Abstract
Market drugs including brand or generic with poor quality, don’t meet the acceptable standard guidelines. Vildagliptin is an important antidiabetic drugs used in monotherapy or in combinations protocols for treatment of diabetes mellites. The main goal of the current study is to assess the pharmaceutical equivalence of two marketed generics of vildagliptin 50 mg tablets compared to the branded product (Galvus 50 mg). The in vitro dissolution test was used as a quality control tool to obtain the dissolution profile of vildagliptin compared to the reference drug. The results revealed that all tested samples showed dissolution behavior like standard drug. Whole samples dissolution reached after 15 min in accordance with the standard. According to the similarity factors records, tested vildagliptin samples showed a comparable dissolution to the reference drug. The current work presents an in vitro protocol for quality evaluation of recently released generic drugs.
Similar content being viewed by others
Introduction
In pharmaceutical industry, Brand-name medicines (Innovator drugs) are those generated by a company and patented to increase the economic gain of being the exclusive manufacturer of such a drug1. When the patency of the Innovator drug product expires, another pharmaceutical manufacturing companies can apply to regulatory authorities for approval to market generic versions of the original medicines2. Pharmaceutical companies that produce generic drugs have not devoted much to the research and development of a new drug; they simply copied the patented formula of the original branded drug2. Generic products are chemically identical to the original drug and have the same active ingredients1. However, the inactive ingredients can be different but at a certain potency2. Today, the use of generic drugs has increased and many countries have introduced regulations to provide safe, effective and good quality medicines to their people3,4. The lower cost of generics compared to Innovator drugs has increased their existence in the local market. Therefore, it is essential to test the interchangeability of the marketed generic products using a suitable approach. The interchangeability of generics is understood to mean the possibility for their mutual replacement or replacement of the original drug in clinical practice5. Bioequivalence studies is considered to be costly , time consuming and involve subjecting healthy volunteers to risks of side effects6. Accordingly, the biopharmaceutics classification system (BCS)7 was developed as a substitute for the in vivo bioequivalence studies and is applicable to highly soluble drugs with known absorption rate and extent in humans, drugs with a wide therapeutic index, and orally administered drugs that are immediate release8. Dissolution test is a valuable quality control tool to monitor batch-to-batch consistency during drug development9. Additionally, dissolution testing can be used for optimization of formulations and monitoring drug stability over time10,11. Vildagliptin (Fig. 1) is an antidiabetic drug belonging to Dipeptidyl Peptidase-4 (DPP-4) inhibitors and approved to be used in monotherapy and combination therapy to control type 2 diabetes mellitus12. Vildagliptin is a BCS-I compound with high solubility and permeability13 and does not to have a narrow therapeutic index which account for its applicability to be assessed using in vitro dissolution approach14. In this work, the in vitro dissolution test is used as a quality control tool to obtain the dissolution profile and assess the equivalency of two marketed generics of vildagliptin 50 mg tablets compared to the branded product (Galvus 50 mg).
Results and discussions
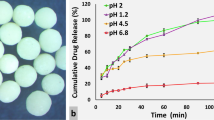
The dissolution pattern of six generic products was evaluated in the current experiment then compared to the dissolution profile of reference product (Galvus). As shown in Table 1, almost a similar percentage drug release is derived for all the products which confirm a fast drug release pattern accounting for the physiochemical property of vildagliptin as BCS class 1. The results of dissolution studies are presented graphically in Fig. 2. Results revealed that V-1 and V-2 samples showed the fastest drug release % reaching 100% within 15 min. Additionally for all samples, the complete dissolution reached after 20 min. As observed the first product showed a slight better and faster release than the brand and this observation was in accordance with the previous reports which is due to tablet hardness15.
After performing the dissolution test, a mathematical comparison is calculated using the difference factor (f1) and similarity factor (f2). The FDA guidelines stated that f1 values up to 15 (0–15) and f2 values greater than 50 (50–100) ensure sameness or equivalence of generic product to the reference product16. All tested samples showed f1 value range from 0.33 in V-3 to 3.94 in V-2. Regarding f2 all samples ranged from 85 to 98. According to the similarity factors records, tested vildagliptin samples showed a comparable dissolution to the reference drug. As shown in Table 2, the obtained values have revealed the sameness of tested generics when compared to the reference product. Compared to the previous studies on vildagliptin dissolution test, the study done by Barden et al., showed that the release profile was satisfactory, and the dissolution was quite fast, as about 80% of vildagliptin was dissolved within 10 min and whole drug dissolution was reached within 30 min17. A generic drug must demonstrate adequate pharmaceutical equivalence to a reference drug that has previously received market authorization depending on clinical trials18,19. To decrease post marketing quality issues, a surveillance system establishment for generic products in the market is encouraged20. In vitro dissolution test for immediate-release solid dosage forms including tablets is employed to assure product quality and performance following changes in manufacturer parameters21.
Materials and methods
Materials and instruments
The reference product Galvus and six generic products of vildagliptin 50 mg tablet where tested (3 batches for each generic were tested for statistical analysis) were collected by the Saudi food and drug authority. The reference standard of vildagliptin was obtained from (Batch #20200808Q). Hydrochloric acid 37% was purchased from sigma Aldrich. Dissolution test was performed on Erweka dissolution tester (DT 1410). High performance liquid chromatography (HPLC) by Shimadzu was used for chemical analysis of samples.
HPLC Instrumentation
Liquid chromatography (LC) method was carried as previously reported17. In brief, a liquid chromatography (LC) Shimadzu 20-A system equipped with a CBM20A system controller, LC-20AT pump, SIL20A/C auto sampler, CTO-20A/C column oven and SPD-M2OA PDA detector. The experiments were performed on an analytical column Zorbax Eclipse Plus RP-C8 (150 mm × 4.6 mm, 5 µm). The LC system was operated isocratically at 25 °C in the column oven, using a mobile-phase composed by a solution of 50 mM potassium phosphate buffer and acetonitrile (85:15 v/v), at a flowrate of 1.0 ml min−1, using detection at 207 nm. The pH of mobile phase was adjusted at 7.0 using phosphoric acid in the aqueous phase (by adding 0.1 mL and monitoring of pH) and then, it was done the mixture of both aqueous and organic phases. The injection volume was 20 μL. The peak areas were integrated automatically by computer using LC-Solution manager system software.
Standard preparation
Vildagliptin standard stock solution was prepared by weighing 12.84 mg of Vildagliptin standard in 25 ml volumetric flask, then complete to volume with water. Final standard solution is obtained by pipetting 2 ml of Vildagliptin standard stock solution into a 20 mL volumetric flask then complete to volume with dissolution medium.
In vitro dissolution test
The dissolution test of each product was performed according to the dissolution method of reference product (Innovator) Galvus. The dissolution parameters are detailed in Table 3. Where the drug release is determined using (USP) dissolution testing apparatus type 2 (paddle method) at 50 rpm. The dissolution medium volume is 0.01 N HCl, 1000 mL and maintained at 37 ± 0.5 C. Dissolution profile was obtained at specific time intervals (10, 15, 20, 30, 45) where an aliquot of 10 ml was withdrawn from the dissolution apparatus. The sample solutions were filtered using 10 μm filters. The resultant samples were chromatographically analyzed by HPLC using photodiode array detector at 210 nm. Dissolution percentage was calculated, and dissolution profile was plotted after comparing the response of vildagliptin standard solution.
Dissolution profile comparison
Moore and Flanner proposed a model independent mathematical approach to compare the dissolution profile using two factors, difference factor (f1) and similarity factor (f2)22. To evaluate the in vitro pharmaceutical equivalence, mean dissolution values were employed to calculate f1 and f2. The following equations were used to calculate f1 and f2 for the tested batches:
Where n is the number of time points, Rt is the dissolution value of the reference product at time t, and Tt is the dissolution value of the test product at time t22.
Conclusion
A dissolution test for six generic vildagliptin tablets was evaluated and presented in this study. The current study presents an in vitro dissolution study for vildagliptin for the first time in Saudi Arabia. The dissolution profile of tested vildagliptin samples showed significant similarity to the reference drug and meets the FDA guidelines. Such study could be considered to present a quality control tool for generic drugs to enhance post-production quality control.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Nandyala, S. et al. Comparative evaluation of branded and generic medicines-ranitidine & metformin HCl. Int. Res. J. Pharmacy Med. Sci. 1, 24–28 (2018).
Igbinovia, M. E. The Perceived Benefits of Generic Versus Branded Medicines (University of Pretoria, 2010).
Stuart, A. V. et al. Comparing the dissolution profiles of seven metformin formulations in simulated intestinal fluid. Dissolut. Technol 22, 17–21 (2015).
Young, A. R. Generic pharmaceutical regulation in the United States with comparison to Europe: innovation and competition. Wash. U. Global Stud. L. Rev. 8, 165 (2009).
Shokhin, I., Ramenskaya, G., Vasilenko, G. & Malalshenko, E. Assessment of the possibility of using comparative in vitro dissolution kinetics (biowaiver) instead of in vivo bioequivalence evaluation for establishing the interchanbeability of generic drugs. Pharm. Chem. J. 45, 107–109 (2011).
Shraim, N. et al. Assessment of dissolution performance of immediate release ibuprofen products: Screening of products available on the Palestinian market. Palestinian Med. Pharm. J. 3, 5 (2018).
Amidon, G. L., Lennernäs, H., Shah, V. P. & Crison, J. R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. J. Pharm. Res. 12, 413–420 (1995).
Anand, O., Yu, L. X., Conner, D. P. & Davit, B. M. Dissolution testing for generic drugs: an FDA perspective. AAPS J. 13, 328–335 (2011).
Rozet, E., Ziemons, E., Marini, R., Boulanger, B. & Hubert, P. Validation of analytical methods involved in dissolution assays: Acceptance limits and decision methodologies. Analytica Chimica Acta 751, 44–51 (2012).
Ku, M. S. Use of the biopharmaceutical classification system in early drug development. AAPS J. 10, 208–212. https://doi.org/10.1208/s12248-008-9020-0 (2008).
Berthelsen, R. et al. Dissolution model development: Formulation effects and filter complications. Dissolut. Technol. 23, 1–12 (2016).
Wiciński, M. et al. Vasculoprotective effects of vildagliptin. Focus on atherogenesis. Int. J. Mol. Sci. 21, 2275 (2020).
Pasha, M. et al. A systematic review on the clinical pharmacokinetics of vildagliptin in healthy and disease populations. J. Expert Opinion Drug Metabolism Toxicol., 1–13 (2023).
Area, R. R. S. P. Assessment report. Prepared for UK Department for International (2012).
Al Ameri, M. et al. The differences between the branded and generic medicines using solid dosage forms: In-vitro dissolution testing. Results Pharma Sci. 2, 1–8 (2012).
Guidance, F. Guidance for industry: Dissolution testing of immediate release solid oral dosage forms. US Department of Health and Human Services. Food Drug Administration, Center for Drug Evaluation Research (1997).
Barden, A. T., Piccoli, B. L., Volpato, N. M. & Steppe, M. Second-order derivative UV spectrophotometric and RP-HPLC methods for the analysis of vildagliptin and application for dissolution study. Drug Anal. Res. 2, 46–53 (2018).
Davit, B., Braddy, A. C., Conner, D. P. & Yu, L. X. International guidelines for bioequivalence of systemically available orally administered generic drug products: A survey of similarities and differences. AAPS J. 15, 974–990 (2013).
Kaushal, N., Singh, S. K., Gulati, M., Vaidya, Y. & Kaushik, M. Study of regulatory requirements for the conduct of bioequivalence studies in US, Europe, Canada, India, ASEAN and SADC countries: impact on generic drug substitution. J. Appl. Pharm. Sci. 6(4), 206–222 (2016).
Hassali, M. A. et al. The experiences of implementing generic medicine policy in eight countries: A review and recommendations for a successful promotion of generic medicine use. Saudi Pharm. J. 22, 491–503 (2014).
Younes, H., Adib, S. S. A., Ibrahim, M. I. M. & Shalash, A. A. Dissolution testing and content uniformity analysis for metformin tablets using vildagliptin as an internal standard. J. Hunan Univ. Nat. Sci. 50(1), 1–9. https://doi.org/10.55463/issn.1674-2974.50.1.1 (2023).
Moore, J. & Flanner, H. Mathematical comparison of dissolution profiles. Pharm. Technol. 20, 64–74 (1996).
Author information
Authors and Affiliations
Contributions
Conceptualization N.A.; Data curation N.A., G.H.A.; Methodology, F.K.A. and N.A. and; Supervision N.A.; Writing-original draft N.A., M.M.A.; Writing-review and editing, N.A. and R.A.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Altoum, G.H., AL-Enazi, F.K., Abudahash, M.M. et al. A comparative study on vildagliptin brand and its generic equivalents using dissolution test as quality control measure tool. Sci Rep 14, 2636 (2024). https://doi.org/10.1038/s41598-024-52674-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52674-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.