drinking water by an inhabitant is below 0.5 μSv/y. Studies of natural radioactivity in spring water in the Sudety Mountains47, where uranium exploration was conducted in the early 1950s. Annual effective doses due to radionuclide intake calculated for 4 out of 20 spring waters used for consumption by spa patients and inhabitants without 222Rn were of the range 0.4 μSv to 9.2 μSv, for patient for of a 20-day duration stay and from 1.3 μSv/y to 26.7 μSv/y for an inhabitant. The contribution of radon consumed with water raises these values to 209.4 μSv per 20 days and 608.3 μSv/y for a patient and inhabitant, respectively. This shows how water ingestion dose may highly varying in certain places depending on considered factors of irradiation and for different categories of public.
Geological conditions bring certain composition of natural radionuclides into the groundwater, which cause corresponding internal human exposure when opportunities for consumption of this water created.
Conclusions
The aim of our work was generalizing the activity concentration of 226Ra and the ∑U in a set of 1240 drinking water samples tested in the laboratory for the period 1998–2023.
Comparison of the data set with previous studies2 shows that the sample set is wider in terms of sampling locations; the averages here are lower, although the sample resembles the previous one in terms of regional maxima.
The consolidated table clearly shows the regions where the concentration of activity is higher, in particular, where the regulations exceeded.
The given data show the expediency of researching water for radioactivity when assessing the need for water purification in high radiation areas, in particular, for Zhytomyr, where excess of permissible level was 71% for 222Rn, 5%—for 226Ra, and 10%—for ∑U.
The assessment of the average annual irradiation dose from lifetime water consumption performed for the sample set shows that the WHO recommended dose limit (0.1 mSv per year) exceeded here for 76 cases or 6.13% (Supplementary Information).
References
Zelensky, A., Buzinny, M., Los, I. (1993). Measurement of Radium-226, Radon-222, and Uranium-238, 234 in Underground Water of the Ukraine with ultra-low-level liquid scintillation counter. Radiocarbon, 405–411. Google scholar: https://scholar.google.com.ua/scholar?oi=bibs&cluster=870539071946204174&btnI=1&hl=en.
Dickinson B.L. Chapter 4–2. Radium in groundwater. In: The environmental behaviour of radium. — Vienna : International Atomic Energy Agency, 2014. Series: Technical reports series (International Atomic Energy Agency), ISSN 0074–1914 ; no. 310. 335–372.
Izotopy radiya i radon v prirodnykh vodakh (Radium isotopes and radon in natural waters). Gudzenko, V.V.; Dubinchuk, V.T.; Polyakov, V.A. (eds.) AN SSSR, Moscow; AN Ukrainskoj SSR, Kiev. Google scholar: https://scholar.google.com.ua/scholar?hl=en&as_sdt=0,5&cluster=733593895605204006.
Radiation safety standards of Ukraine (NRBU-97): state health and safety standards, Kyiv, 1997, 121. Google scholar: https://scholar.google.com.ua/scholar?oi=bibs&cluster=270268710775278472&btnI=1&hl=en.
EPA: Natural Radionuclides in Public Drinking Water. URL: https://www.epa.gov/radtown/natural-radionuclides-public-drinking-water.
Chen, J., Cooke, M. W. & Mercier, J.-F. A review of natural radionuclides in Canadian drinking water (1975–16). Radiat. Prot. Dosimetry 179(1), 26–36. https://doi.org/10.1093/rpd/ncx204 (2018).
Vesterbacka, P., Mäkeläinen, I. & Arvela, H. Natural radioactivity in drinking water in private wells in Finland. Radiat. Prot. Dosimetry 113(2), 223–232. https://doi.org/10.1093/rpd/nch446 (2005).
Hygienic requirements for drinking water intended for human consumption: state health and safety standards and rules DSanPiN 2.2.4-171-10, Кyiv, 2010, 32. URL: https://zakon.rada.gov.ua/laws/show/z0452-10#Text.
Zoltan Szabo, Vincent T. De Paul, Thomas F. Kraemer, and Bahman Parsa. Occurrence of Radium-224, Radium-226, and Radium-228 in Water of the Unconfined Kirkwood-Cohansey Aquifer System, Southern New Jersey. Scientific Investigations Report 2004–5224. Department of the Interior Geological Survey. 101 p. https://pubs.usgs.gov/sir/2004/5224/.
Shiraishi, K. et al. Concentrations of thorium and uranium in freshwater samples collected in the former USSR. J. Radioanal. Nucl. Chem. Art. 185, 157–165. https://doi.org/10.1007/BF02042962 (1994).
Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Official Journal of the European Community, 1998, L330/32.
Sources and effects of ionizing radiation: UNSCEAR report/United Nation Scientific Committee on the Effects of Atomic Radiation. New York, 2000, Annex A, 93–108.
Council Directive 2013/51/EURATOM of 22 October 2013 laying down requirements for the protection of the health of the general public with regard to radioactive substances in water intended for human consumption. Official Journal of the European Community, 2013, L296/12.
Management of Radioactivity in Drinking-Water. Geneva: World Health Management of Radioactivity in Drinking Water. Geneva. World Health Organization, 2018, 124 p. https://www.who.int/publications/i/item/9789241513746.
Nuccetelli, C., Rusconi, R. & Forte, M. Radioactivity in drinking water: Regulations, monitoring results and radiation protection issues. Ann. Ist. Super Sanita 48(4), 362–373. https://doi.org/10.4415/ANN_12_04_04 (2012).
Beyermann, M., Bünger, T., Schmidt, K. & Obrikat, D. Occurrence of natural radioactivity in public water supplies in Germany: 238U, 234U, 235U, 228Ra, 226Ra, 222Rn, 210Pb, 210Po and gross α activity concentration. Radiat. Prot. Dosimetry 141(1), 72–81. https://doi.org/10.1093/rpd/ncq139 (2010).
Wallner, G. & Steininger, G. Radium isotopes and 222Rn in Austrian drinking waters. J. Radioanal. Nucl. Chem. 274(3), 511–516. https://doi.org/10.1007/s10967-006-6939-4 (2007).
Forte, M., Bagnato, L., Caldognetto, E., Risica, S. & Rusconi, R. Radium isotopes in Estonian groundwater: Measurements, analytical correlations, population dose and a proposal for a monitoring strategy. J. Radiol. Prot. 30, 761–780. https://doi.org/10.1088/0952-4746/30/4/009 (2010).
Dowdall, A., Currivan, L., Hanley, O. (2013). Radioactivity levels in groundwater sources in Ireland. Radiological Protection Institute of Ireland. https://inis.iaea.org/collection/NCLCollectionStore/_Public/44/122/-44122590.pdf?r=1&r=1.
Przylibski, T. A. et al. 222Rn and 226Ra activity concentrations in groundwaters of southern Poland: New data and selected genetic relations. J. Radioanal. Nucl. Chem. 301, 757–764. https://doi.org/10.1007/s10967-014-3215-x (2014).
Martín Sánchez, A., Rubio Montero, M. P., Gómez Escobar, V. & Jurado, V. M. Radioactivity in bottled mineral waters. Appl. Radiat. Isot. 50(6), 1049–1055. https://doi.org/10.1016/s0969-8043(98)00126-2 (1999).
Fatima, I., Zaidi, J. H., Arif, M. & Tahir, S. N. Measurement of natural radioactivity in bottled drinking water in Pakistan and consequent dose estimates. Radiat. Prot. Dosimetry 123(2), 234–240. https://doi.org/10.1093/rpd/ncl093 (2007).
Buzynnyi, M. & Mykhailova, L. Radioactivity of bottled water from trade network in Kyiv. Nucl. Radiat. Saf. 4(88), 77–80. https://doi.org/10.32918/nrs.2020.4(88).10 (2020).
Piñero-García, F., Thomas, R., Mantero, J., Forssell-Aronsson, E. & Isaksson, M. Radiological impact of naturally occurring radionuclides in bottled water. Food Control 130, 108302. https://doi.org/10.1016/j.foodcont.2021.108302 (2021).
Council Directive 96/29/Euratom of 13 May 1996 laying down basic safety standards for the protection of the health of workers and the general public against the dangers arising from ionizing radiation. Official Journal of the European Communities, 1996, L159/1.
Zelensky, A., Buzinny, M. & Los, I. Radon-222 in water: Concentrations, doses, standardization. Problems Radiat. Med. 5, 573–581 (1993).
Map of Tectonic structure of Ukraine: Author: Alex Tora - based on CC BY-SA 3.0 URL: https://commons.wikimedia.org/w/index.php?curid=8341663.
Buzynnyi, M. (2004). Natural radioactivity of drinking water wells in Ukraine. Hygienic science and practice at the turn of the century: materials of the XIV Congress of Ukrainian Hygienists (May 19–21, 2004, Dnipropetrovsk). Kyiv, 308–310. Google scholar: https://scholar.google.com.ua/scholar?oi=bibs&cluster=1393528209829818671&btnI=1&hl=en.
Buzinny, M., Sakhno, V., Romanchenko, M. (2010). Natural radionuclides in underground water in Ukraine. LSC 2010. Advances in Liquid Scintillation Counting, edited by Philippe Cassette, 81–85. Google scholar: https://scholar.google.com.ua/scholar?oi=bibs&cluster=1544418838744645941&btnI=1&hl=en.
Buzynnyi, M., Mykhailova, L., Sakhno, V. & Romanchenko, M. Study of natural radionuclides in underground water in Ukraine. Environ. Health 1, 31–35 (2011).
Mykhailova, L., Buzynnyi, M., Sakhno, V. & Romanchenko, M. Statistical analysis of radiation parameters of water investigated in 2012–2014. Hyg. Settl. 65, 179–185 (2015).
Buzynnyi, M. & Mykhailova, L. Generalized data for 20 years of radon-222 monitoring in drinking water of Ukraine. Nucl. Radiat. Saf. 4(96), 29–38. https://doi.org/10.32918/nrs.2022.4(96).04 (2022).
Buzynnyi M.G., Mykhailova L.L., Bondar M.O., Cherniak O.V. Considering of radioactivity of the water during of its purification in private house holdings of Zhytomyr. A collection of abstracts of reports of a scientific and practical conference with international participation for the 140th anniversary of the birth of O.M. Marzieiev (19th Marzieiev readings). 2023. Issue 23, 131–132. http://www.health.gov.ua/www.nsf/all/u03-06-02-12?opendocument.
Buzynnyi, M. G. & Mykhailova, L. L. Total Alpha activity and Radon-222 activity in the underground water of some regions of Ukraine. Environ. Health 2(99), 36–44. https://doi.org/10.32402/dovkil2021.02.036 (2021).
Jobbagy V., Hult M. Performance evaluation report of the REM 2018 radon-in-water proficiency test. European Commission, Geel, 2019, JRC116812. https://publications.jrc.ec.europa.eu/repository/handle/JRC119713.
Jobbagy, V., Dupuis, E., Emteborg, H. and Hult, M., REM 2019 proficiency test on gross alpha/beta activity concentration in drinking water, EUR 30822 EN, Publications Office of the European Union, Luxembourg, 2021, ISBN 978-92-76-41388-2, https://publications.jrc.ec.europa.eu/repository/handle/JRC121498.
O.A. Bondarenko, A.V. Zelensky, V.S. Repin. Ways to improve and develop the use of 90Sr beta-spectrometric method after the accident at the Chernobyl nuclear power plant. In: Problems of radiation medicine. Republican interdepartmental collection. Issue 5. Kyiv. 1993.- pp. 44–47.
File:Map of Ukraine political simple blank.svg. Author: Sven Teschke. - based on CC BY-SA 3.0 URL: https://commons.wikimedia.org/wiki/File:Map_of_Ukraine_political_simple_blank.svg.
Aakerblom, G., Falk, R., Lindgren, J., Mjoenes, L., Oestergren, I., Soederman, A.L., Nyblom, L., Moere, H., Hagberg, N., Andersson, P., & Ek, B.M. Natural radioactivity in Sweden, exposure to internal radiation (SSI—2005-15). Rahola, T. (Ed.). Sweden (2005).
Asikainen, M. & Kahlos, H. Natural radioactivity of drinking water in Finland. Health Phys. 39(1), 77–83. https://doi.org/10.1097/00004032-198007000-00009 (1980).
Vesterbacka, P. Natural radioactivity in drinking water in Finland. Boreal Environ. Res. 12(1), 11–16 (2007).
Kiisk, M., Suursoo, S., Isakar, K. & Koch, R. Relevant radionuclides in Estonian drinking and ground waters—Measurement techniques and activity concentrations. Radioprotection 46(6), S107–S112. https://doi.org/10.1051/radiopro/20116826s (2011).
Forte M., Rusconi R., Trotti F., Caldognetto, E., Airoldi R., Realini F., Risica S., Bagnato L.,.“Estimation of concentrations of radionuclides in Estonian ground waters and related health risks”, Final Report. Milano: Agenzia Regionale per la Protezione dell’Ambiente della Lombardia (Twinning Light Contract EE06-IB-TWP-ESC-03, 2010).
Tonev, D. et al. Investigation of natural radioactivity in drinking water sources in South-Central Bulgaria. J. Radioanal. Nucl. Chem. 332, 4641–4649. https://doi.org/10.1007/s10967-023-08983-5 (2023).
Dowdall A., Currivan L., Hanley O. Radioactivity levels in groundwater sources in Ireland. Radiological Protection Institute of Ireland, 2013. URL : https://inis.iaea.org/collection/NCLCollectionStore/_Public/44/122/44122590.pdf?r=1&r=1.
Sekudewicz, I. & Gąsiorowski, M. Determination of the activity and the average annual dose of absorbed uranium and polonium in drinking water from Warsaw. J. Radioanal. Nucl. Chem. 319, 1351–1358. https://doi.org/10.1007/s10967-018-6351-x (2019).
Kozłowska, B., Walencik, A. & Dorda, J. Natural radioactivity and dose estimation in underground water from the Sudety Mountains in Poland. Radiat. Prot. Dosimetry 128(3), 331–335. https://doi.org/10.1093/rpd/ncm380 (2008).
Acknowledgements
The authors are sincerely grateful to Tsygankov M.Ya. for his performing of uranium radioactivity analyses for 1998-2003, to Zvarych S.I. for the development and implementation of the extractive method for water samples preparing for measuring the uranium radioactivity, and the staff of the radiation monitoring laboratory for conducting research on the radioactivity of water.
Author information
Authors and Affiliations
Contributions
L.M. collects all data from our databases. M.B. prepares generalized tables and figures. L.M. and M.B. wrote the main text and discuss it. All authors reviewed the manuscript. M.B. prepares English version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buzynnyi, M., Mykhailova, L. Long term studying of uranium and radium-226 activity in drinking water in some regions of Ukraine and assessment of corresponding hypotetical irradiation doses. Sci Rep 14, 2530 (2024). https://doi.org/10.1038/s41598-024-53079-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53079-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.