Abstract
Radiotherapy with deep inspiration breath hold (DIBH) reduces doses to the lungs and organs at risk. The stability of breath holding and reproducibility of tumor location are higher during expiration than during inspiration; therefore, we developed an irradiation method combining DIBH and real-time tumor-tracking radiotherapy (RTRT) (DBRT). Nine patients were enrolled in this study. Fiducial markers were placed near tumors using bronchoscopy. Treatment planning computed tomography (CT) was performed thrice during DIBH, assisted by spirometer-based device. Each CT scan was fused using fiducial markers. Gross tumor volume (GTV) was contoured for each dataset and summed to create GTVsum; adding a 5-mm margin around GTVsum generated the planning target volume. The prescribed dose was mainly 42 Gy in four fractions. The treatment plan was created using DIBH CT (DBRT-plan), with a similar treatment plan created for expiratory CT for cases for which DBRT could not be performed (conv-plan). Vx defined as the volume of the lung received x Gy, and the mean lung dose, V20, V10, and V5 were evaluated. DBRT was completed in all patients. Mean dose, V20, and V10 were significantly lower in the DBRT-plan than in the conv-plan (all p = 0.003). Mean rates of decrease for mean dose, V20, and V10 were 14.0%, 27.6%, and 19.1%, respectively. No significant difference was observed in V5. We developed DBRT, a stereotactic body radiation therapy performed with the DIBH technique; it combines a spirometer-based breath-hold support system with an RTRT system. All patients who underwent DBRT completed the procedure without any technical or mechanical complications. This is a promising methodology that may significantly reduce lung doses.
Similar content being viewed by others
Introduction
Lung tumors can move significantly during respiration. Unless countermeasures are taken, irradiating a tumor that moves with respiration requires that the irradiated area include the entire path of the moving tumor, resulting in a large irradiation volume. Respiratory motion management (RMM) is used to avoid this1. RMM plays an important role in stereotactic body radiation therapy (SBRT), which requires high accuracy. Breath hold (BH), one of several RMM techniques, can be performed using the position of the chest or abdominal wall, the shape of the body surface, or the volume of lung ventilation as indicators2,3,4,5,6,7,8. Methods using ventilation volume as the indicator are more accurate than those using chest or abdominal wall position or body surface shape9,10. BH is more stable and shows greater reproducibility of tumor location in expiration than in inspiration11,12. Therefore, expiration is often employed when irradiation is performed using the BH technique13.
Real-time tumor-tracking radiation therapy (RTRT) is a highly accurate type of RMM14,15. We used an RTRT system with a tracking error of < 0.4 mm16. This method tracks, in real time, fiducial markers (FMs) placed near the tumor to determine the tumor location: irradiation is performed only when the FM is present at a defined position—called a gating box—in three dimensions. The FM is used as an index; because the marker is placed in the body, it has a higher correlation with the position of the tumor than indexing methods that use external information such as the position of the chest or abdominal wall and the body surface17. The speed at which tumors move due to respiration is fast during inspiration and slow during expiration18. In RTRT, there is a slight beam-on delay from the time the marker enters the gating box until the start of irradiation16. As a result, the distance the tumor moves during the beam-on delay is larger during inspiration than during expiration, resulting in a large margin that needs to be added. Therefore, in RTRT, irradiation is performed at the end of expiration, and irradiation with RMM often involves the expiratory phase. However, during expiration, the lung collapses. This collapse may result in a relative increase in lung dose that leads to more adverse events occurring. The lung dose can be reduced if irradiation is performed during inspiration, especially deep inspiration BH (DIBH). Performing SBRT with DIBH requires high positional accuracy; therefore, a high reproducibility of the tumor location must be ensured. Thus, we developed DIBH RTRT (DBRT), a method of irradiation that uses RTRT during DIBH with ventilation volume as the indicator.
Methods
Patients
All procedures performed in this study, which involved human participants, were conducted according to the ethical standards of the institutional review board and the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained from each patient before radiotherapy. Patients who underwent SBRT between May 2022 and January 2023 for lung tumors 5 cm or smaller in size with respiratory motion greater than 10 mm and N0M0 disease were included; respiratory motion was measured using four-dimensional computed tomography (CT). Patients who had difficulty holding their breath for approximately 20 s and those with active interstitial pneumoniae were excluded.
CT simulation
Prior to treatment planning, three to four FMs were implanted near the tumor using a bronchoscopy. The FMs were spherical Disposable Gold Marker (Olympus Medical Systems, Japan) with diameter of 1.5 mm. Reproducible BH measurements were conducted with the SDX system (DYN'R Medical Systems, France): this device monitors patient ventilation and displays it as a respiratory waveform in real time. Respiratory waveforms are fed back to the patient as visual data, and the patient voluntarily holds their breath on instruction from the medical staff. Patients were trained in the SDX system after receiving an overview of the device and method of holding their breath before the procedure was conducted. Treatment planning CT was performed 1 week after FM implantation using the SOMATOM Definition AS (Siemens Healthcare GmbH, Germany). The SDX system was used as recommended by the manufacturer, and patients were instructed to hold their breath at 85% of the maximum inspiratory volume. Actual inspiratory volumes ranged within a safety margin of approximately 100 mL. CT was performed three times using DIBH (IN-CT). To prepare for cases in which irradiation with DIBH could not be performed, we also performed CT with light expiration (EX-CT) so that, if necessary, we could quickly switch to RTRT, which is usually performed at our hospital. Treatment planning CT also confirmed that no implanted FMs had migrated.
Treatment planning
The FM closest to the tumor was tracked during planning and treatment. The treatment planning system Eclipse (Varian Medical Systems, USA) was used for target delineation and dose calculations. Three IN-CT scans were fused: the scans were first registered using the bone structure and then registration was corrected using the FMs in the x-, y-, and z-axis directions. The gross tumor volume (GTV) was contoured for each dataset. A high correlation exists between the FM and tumor location during resting breathing19,20. Since this correlation may decrease with deep inspiration, a GTVsum was created by adding the GTVs for each IN-CT. The clinical target volume was defined as the GTVsum. The planning target volume (PTV) was determined by adding a 5-mm margin around the clinical target volume.
The treatment plan was created with six to seven ports using 6-MV flattening filter-free photons (DBRT-plan) and a dose rate of 1,400 MU/min. Eight patients had peripheral lesions and were prescribed 42 Gy in four fractions at the D95. The leaf margin was adjusted such that D2 was 125–130% of the prescribed dose. D95 and D2 were the doses administered to 95% and 2% of the PTV, respectively. The remaining patient had a central lesion and was prescribed 60 Gy in eight fractions to the isocenter of the PTV. The leaf margin was modified to cover the PTV with approximately 80% of the prescribed dose. The dose calculation algorithm used was Acuros External Beam (version 15.6.06). A similar treatment plan was created for EX-CT in cases for which DBRT could not be performed (conv-plan).
After treatment planning, the patient was placed on the treatment couch to ensure that the gantry did not touch the patient or SDX system. We confirmed that the FM could be tracked in all beam directions.
Irradiation
CT was performed immediately before each irradiation session to confirm that no FM had migrated. Irradiation was performed using TrueBeam (Varian Medical Systems, USA). SyncTraX (Shimadzu Corporation, Japan) was used as the RTRT system: two sets of X-ray tubes are installed under the floor of the treatment room and image intensifiers are installed on the ceiling to provide real-time tracking of the FM near the tumor. The position of the FM is used as a surrogate for the position of the tumor. Irradiation is triggered when an FM enters a position within a 2-mm range in triaxial directions (a cube of 4 mm per side) of the position the FM held at the time of planning CT. Irradiation stops automatically when the FM is no longer in the gating box.
Evaluation
Lung volumes were evaluated using IN-CT and EX-CT. Lungs were contoured automatically with Eclipse, and if the trachea and/or main bronchi were included, they were corrected manually. The left and right lungs were evaluated together. The ratio of lung volume on IN-CT to that on EX-CT was calculated. The mean lung dose, V20, V10, and V5 were calculated from dose-volume histograms: Vx was defined as the percentage of the lung volume that received x Gy. Comparisons between the lung doses in the two plans were made using the Wilcoxon signed-rank test. Correlations between lung volume and lung dose or pulmonary function test parameters were evaluated using the Spearman’s correlation coefficient.
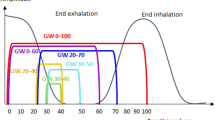
Irradiation time was defined as the time from the start of fluoroscopy for motion tracking after the patient was set up until the end of fluoroscopy. The ratio of irradiation time in the x-th session to that in the first session was calculated as the irradiation time ratio. An overview of the method is shown in Fig. 1.
A p value < 0.05 was considered to indicate a statistically significant difference.
Ethical approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study design was approved by the ethics committee of Yamaguchi University Hospital (approval number 2022-077). All patients provided written informed consent for the use of their data in clinical research before the administration of radiotherapy and had the opportunity to opt out of the study.
Results
Nine patients were enrolled in this study. Patient characteristics are shown in Table 1 and supplementary Table 1. The median age of the patients was 75 (range 70–82) years. Six (66.7%) patients were male and three (33.3%) were female. Eight (88.9%) patients had lesions in the lower lobe, and the remaining patient had a lingual segment. Four (44.4%) patients had a history of lung surgery: patient 1 had undergone two surgeries for lung tumors, both on the contralateral side; patient 5 had a history of ipsilateral surgery in the upper lobe; patients 7 and 8 had a history of contralateral lung tumor surgery; and patient 7 had a history of ipsilateral SBRT to another lobe. None of the other patients had a history of radiation therapy to the chest. Eight (88.9%) patients were smokers. All patients underwent pulmonary function tests before undergoing CT simulation. On an average, 28 (range 10–203) days lapsed between the pulmonary function test and the CT simulation.
DBRT was completed for all patients. The median distance from the tumor center to the marker center was 1.46 (range 0.44–2.66) cm, and the median PTV was 11.0 (range 5.2–120.6) mL. The lung volumes and doses on EX-CT and IN-CT are shown in Table 2. The mean dose, V20, and V10 were significantly lower in the DBRT-plan than in the conv-plan (p = 0.003, 0.003, and 0.003, respectively). No significant differences were observed in V5 (p = 0.074).
The ratio of lung volume was significantly correlated with the percentage of forced expiratory volume in 1 s (%FEV1) (ρ = 0.917, p = 0.001; supplementary Fig. 1). A strong negative correlation was observed between the ratio of lung volume and lung volume on EX-CT (ρ = − 0.800, p = 0.013; supplementary Fig. 2).
Lung volume on EX-CT and the decrease in the mean lung dose showed a strong negative correlation (ρ = − 0.850, p = 0.006; supplementary Fig. 3). Similarly, lung volume on EX-CT had a strong negative correlation with the decrease in both V20 and V10 (ρ = − 0.800, p = 0.013 and ρ = − 0.833, p = 0.008, respectively; supplementary Figs. 4 and 5). No significant correlation was observed between lung volume on EX-CT and the decrease in V5 (ρ = − 0.617, p = 0.086; supplementary Fig. 6).
A positive correlation was observed between the ratio of lung volume and the decrease in mean lung dose or V20 (ρ = 0.648, p = 0.049 and ρ = 0.879, p = 0.019, respectively; supplementary Figs. 7 and 8). No significant correlation was found between the ratio of lung volume and the decrease in V10 or V5 (ρ = 0.624, p = 0.060 and ρ = 0.297, p = 0.407, respectively; supplementary Figs. 9 and 10).
The average irradiation time for one session was 31 (range 25–49) min. Figure 2 shows the irradiation time ratio for each patient.
Discussion
In this study, the increased lung volume, made possible with DBRT, enabled a significant decrease in the mean lung dose, V20, and V10. This is the greatest advantage of irradiation using DIBH. However, there was no significant difference in V5 between the DBRT-plan and conv-plan. The area irradiated with more than 20 or 10 Gy was relatively limited around the PTV, but the area irradiated with more than 5 Gy included most of the area through which the beam passed. Therefore, when DIBH is used, the distance and volume that X-rays pass through also increase, making it difficult to achieve a reduction in the low-dose area (Fig. 3).
The ratio of lung volume significantly correlated with %FEV1. %FEV1 is a value used for staging chronic obstructive pulmonary disease (COPD), and low %FEV1 indicates advanced disease21; that is patients with severe COPD tend to have a low ratio of lung volume. A strong negative correlation was also observed between the ratio of lung volume and lung volume on EX-CT. Patients with a large lung volume on EX-CT are less likely to have further infiltration. As COPD progresses, adequate expiration becomes difficult and expiratory lung volume increases22. Thus, patients with COPD are unlikely to achieve further volume expansion.
A negative correlation was found between the lung volume on EX-CT and the decrease in mean lung dose, V20, or V10, with a positive correlation observed between the ratio of lung volume and the decrease in mean lung dose or V20. An increase in lung volume because of deep inspiration produces a relative decrease in the area exposed to moderate or high doses. As data accumulation and analysis progress, clinicians may be able to use lung volume to determine whether irradiation with DIBH is beneficial for patients before treatment planning.
The volume of the lungs changes considerably respiration. As lung volume increases with inspiration, the relative proportion of tumors in the lung decreases, and as a result, radiotherapy with DIBH can use a lower dose than radiotherapy with expiration (Fig. 4).
Radiotherapy with deep inspiration breath hold reduces the dose the lung and organs at risk are irradiated with. This technique allows for the distance between irradiated sites to be increased in patients with a history of irradiation. D1, d1: distance between tumor and organ at risk. D2, d2: distance between tumor and past irradiated site.
In addition, the expansion of the lungs increases the distance between the tumor and the organs at risk (OARs) such as the spinal cord, esophagus, bronchi, and heart; thus, the distance is increased between the OAR and the irradiation field, thereby reducing the doses to these organs. For patients with a history of radiotherapy to the lung, the distance from the previously irradiated site can also be maintained, thereby reducing the possibility of irradiation fields and areas overlapping and increasing the safety of the procedure. Despite the many advantages of performing SBRT for lungs with DIBH, it is often performed during expiration13. Radiotherapy is not performed with DIBH because of the type of RMM it is. With the BH method, inhalation is less stable than exhalation and reproducibility of tumor location is poorer during inspiration than during expiration11,12. Nearly half of the cases reported in the literature are ineligible for irradiation with DIBH owing to reproducibility issues7,13. If the variation in the tumor location for each BH is large, the range of variation is included in the target, which leads to an expansion of the irradiation field. In SBRT, a large dose is administered in one session, making expansion of the irradiation field undesirable. In addition, DIBH is an unfavorable RMM technique in RTRT because tumors move faster during inspiration than during expiration17. In RTRT, the beam is turned on when the marker enters the gating box, but a slight time lag occurs between when the beam-on signal is issued and when the beam is actually turned18. The faster the tumor moves, the larger the distance it moves during this time lag. Similarly, there is a slight time lag when the beam, triggered by the marker leaving the gating box, is turned off. A margin for this beam-on and beam-off delay, is provided, but the margin inevitably becomes large for inspiration in cases with a high tumor velocity. Moreover, with free breathing, the reproducibility of the intrafractional positioning is higher at end of expiration than at end of inspiration23. Therefore, irradiation is aimed to occur at the end of expiration when movement is the slowest and reproducibility is the highest in the RTRT24. We developed and successfully performed DBRT, in which RTRT is performed during DIBH. DBRT addresses all these problems. The high reproducibility of the tumor location during DIBH is guaranteed by tracking the FM, which is an internal respiration signal in real-time, and using it as a beam-on trigger. The beam is not turned on if the location has not been reproduced. Although previous attempts have been made to perform SBRT during DIBH using an external respiration signal25,26,27,28, few reports exist on the use of SBRT for lung tumors that utilize DIBH with real-time tracking of internal respiration signals29. Concerns about errors in positional correlation between the FM and tumor are addressed by summing the GTV in three IN-CTs with marker-based registration. Extremely poor reproducibility of the tumor location during DIBH raises the concern that the beam will not turn on and irradiation will not end. We used an SDX system to improve the reproducibility of the tumor location during DIBH and to increase the frequency of beam-on. All patients in this study could complete all treatment sessions with DBRT. Theoretically, the spirometer-based BH method, as well as other devices that support BH, can be used.
DBRT can resolve two additional problems. First, during irradiation with BH, “residual motion,” in which the tumor moves slowly despite BH, is problematic30. Even if the tumor is within the irradiation field at the start of irradiation, a risk exists that residual motion will cause the tumor to move out of the field during irradiation. Even if residual motion occurs during DBRT, irradiation stops when the FM moves out of the gating box. Thus, missed hits arising from residual motion are avoided. Second, sudden tumor motion may be problematic, for example, when a patient cannot maintain BH and exhales or when there is body movement for some reason. If the beam is manually turned on and off without gating with the respiratory waveform, detecting these abnormalities and turning the beam off will take time. This may impact tumor control because a missed hit to the tumor will occur. With DBRT, the beam is turned off as soon as the FM leaves the gating box. In summary, the advantages of DBRT include: reduction in lung dose due to larger lung volume, higher accuracy of the RTRT system with a tracking error of < 0.4 mm16, reduction on OAR dose due to a greater distance to this OAR, and detection ability of residual motion (Table 3).
A disadvantage of DBRT is that it requires a long irradiation time: on average, 31 min for each session. Patients with the longest irradiation times required an average of 49 min, with some sessions lasting up to 57 min. The average irradiation time for conventional RTRT at our hospital, which irradiates the terminal expiration, is approximately 19 min. Checking for FM migration, setting up the patient, and moving the gantry and couch add to the time spent for a DBRT session. The ratio of the second and subsequent irradiation times to the first irradiation time for each patient was expected to gradually shrink, but in practice it generally remained unchanged. Regarding the conventional RTRT, fluoroscopy is always on due to performing treatment under free breath. Using the DBRT, fluoroscopy is turned on only during breath hold by patients as instructed by the medical staff. The majority of irradiation time is spent regulating patient's breathing after breath hold, whereas only a small amount of the time is actually spent for breath hold. Therefore, the time when fluoroscopy is turned on is almost as the same as that using the conventional method.
This study was limited by its retrospective nature, small sample size, and lack of clinical assessment of adverse events and treatment outcomes. The appropriate sample size was estimated to be 37 cases with an alpha error of 0.05 and power of 0.80. Future research that uses a larger sample size and includes an analysis of follow-up data to evaluate the clinical assessment is needed.
Conclusions
We developed DBRT, a SBRT that is administered using the DIBH technique. It combines a spirometer-based BH support system with an RTRT system. All patients who underwent DBRT completed the procedure without any complications. DBRT is a promising method of irradiation that can significantly reduce lung doses. The long irradiation time that this method uses is an issue that requires future research.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- RMM:
-
Respiratory motion management
- SBRT:
-
Stereotactic body radiation therapy
- BH:
-
Breath hold
- RTRT:
-
Real-time tumor-tracking radiation therapy
- FM:
-
Fiducial marker
- DIBH:
-
Deep inspiration breath hold
- DBRT:
-
Deep inspiration breath hold real-time tumor-tracking radiotherapy
- CT:
-
Computed tomography
- GTV:
-
Gross tumor volume
- PTV:
-
Planning target volume
- COPD:
-
Chronic obstructive pulmonary disease
References
Boda-Heggemann, J. et al. Deep inspiration breath hold-based radiation therapy: A clinical review. Int. J. Radiat. Oncol. Biol. Phys. 94, 478–492 (2016).
Korreman, S. S., Pedersen, A. N., Nøttrup, T. J., Specht, L. & Nyström, H. Breathing adapted radiotherapy for breast cancer: Comparison of free breathing gating with the breath-hold technique. Radiother. Oncol. 76, 311–318 (2005).
Schönecker, S. et al. Treatment planning and evaluation of gated radiotherapy in left-sided breast cancer patients sing the Catalyst™/Sentinel™ system for deep inspiration breath-hold (DIBH). Radiat. Oncol. 11, 143 (2016).
Tarohda, T. I. et al. The management of tumor motions in the stereotactic irradiation to lung cancer under the use of Abches to control active breathing. Med. Phys. 38, 4141–4146 (2011).
Hoisak, J. D. P., Sixel, K. E., Tirona, R., Cheung, P. C. F. & Pignol, J. P. Correlation of lung tumor motion with external surrogate indicators of respiration. Int. J. Radiat. Oncol. Biol. Phys. 60, 1298–1306 (2004).
Wong, J. W. et al. The use of active breathing control (ABC) to reduce margin for breathing motion. Int. J. Radiat. Oncol. Biol. Phys. 44, 911–919 (1999).
Mah, D. et al. Technical aspects of the deep inspiration breath-hold technique in the treatment of thoracic cancer. Int. J. Radiat. Oncol. Biol. Phys. 48, 1175–1185 (2000).
Yang, W. et al. Clinical experience using a video-guided spirometry system for deep inspiration breath-hold radiotherapy of left-sided breast cancer. J. Appl. Clin. Med. Phys. 16, 5218 (2015).
Giraud, P. et al. Respiratory gating techniques for optimization of lung cancer radiotherapy. J. Thorac. Oncol. 6, 2058–2068 (2011).
Prado, A. et al. Intrafraction target shift comparison using two breath-hold systems in lung stereotactic body radiotherapy. Phys. Imaging. Radiat. Oncol. 22, 57–62 (2022).
Kimura, T. et al. Reproducibility of organ position using voluntary breath-hold method with spirometer for extracranial stereotactic radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 60, 1307–1313 (2004).
Kimura, T. et al. Interbreath-hold reproducibility of lung tumour position and reduction of the internal target volume using a voluntary breath-hold method with spirometer during stereotactic radiotherapy for lung tumours. Br. J. Radiol. 80, 355–361 (2007).
Keall, P. J. et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med. Phys. 33, 3874–3900 (2006).
Shirato, H., Shimizu, S., Shimizu, T., Nishioka, T. & Miyasaka, K. Real-time tumour-tracking radiotherapy. Lancet 353, 1331–1332 (1999).
Shirato, H. et al. Four-dimensional treatment planning and fluoroscopic real-time tumor tracking radiotherapy for moving tumor. Int. J. Radiat. Oncol. Biol. Phys. 48, 435–442 (2000).
Shiinoki, T. et al. Evaluation of a combined respiratory-gating system comprising the TrueBeam linear accelerator and a new real-time tumor-tracking radiotherapy system: A preliminary study. J. Appl. Clin. Med. Phys. 17, 202–213 (2016).
Korreman, S., Mostafavi, H., Le, Q. T. & Boyer, A. Comparison of respiratory surrogates for gated lung radiotherapy without internal fiducials. Acta Oncol. 45, 935–942 (2006).
Shirato, H. et al. Speed and amplitude of lung tumor motion precisely detected in four-dimensional setup and in real-time tumor-tracking radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 64, 1229–1236 (2006).
Ueki, N. et al. Intra- and interfractional variations in geometric arrangement between lung tumours and implanted markers. Radiother. Oncol. 110, 523–528 (2014).
Willmann, J. et al. Four-dimensional computed tomography-based correlation of respiratory motion of lung tumors with implanted fiducials and an external surrogate. Adv. Radiat. Oncol. 7, 100885 (2021).
Vogelmeier, C. F. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am. J. Respir. Crit. Care Med. 95, 557–582 (2017).
Ferguson, G. T. Why does the lung hyperinflate?. Proc. Am. Thorac. Soc. 3, 176–179 (2006).
Shiinoki, T. et al. Interfractional reproducibility in pancreatic position based on four-dimensional computed tomography. Int. J. Radiat. Oncol. Biol. Phys. 80, 1567–1572 (2011).
Tanaka, H. et al. Anemia is a prognostic factor for overall survival rate in patients with non-small cell lung cancer treated with stereotactic body radiation therapy. Cancer Manag. Res. 13, 7447–7453 (2021).
Zeng, C. et al. Intrafraction motion during deep inspiration breath hold pancreatic cancer treatment. J. Appl. Clin. Med. Phys. 20, 37–43 (2019).
Josipovic, M. et al. Deep inspiration breath hold in locally advanced lung cancer radiotherapy: Validation of intrafractional geometric uncertainties in the INHALE trial. Br. J. Radiol. 92, 20190569 (2019).
Mørkeset, S. T., Lervåg, C., Lund, J. Å. & Jensen, C. Clinical experience of volumetric-modulated flattening filter free stereotactic body radiation therapy of lesions in the lung with deep inspiration breath-hold. J. Appl. Clin. Med. Phys. 23, e13733 (2022).
Naumann, P. et al. Feasibility of optical surface-guidance for position verification and monitoring of stereotactic body radiotherapy in deep-inspiration breath-hold. Front. Oncol. 10, 537279 (2020).
Jaccard, M. et al. Clinical experience with lung-specific electromagnetic tranpinders for real-time tumor tracking in lung stereotactic body radiotherapy. Phys. Imaging Radiat. Oncol. 12, 30–37 (2019).
Vogel, N. et al. Intra-breath-hold residual motion of image-guided DIBH liver-SBRT: An estimation by ultrasound-based monitoring correlated with diaphragm position in CBCT. Radiother. Oncol. 129, 441–448 (2018).
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
H.T., T.Ser., Y.Y., and T.Shi. were contributed to the study conception and design. Data collection was performed by H.T., T.O., K.U., M.Kar., Y.M., M.Kaj., T.Ser., K.F., Y.Y., and T.Shi. Data analysis was performed by H.T., T.O., T.Ser., and T.Shi. The first draft of manuscript was written by H.T., T.O., and T.Shi. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tanaka, H., Ono, T., Ueda, K. et al. Deep inspiration breath hold real-time tumor-tracking radiation therapy (DBRT) as a novel stereotactic body radiation therapy approach for lung tumors. Sci Rep 14, 2400 (2024). https://doi.org/10.1038/s41598-024-53020-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53020-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.