Abstract
In this study, high-throughput sequencing of 16S rRNA amplicons and predictive PICRUSt functional profiles were used to perform a comprehensive analysis of the temporal bacterial distribution and metabolic functions of 19 bimonthly samples collected from July 2019 to January 2020 in the surface water of Billings Reservoir, São Paulo. The results revealed that most of the bacterial 16S rRNA gene sequences belonged to Cyanobacteria and Proteobacteria, which accounted for more than 58% of the total bacterial abundance. Species richness and evenness indices were highest in surface water from summer samples (January 2020), followed by winter (July 2019) and spring samples (September and November 2019). Results also showed that the highest concentrations of sulfate (SO4–2), phosphate (P), ammonia (NH3), and nitrate (NO3-) were detected in November 2019 and January 2020 compared with samples collected in July and September 2019 (P < 0.05). Principal component analysis suggests that physicochemical factors such as pH, DO, temperature, and NH3 are the most important environmental factors influencing spatial and temporal variations in the community structure of bacterioplankton. At the genus level, 18.3% and 9.9% of OTUs in the July and September 2019 samples, respectively, were assigned to Planktothrix, while 14.4% and 20% of OTUs in the November 2019 and January 2020 samples, respectively, were assigned to Microcystis. In addition, PICRUSt metabolic analysis revealed increasing enrichment of genes in surface water associated with multiple metabolic processes rather than a single regulatory mechanism. This is the first study to examine the temporal dynamics of bacterioplankton and its function in Billings Reservoir during the winter, spring, and summer seasons. The study provides comprehensive reference information on the effects of an artificial habitat on the bacterioplankton community that can be used to interpret the results of studies to evaluate and set appropriate treatment targets.
Similar content being viewed by others
Introduction
Pollution of water resources remains a critical environmental concern across the globe. In developing countries, rapid urbanization, poor urban planning, and inadequate waste management exacerbate water quality deterioration, leading to increased microbial concentrations1. Bacterioplankton communities play a vital role in controlling energy flow and biogeochemical cycles within aquatic ecosystems. Their dynamics are subject to environmental factors such as total phosphorus2, pollution3, pH4, and dissolved oxygen (DO)5. Temporal variations in marine bacteria distribution and abundance suggest a seasonal influence on microbial diversity6. The biogeochemical cycling of nutrients by sediment bacteria is essential for maintaining the trophic state of water bodies7,8,9.
Understanding bacterioplankton diversity and its response to anthropogenic disturbances is key to detecting environmental changes in local ecosystems. Urbanization, for instance, has been linked to alterations in the physicochemical properties of aquatic environments, leading to biodiversity loss and reduced ecological function10,11. Changes in bacterioplankton community structure can significantly impact water quality, particularly due to the threat posed by toxin-producing cyanobacteria12,13,14,15. The interaction between cyanobacteria and heterotrophic bacteria influences the structure of bacterioplankton communities16, while bacteria serve as indicators of surface water contamination17. Pathogenic microbes and heavy metals also pose a significant threat to water quality, affecting human and animal health18.
Water properties fluctuate daily, with noticeable temporal and spatial variations19,20. Therefore, comprehensive monitoring of freshwater sources is crucial for maintaining suitable water quality. Reservoirs, which are limnologically a mix between a lake and a river, exhibit pronounced stratification in summer and potential reverse stratification in winter, affecting the distribution of chemicals and nutrients in the water21,22.
São Paulo, the largest urban area in Brazil, faces water stress with an annual water availability of less than 1,700 m3 per person in the São Paulo Metropolitan Region (SPMR)23. The Billings Reservoir, a significant freshwater source for the SPMR, serves multiple functions, including irrigation and flood control24. Despite its importance, the microbial community structure within the Billings Reservoir has been understudied since its construction in 194025. Previous studies primarily focused on physicochemical variables and the presence of bacterial strains using culture-dependent methods22,26,27,28,29. Recent culture-independent studies conducted by our group revealed a higher prokaryotic diversity in Billings Reservoir compared to similar water systems and also a high abundance of cyanobacteria24,30. These bacteria are considered the most widespread photosynthetic organisms due to their global distribution and their significant ecological presence in nutrient-rich habitats, which often lead to the formation of blooms31. Cyanobacterial blooms are considered harmful to aquatic ecosystems as they are able to alter critical ecological parameters such as dissolved oxygen concentration, pH balance and light availability. Most species contributing to these blooms are capable of synthesizing cyanotoxins, leading to their classification as harmful cyanobacterial blooms31. The presence of cyanotoxins at Billings Reservoir was also shown by additional studies21,25,32. It is possible that nutrient inputs from agriculture and a rise in water temperature are among the triggers of the algal bloom in the reservoir, but this needs to be confirmed by further investigations. Without a complete phytoplankton succession series, however, the data are not yet sufficient to reveal the ecological processes of the algal bloom.
Therefore, this study involved bi-monthly sampling to examine the phytoplankton dynamics in the surface waters of Billings Reservoir, spanning from June 2019 to January 2020. The study hypothesizes that the Billings Reservoir exhibits significant seasonal shifts in phytoplankton composition due to environmental factors, with species displaying distinct adaptive responses to optimize survival and dominance in fluctuating conditions. The objective is to report on seasonal phytoplankton composition shifts and their adaptive responses to environmental conditions. The findings from this study offer a preliminary assessment of the unique ecological conditions formed by the seasonal activities within the reservoir.
Material and methods
Study area and sampling
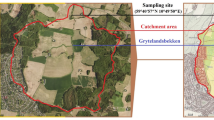
Located in southeastern São Paulo, Billings Reservoir is fed by ten main tributaries, namely Rio Grande, Ribeirão Pires, Rio Pequeno, Rio Pedra Branca, Rio Taquacetuba, Ribeirão Bororé, Ribeirão Cocaia, Ribeirão Guacuri, Córrego Grota Funda, and Córrego Alvarenga. This reservoir is classified in the II class of Resolution 357 of the Brazilian Environmental Council (CONAMA), pursuant to Decree 10.755/77. The tree-shaped Billings Reservoir was built in 1925 during the water crisis as one of the solutions to minimize the impact of the crisis on public supply. Billings Reservoir is the largest water storage reservoir in the SPMR. It covers 127 km2 in six counties and has a total volume of 1228.7 × 106 m3 with a water surface area of 10,814.20 ha and a maximum depth of 18 m33,34,35. It includes eight arms (or subregions): Rio Grande, Rio Pequeno, Rio Capivari, Rio Pedra Branca, Taquacetuba, Bororé, Cocaia, and Alvarenga. The reservoir's water quality is poor because the Pinheiro River, Brazil's most polluted aquatic ecosystem, is diverted into the reservoir to increase electricity generation (by water pumping) at the Henry Borden Power Plant on the São Paulo coast27,30,36. Since 1922, however, such pumping has been permitted only in the event of a flood in the capital city30,37. Currently, the reservoir is used as a water source, for fishing, navigation, recreation and hydroelectric power generation38. Approximately 250 ml of surface water (10 cm depth) was collected in triplicate at 30 locations approximately 18 km apart along the reservoir using a 5-L van spike sampler. Samples were collected in July 2019 (winter month), September 2019 (spring month), November 2019 (hot spring month), and January 2020 (summer month). At the beginning of the study, the geographic coordinates of the collection sites were recorded to ensure that subsequent water samples and measurements were taken on water from specific locations. Collected samples were stored in cool boxes at 4 °C in the dark and transported to the laboratory, where they were immediately processed as previously described24,30. The number of sites on the reservoir and their locations are shown in Fig. 1.
Physicochemical analysis
Samples collected bimonthly were analyzed on site using a multiparameter water meter (YSI, USA) to determine the main physicochemical parameters, namely turbidity, temperature (Temp), dissolved oxygen (DO), and pH. Concentrations of nitrate (NO3-), sulfate (SO42−), phosphate (PO43− (designated here as “P”) and ammonia (NH3) were analyzed from another subsample (250 ml) in the laboratory according to standard procedures (CONAMA 2005). These physicochemical parameters were frequently used to evaluate the ecological status of the reservoir according to the Brazilian standard (Order of the Minister of Environment, 20005).
DNA extraction, library preparation, and sequencing
DNA was extracted from approximately 600 µl of each concentrated water sample by centrifugation of the surface water samples as detailed in our recently published study24. The PowerSoil DNA isolation kit (Mobio Laboratories Inc., Carlsbad, CA, USA) was used according to the manufacturer's protocol. DNA quality was checked by 1% (w/v) agarose gel electrophoresis, and the amount was determined using a Qubit 2.0 fluorometer (Life Technologies: Carlsbad, CA, USA). The extracted DNA was stored at -20 °C until further processing. PCR of the V3-V4 variable region of the 16S rRNA gene was performed using the Bakt_341F/Bakt_805R39, as previously described40. The amplicons of all samples were purified using a PCR clean-up kit (D4014, Zymo research) and then used as DNA template for a second round of PCR to index the DNA and prepare libraries as described previously41,42. The purified indexed libraries were pooled in equimolar amounts, diluted to four nM, and finally loaded onto an Illumina MiSeq cartridge for paired-end 300 sequencing.
Data analysis pipeline and statistical analysis
Processing raw reads started with quality check and filtering of low quality (< Q25) reads by Trimmomatic ver. 0.3243. After QC pass, paired-end sequence data were merged together using fastq_mergepairs command of VSEARCH version 2.13.444 with default parameters. Primers were then trimmed with the alignment algorithm of Myers and Miller45 at a similarity cut off of 0.8. Non-specific amplicons that do not encode 16S rRNA were detected by nhmmer46 in HMMER software package ver. 3.2.1 with hmm profiles. Unique reads were extracted and redundant reads were clustered with the unique reads by derep_fulllength command of VSEARCH44. The EzBioCloud 16S rRNA database47 was used for taxonomic assignment using usearch_global command of VSEARCH44 followed by more precise pairwise alignment45. Chimeric reads were filtered on reads with < 97% similarity by reference based chimeric detection using UCHIME algorithm48 and the non-chimeric 16S rRNA database from EzBioCloud. After chimeric filtering, reads that are not identified to the species level (with < 97% similarity) in the EzBioCloud database were compiled and cluster_fast command44 was used to perform de-novo clustering to generate additional OTUs. Following chimeric filtering, reads that could not be identified to the species level (with < 97% similarity) in the EzBioCloud database were compiled, and the cluster_fast command44 was used to perform de novo clustering to generate additional OTUs. Finally, OTUs with single reads (singletons) are omitted from further analysis. The secondary analysis which includes diversity calculation and biomarker discovery was conducted by in-house programs of Chunlab, Inc (Seoul, South Korea). The alpha diversity indices (ACE49, Chao150, Jackknife51, Shannon52, NPShannon53, Simpson52 and Phylogenetic diversity54), rarefaction curves55, rank abundance curves56 are estimated. To visualize the sample differences, beta diversity distances were calculated by Jensen–Shannon algorithm57. PERMANOVA test was used to determine significant differences in beta diversity. With functional profiles that are predicted by LEfSe58 and Kruskal–Wallis H Test59). All analytics mentioned above were performed in EzBioCloud 16S-based Microbiome Taxonomic Profiling (MTP), which is a ChunLab’s bioinformatics cloud platform that automatically process the uploaded fastq data which are converted to MTP data unit. An MTP represents single metagenomic or microbiome sample.
Comparison of differences between water samples was performed using the one-way analysis of variance test (ANOVA). Samples with significant differences were subjected to the Turkey post-hoc test to determine which of the variables were statistically significant. To determine the causes of bacterial community dominance, principal component analysis (PCA) in Past4.07b software was used to examine the correlation between bacterial communities and environmental variables.
The map was created using ArcGIS software, version 10.2, licensed (ESRI, Redlands, California, USA). The administrative shapefiles used to create the map were obtained from the open-access domain of DataGEO: http://datageo.ambiente.sp.gov.br/. To display the study sites on the map, Global Positioning System (GPS) coordinates for the study sites were converted to shapefiles that were combined with the administrative shapefiles for the sites.
Results
Variation of water physicochemical properties
To obtain information on the diversity and composition of the bacterioplankton community in the surface waters of Billings Reservoir, 30 sites along the reservoir were sampled bimonthly. Significant variations in physicochemical parameters were observed during the study period (Fig. 2). For example, the average water depth or level of the reservoir decreased from 7.74 m in July and September (winter season) to 5.47 m in November 2019 and to 5.89 m in January 2020 (dry season), as shown in Fig. 3. In addition, the highest median temperature of 26.85 °C was observed in January (range 21.9–29.20 °C). The lowest and highest water temperatures were recorded at sites B14 (minimum of 20.90 °C in July 2019 and maximum of 27.70 °C in January 2020) and B18 (minimum of 19.90 °C in September 2019 and maximum of 27.10 °C in January 2020). No significant differences in DO were observed over time (Fig. 3). Site B06 showed the greatest variation at DO with the highest value of 9.4 mg/L in November 2019 and the lowest of 1.7 mg/L in September 2019 (Fig. 2). As shown in Fig. 3, pH was constant at most sites in July 2019, November 2019, and January 2020. A significant decrease in pH was observed for samples collected in September 2019. The same figure shows the greatest variation in pH in November 2019 (median 7.8; range 6–8.6) and January 2020 (median 7.9; range 6.45–8.6). The greatest variation in pH within the same site was observed at B26 (minimum of 6 in November 2019 and maximum of 8.3 in January 2020), B12 (minimum of 6.5 in September 2019 and maximum of 8.2 in November 2019), and B16 (minimum of 6.8 in September and maximum of 8.63 in July) (Fig. 2). Water turbidity varied over time, which was particularly evident in samples collected in January 2020 compared to samples collected in July 2019, namely B15, B12, and B09 (Figs. 2 and 3). The highest concentrations of SO42−, P, NH4+ –N, and NO3− were detected in November 2019 and January 2020 (Fig. 4).
The measured physicochemical water parameters were ordinated in a PCA (Fig. S1). Our interpretation on loading was considered significant if was greater than 0.40 or less than − 0.40 60. In the July 2019 PCA, the first two principal components (PCs) accounted for 44.5% of the variability in the data set, with pH and DO along PC1 and water temperature and NH4+ –N along PC2 showing strong positive associations with water quality (Fig. S1a). However, it is important to consider that the increase in pH may be influenced by cyanobacterial blooms, which are known to elevate pH levels as a result of CO2 uptake during photosynthesis. Thus, the role of pH as a response variable rather than an explanatory one in the context of cyanobacterial abundance must be carefully considered when interpreting these PCA results. PC1 and PC2 explained 50.6% of the variability in the data set in the September 2019 samples, and phosphate concentrations and turbidity were most strongly and positively associated with PC1 (Fig. S1b). Along PC2, sulfate concentrations had a much greater loading than the eight other variables in this component. Analysis of PC1 and PC2 in the November 2019 samples explained 51.2% of the variance. As shown in Fig. S1c, PC1 showed a strong positive correlation with phosphate, sulfate, and turbidity, while PC2 was strongly associated with water depth. Individual loading analysis of the January 2020 samples showed that PC1 and PC2 explained 53.6% of the variability in the data set. Sulfate and nitrate concentrations were most strongly associated with PC1 and pH and phosphate concentrations were most strongly associated with PC2 (Fig. S1d). PC1 scores of water turbidity and nutrient variables followed similar patterns in bi-monthly samples collected from September 2019 to January 2020. DO Concentrations radiated in opposite directions, indicating a strong negative correlation of these variables. Algae were observed floating on the surface of the reservoir at some locations during the January 2020 sampling.
Sequencing depth and diversity analysis
Of the 30 sites, 76 site-matched water samples (19 × 4 bimonthly samples) were successfully amplified and sequenced from surface layers and submitted for 16S rRNA analysis. Up to 100,000 MPS reads from each sample were uploaded, quality controlled, and profiled using the EzBioCloud tool. Trimming-based quality control removed 142,347 low-quality amplicons from all samples. The taxonomic approach detected and removed 243,108 and 1,629,579 non-target and chimera amplicons, respectively. The total quality assessment and trimming steps resulted in 4 945 059 valid reads from all samples considered for further analysis. On average, each sample or MTP was represented by 65,066 ± 7,604 valid reads. The average length of these reads was approximately 435 (± 17.22) nucleotides, with average minimum and maximum lengths of 409 (± 2.2) and 455 (± 2.3) nucleotides, respectively. Of the valid reads, 3,489,504 (70.6%) sequences were identified at the species level with a similarity cutoff of 97%. The average of Good's coverage estimator for OTUs in all MTPs was 99.6 (± 0.22), indicating that the diversity of bacterioplankton in all MTPs was adequately covered by the generated sequences. The number of observed OTUs determined in the water samples of the January 20 samples was higher than that of the September 19 samples (Bonferroni-corrected p = 0.049). When analyzing alpha diversity using the diversity indices ACE, Chao 1, Jackknife, NPShannon, or Simpson, we found no significant differences among the four sample groups (p > 0.05) (Fig. 5). For beta diversity, PERMANOVA analysis revealed significant differences in bacterioplankton structure between sampling period (Fig. 6a). Pairwise comparisons revealed significant differences in beta diversity between July 2019 samples compared to all other sampling sites (p < 0.001) and between Sep 2019 and Jan 2020 (p = 0.019) (Fig. 6b).
Bacterial community identification at phylum and genus levels
Sequences from all data sets of the four sample groups could be assigned to 48 phyla. The overall composition of bacterioplankton at the phylum level of all surface samples is shown in Fig. S2. Among these groups, cyanobacteria were the phyla with the most assigned sequences, averaging 31.75%. Other dominant bacterial phyla were Proteobacteria (26.5%), Actinobacteria (15.5%), Bacteroidetes (12.25%), Verrucomicrobia (6%), Planctomycetes (3%), and Chlorobi (1.25%) (Fig. 7). At the genus level, 18.3% and 9.9% of OTUs in the July and September 2019 samples, respectively, were assigned to the genus Planktothrix, while 14.4% and 20% of OTUs in the November 2019 and January 2020 samples, respectively, were assigned to the genus Microcystis within the family Chroococcaceae. The distribution of the 10 most frequently detected bacterioplankton genera in all samples analyzed at four time points is shown in Fig. 8. The relative abundance of cyanobacteria generally increased from July to September (26.7%) and increased in the following months to > 35% in November and January (Wilcoxon rank sum test, p < 0.05) (Fig. 9A). It is noteworthy that the different sampling sites showed different trends in the relative abundance of cyanobacteria. For example, in July, the lowest concentration of cyanobacteria was detected in sample B04, while the highest concentration was measured in sample B12 (Fig. S2). This scenario was also observed in January, when sample B04 had the lowest concentration of cyanobacteria. Strong concentrations of cyanobacteria were observed in all samples collected in November, with their relative abundance exceeding > 50% in sample B15 and > 20% as the lowest concentration in sample B29. A greater amount of Proteobacteria was observed in September 2019 than in January 20 (Wilcoxon rank sum test, p < 0.05) (Fig. 9B). At the same time, the relative abundance of Actinobacteria in the July 2019 samples was remarkably higher than the relative abundances calculated at other sampling times (Fig. 9C). In addition, the relative abundance of Bacteroidetes was significantly lower in the July 19 samples than in September 19 and November 19 (Fig. 9D). The abundance of the remaining bacterial phyla, including Verrucomicrobia, Planctomycetes, and Chlorobi, did not differ significantly in the bi-monthly samples.
In addition to the predominant phyla in Billings Reservoir, there were approximately 3.98% unclassified OTUs in the surface water, indicating as yet unidentified bacterial populations. The detailed distribution of these new bacteria by location and date of collection is shown in Fig. S3.
We then investigated the relationships between physicochemical variables and bacterial composition of the seven major phyla. Analysis of the July 2019 samples showed that the abundance of cyanobacteria was positively related to water depth and turbidity, and that Proteobacteria and Bacteroidetes were strongly related to temperature and nitrate levels, respectively (Fig. 10A). The abundance of Proteobacteria was most strongly and positively associated with PC1, explaining 27% of the variance in this component. Along PC2, the concentrations of DO, the relative abundances of Planctomycetes and Verrucomicrobia had a much greater loading than the 13 other variables in this component. Analysis of PC1 and PC2 in the September 2019 samples explained 49.5% of the variance. PCA showed that the abundance of Actinobacteria, Planctomycetes, and Verrucomicrobia was most positively associated with pH and temperature (Fig. 10B) and that taxa belonging to Proteobacteria and Bacteroidetes had the strongest loadings on PC1. The first two principal components of the Nov-2019 samples explained 45.3% of the variability in the data set, with P concentrations most strongly and positively associated with PC1 and Proteobacteria abundance negatively associated with PC2. Figure 10C shows that Proteobacteria, Bacteroidetes, and Cyanobacteria were positively associated with nitrate concentration in water, while Actinobacteria were related to pH. The abundance of Planctomycetes and Verrucomicrobia was related to the concentrations of DO. Finally, PC1 and PC2 of Jan-2020 samples explained 53.6% of the variance, and the individual variable loadings showed that temperature and pH were the variables most positively associated with PC1, while Proteobacteria and Verrucomicrobia were negatively associated with PC1 and PC2, respectively. The PCA shown in Fig. 10D indicated that Actinobacteria and Verrucomicrobia were associated with the concentrations of DO and water depth, and that the abundance of Planctomycetes was affected by temperature and pH.
Taxonomic biomarker discovery and functional analysis
LEfSe analysis was performed on bimonthly samples using KEGG orthology (KO) of metabolic functions predicted by PICRUSt to assess taxonomic biomarkers. The Kruskal–Wallis method was used to examine grouped data with a significance of 0.05, and the statistically differentially distributed KO Ids were used for LDA model analysis, with relative frequency of significance assessed by an LDA threshold of 2.0. A total of 32 genera and 41 species were significantly more abundant in all paired comparisons between sampling dates (p value (FDR) < 0.05, LDA effect size ≥ 3.0) (Table S1). Cytophagaceae (GU454944_g species) and Planktophila were among the bacteria whose relative abundances differed significantly (Fig. 11A and B). For example, the September 19 and November 19 samples were characterized by a preponderance of Cytophagaceae (LDA score 4.37, p value (FDR) = 0.00036), while the July 19 bacteriome was characterized by a preponderance of Planktophila compared to the other sampling dates (LDA score 3.98, p value (FDR) = 0.004). LEfSe analysis also showed that Planctomycetes were significantly less abundant in the January 20 samples compared to the other sampling dates (LDA score 3.92, p value (FDR) < 0.0001) (Fig. 11C). The analysis also showed that the Microcystis aeruginosa cyanobacteria group was significantly more abundant in the January 20 samples (Fig. 11d Microcystis aeruginosa cyanobacteria group), while C. Planktothrix_uc species were detected significantly more often in the July 19 samples (Fig. 11C and E. Planktothrix_uc).
Analysis of the potential functions of the metagenomes between collection dates yielded a total of 5575 significant KO Ids for all paired comparisons between collection dates (p value (FDR) ≤ 0.05) (Table S2). Metabolic pathways (ko01100) was the most abundant functional gene category, followed by biosynthesis of secondary metabolites (ko01110), microbial metabolism in different environments (ko01120), two-component system (ko02020), ABC transporters (ko02010), biosynthesis of cofactors (ko01240), and carbon metabolism (ko01200).
Discussion
In this study, we examined the spatiotemporal changes in bacteriological and physicochemical parameters over the course of a year in 19 bi-monthly surface water samples collected from Billings Reservoir. Genome sequencing of the 16 s rRNA gene was generated using the Illumina MiSeq platform and used to analyze variance in bacterial community structure.
Variation in physicochemical parameters
The characteristics of the surface water from Billings Reservoir showed seasonal differences in physicochemical composition. For example, as expected, water surface temperature, an important abiotic factor affecting aquatic microbial communities, was on average 4.70 °C warmer during the summer months than during the winter months. A temperature fluctuation of this magnitude can greatly accelerate the growth rates of cyanobacteria and mesophilic bacteria61,62,63. Seasonal changes in temperature have also been reported as the principal factor driving alterations of the bacterial populations in freshwater Lake Taihu64 and in Pearl River Estuary sediments65. In addition to temperature fluctuations, we have found that the amount of nutrients in the reservoir changes with the seasons. This may have affected not only the bacterioplankton communities66,67 but also led to seasonal responses in the fish communities68. Our results are consistent with a previous study by Mankiewicz et al.69 who described the dynamics of Microcystis in the summer of five consecutive years at Sulejów Reservoir and concluded that optimal nutrient concentrations, high water retention, and temperature were the main causes of the prevalence of Cyanobacteria producing toxic microcystins. In other studies, the availability of nitrogen, not phosphate, was considered one of the most important factors in the dominance of toxic over nontoxic Microcystis populations, usually caused by gradual anthropogenic eutrophication70,71. In contrast, DO, which often affects bacterial communities in aquatic ecosystems72, does not vary significantly over the course of the year, so it was not considered to affect bacterioplankton communities in Billings surface water. Consistent with our results on the water surface from Billings reservoir, Pierangeli et al.73 recently found that DO and groundwater pH showed no statistical differences among sampling sites. According to our results, the water was neutral, with a relatively large increase in pH in September, the month of transition from the dry winter to the hot rainy season. All surface water samples collected from the reservoir, regardless of season, had an average pH of 7.6 and a range of 6.0 to 8.86, which is within the pH range determined by WHO for most natural waters. The higher pH than the upper limit of 8.5 could be due to abundant rainfalls which promotes leaching and dissolution of salts74 or to algal growth, which could be promoted by increased CO2 consumption during photosynthesis75. Our results also showed that the water level in the reservoir was lowest during the summer season due to increasing temperatures, increasing evaporation, and increased demand for human consumption. Many studies have shown that water depth affects the physicochemical composition of the water as well as the release of nutrients from the sediment and favors the growth of certain bacteria76,77,78,79.
Bacterioplankton dynamics and community structure
As expected, the results showed that seasonal variations dominate the spatial patterns of bacterioplankton communities in the reservoir, as evidenced by the diversity indices and community composition. In particular, summer showed higher diversity indices and different bacterioplankton community composition than the other seasons. These differences in abundance could have been caused by significant seasonal variations in temperature and the availability of inorganic nutrients such as nitrogen in the reservoir.
The phytoplankton of Billings Reservoir showed that the cyanobacteria group quantitatively dominated the community structure, especially in summer. These results are consistent with those of other studies in Carpina Reservoir (Pernambuco, Brazil)80, Lake Tanganyika (Central Africa)81, Lake Victoria (Kenya)82, Dongping Lake (China)83, and Nui Coc Reservoir (Vietnam)84. The significant abundance of cyanobacteria in all seasons reported in this study suggests that these strains have the ability to adapt to the changing environmental circumstances of reservoirs. The fact that the cyanobacteria dominated in summer and could reach higher concentrations than the cyanobacteria that dominated in spring and winter indicates greater eutrophication. The increase in cyanobacteria can have both positive and negative ecological implications. On the one hand, they contribute to primary production and are a fundamental component of the aquatic food web85. On the other hand, some cyanobacteria can produce toxins that are harmful to aquatic life and human health, leading to issues when they become overly abundant. Our findings suggest that the proliferation of cyanobacteria in the Billings Reservoir can be traced to a confluence of environmental and ecological variables. These blue-green algae are remarkably adaptable to a spectrum of aquatic habitats due to their photosynthetic nature, enabling them to flourish under diverse light conditions and nutrient availabilities, thus outcompeting other species in numerous ecosystems. Notably, the study highlighted a surge in key nutrients such as sulfate, phosphate, ammonium nitrogen, and nitrate nitrogen during November 2019 and January 2020, which cyanobacteria efficiently utilize, particularly phosphate, to thrive in eutrophic conditions86. Moreover, the optimal median water temperature of 26.85 °C recorded in January bolsters their metabolic activities, accelerating growth and proliferation. Additionally, the reduction in water depth during the dry season presumably enhances light penetration, a boon for the photosynthesis-dependent cyanobacteria79. The consistent dissolved oxygen (DO) levels observed by the study imply that oxygen availability was not a constraining factor, and the photosynthetic activity of cyanobacteria could have contributed to maintaining these stable DO concentrations87. The proliferation of cyanobacteria leads to the frequent occurrence of algal blooms88. During algal blooms, cyanobacteria release various low molecular weight compounds such as glucose, organic acids, amino acids, and sugar alcohols, benefiting bacteria in the phycosphere89. Additionally, the proliferation and decomposition of algae not only elevate the pH of water but also promote microbial growth, resulting in an increased presence of certain microbial groups in eutrophic waters90. However, it is crucial to acknowledge that the abundance of cyanobacteria in inland waters, including the observed higher concentrations in the reservoir during summer, is primarily attributed to nutrient loading from agricultural fertilizers, particularly phosphorus, in the catchment area. The decomposition of harmful algal blooms can also release toxins and negatively impact microbial species diversity91. Acknowledging the predominant role of fertilizer-driven eutrophication in cyanobacterial dominance is essential for a comprehensive understanding of these blooms92. In our study, Planktothrix was the numerically dominant bacterial genus in the reservoir in the July and September 2019 samples, while Microcystis was the most abundant bacterial species detected in the November and January 2020 samples. Changes in cyanobacterial community structure may be influenced in large part by nutrients in aquatic ecosystems. For example, abundances of Synechococcus and Cyanobium in East Fork Lake and Delaware Lake were comparable to those in other oligotrophic freshwater lakes93,94. According to a study of cyanobacteria in Lake Erie, Synechococcus and Cyanobium were absent for the previous two decades but have since become the dominant taxa as a result of the transition from eutrophic to oligo-mesotrophic conditions95. On the other hand, Planktothrix has been identified as a bacterial biomarker for lakes that are either eutrophic or hypertrophic80. Recently, Zhang et al.96 found that Planktothrix may live symbiotically with antibiotic-resistant bacteria and secrete antifungal chemicals to promote the emergence and spread of these bacteria. Thus, the detection of Planktothrix in the reservoir has far-reaching human health implications beyond conventional cyanotoxin risks.
The second most abundant phylum in the reservoir was Proteobacteria. This phylum was indicated as the most abundant phylum in sediments or soils because of its ability to adapt to a variety of toxic conditions and its involvement in organic matter degradation and metabolic processes in lake sediments97.
The overall function of the microbial population in surface water of the Billings reservoir increased as eutrophication progressed. Eutrophication had the greatest impact on metabolic functions such as biosynthesis of secondary metabolites and microbial metabolism in different environments. This finding is in agreement with the results of Wan et al.98, who studied the diversity of the bacterioplankton population in Lake Nanhu before and after dredging and identified several metabolic pathways.
Conclusions
The MPA data obtained in this study provide a detailed description of seasonal changes in the taxonomic composition and quantitative ratios of various bacterial groups in the surface waters of Billings Reservoir. The most abundant OTUs belong to the phyla cyanobacteria and proteobacteria, which constitute a major proportion of each community; then follow the phyla Actinobacteria, Bacteroidetes, Verrucomicrobia, Planctomycetes, and Chlorobi. Bacterial communities found in surface waters during the winter season are dominated by Planktothrix, in contrast to those found during the summer season, which are dominated by Microcystis, which may be explained by seasonal physical and chemical factors. On the other hand, the seasonal variations in the physico-chemical properties of the surface water of the reservoir create new conditions according to which the concentrations of the various bacterial groups change.
Data availability
The sequencing data generated during the current study are available in the Zenodo repository (DOI https://doi.org/https://doi.org/10.5281/zenodo.8298073).
References
Wang, J., Da, L., Song, K. & Li, B. L. Temporal variations of surface water quality in urban, suburban and rural areas during rapid urbanization in Shanghai, China. Environ. Pollut. 152, 387–393. https://doi.org/10.1016/j.envpol.2007.06.050 (2008).
Song, H., Li, Z., Du, B., Wang, G. & Ding, Y. Bacterial communities in sediments of the shallow Lake Dongping in China. J. Appl. Microbiol. 112, 79–89. https://doi.org/10.1111/j.1365-2672.2011.05187.x (2012).
Haller, L. et al. Composition of bacterial and archaeal communities in freshwater sediments with different contamination levels (Lake Geneva, Switzerland). Water Res. 45, 1213–1228. https://doi.org/10.1016/j.watres.2010.11.018 (2011).
Mendez-Garcia, C. et al. Microbial stratification in low pH oxic and suboxic macroscopic growths along an acid mine drainage. ISME J. 8, 1259–1274. https://doi.org/10.1038/ismej.2013.242 (2014).
Dong, S., Liu, L., Zhang, Y. & Jiang, F. Occurrence and succession of bacterial community in O3/BAC process of drinking water treatment. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph16173112 (2019).
Fuhrman, J. A. et al. Annually reoccurring bacterial communities are predictable from ocean conditions. Proc. Natl. Acad. Sci. U. S. A. 103, 13104–13109. https://doi.org/10.1073/pnas.0602399103 (2006).
Sinkko, H. et al. Bacteria contribute to sediment nutrient release and reflect progressed eutrophication-driven hypoxia in an organic-rich continental sea. PloS One 8, e67061. https://doi.org/10.1371/journal.pone.0067061 (2013).
Hudon, C. & Carignan, R. Cumulative impacts of hydrology and human activities on water quality in the St. Lawrence River (Lake Saint-Pierre, Quebec, Canada). Can. J. Fish. Aquat. Sci. 65, 1165–1180 (2008).
Hou, J., Song, C., Cao, X. & Zhou, Y. Shifts between ammonia-oxidizing bacteria and archaea in relation to nitrification potential across trophic gradients in two large Chinese lakes (Lake Taihu and Lake Chaohu). Water Res. 47, 2285–2296. https://doi.org/10.1016/j.watres.2013.01.042 (2013).
Wagner, R., Zona, D., Oechel, W. & Lipson, D. Microbial community structure and soil pH correspond to methane production in Arctic Alaska soils. Environ. Microbiol. 19, 3398–3410. https://doi.org/10.1111/1462-2920.13854 (2017).
Utz, R. M., Eshleman, K. N. & Hilderbrand, R. H. Variation in physicochemical responses to urbanization in streams between two Mid-Atlantic physiographic regions. Ecol. Appl. Publ. Ecol. Soc. Am. 21, 402–415. https://doi.org/10.1890/09-1786.1 (2011).
Muluye, T., Fetahi, T., Engdaw, F. & Mohammed, A. Cyanotoxins in African waterbodies: Occurrence, adverse effects, and potential risk to animal and human health. Environ. Geochem. Health 45, 7519–7542. https://doi.org/10.1007/s10653-023-01724-3 (2023).
McElhiney, J., Lawton, L. A. & Leifert, C. Investigations into the inhibitory effects of microcystins on plant growth, and the toxicity of plant tissues following exposure. Toxicon Off. J. Int. Soc. Toxinol. 39, 1411–1420. https://doi.org/10.1016/s0041-0101(01)00100-3 (2001).
Crush, J. R., Briggs, L. R., Sprosen, J. M. & Nichols, S. N. Effect of irrigation with lake water containing microcystins on microcystin content and growth of ryegrass, clover, rape, and lettuce. Environ. Toxicol. 23, 246–252. https://doi.org/10.1002/tox.20331 (2008).
Dos Santos Machado, L. et al. Permanent occurrence of Raphidiopsis raciborskii and cyanotoxins in a subtropical reservoir polluted by domestic effluents (Itupararanga reservoir Sao Paulo Brazil). Environ. Sci. Pollut. Res. Int. 29, 18653–18664. https://doi.org/10.1007/s11356-021-16994-6 (2022).
Svircev, Z., Krstic, S., Miladinov-Mikov, M., Baltic, V. & Vidovic, M. Freshwater cyanobacterial blooms and primary liver cancer epidemiological studies in Serbia. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 27, 36–55. https://doi.org/10.1080/10590500802668016 (2009).
Bower, P. A., Scopel, C. O., Jensen, E. T., Depas, M. M. & McLellan, S. L. Detection of genetic markers of fecal indicator bacteria in Lake Michigan and determination of their relationship to Escherichia coli densities using standard microbiological methods. Appl. Environ. Microbiol. 71, 8305–8313. https://doi.org/10.1128/AEM.71.12.8305-8313.2005 (2005).
Pandey, P. K., Kass, P. H., Soupir, M. L., Biswas, S. & Singh, V. P. Contamination of water resources by pathogenic bacteria. AMB Express 4, 51. https://doi.org/10.1186/s13568-014-0051-x (2014).
Erkkilä, A. & Kalliola, R. Patterns and dynamics of coastal waters in multi-temporal satellite images: Support to water quality monitoring in the Archipelago Sea, Finland. Estuar. Coast. Shelf Sci. 60, 165–177. https://doi.org/10.1016/j.ecss.2003.11.024 (2004).
Vasistha, P. & Ganguly, R. Water quality assessment of natural lakes and its importance: An overview. Mater. Today Proc. 32, 544–552. https://doi.org/10.1016/j.matpr.2020.02.092 (2020).
Ribeiro, M. S. F., Tucci, A., Matarazzo, M. P., Viana-Niero, C. & Nordi, C. S. F. Detection of cyanotoxin-producing genes in a eutrophic reservoir (Billings Reservoir, São Paulo, Brazil). Water 12, 903 (2020).
Sant’Anna, C. L. et al. Highly toxic Microcystis aeruginosa strain, isolated from Sao Paulo-Brazil, produce hepatotoxins and paralytic shellfish poison neurotoxins. Neurotox. Res. 19, 389–402. https://doi.org/10.1007/s12640-010-9177-z (2011).
Ussami, K. A. & Guilhoto, J. J. M. Economic and water dependence among regions: The case of Alto Tiete, Sao Paulo State, Brazil. EconomiA 19, 350–376. https://doi.org/10.1016/j.econ.2018.06.001 (2018).
Marcondes, M. A. et al. Characterization of bacterial communities from the surface and adjacent bottom layers of water in the billings reservoir. Life https://doi.org/10.3390/life12081280 (2022).
de Carvalho, L. R., Sant’Anna, C. L., Gemelgo, M. C. P. & Azevedo, M. T. D. P. Cyanobacterial occurrence and detection of microcystin by planar chromatography in surface water of Billings and Guarapiranga Reservoirs, SP, Brazil. Braz. J. Bot. 30, 141–148. https://doi.org/10.1590/S0100-840420070001000144 (2007).
Gomes, A. D. et al. Fatty acid composition of tropical fish depends on reservoir trophic status and fish feeding habit. Lipids 51, 1193–1206. https://doi.org/10.1007/s11745-016-4196-z (2016).
Leme, E. et al. Billings reservoir water used for human consumption presents microbiological contaminants and induces both behavior impairments and astrogliosis in zebrafish. Ecotoxicol. Environ. Saf. 161, 364–373. https://doi.org/10.1016/j.ecoenv.2018.06.009 (2018).
Bittencourt-Oliveira Mdo, C., Piccin-Santos, V., Moura, A. N., Aragao-Tavares, N. K. & Cordeiro-Araujo, M. K. Cyanobacteria, microcystins and cylindrospermopsin in public drinking supply reservoirs of Brazil. Anais Acad. Bras. Cienc. 86, 297–310. https://doi.org/10.1590/0001-3765201302512 (2014).
Rocha-e-Silva, T. A. et al. Dynamics of cytochrome P450 inducers in polluted sites of Sao Paulo city reservoirs. Ecotoxicol. Environ. Saf. 59, 109–115. https://doi.org/10.1016/S0147-6513(03)00097-6 (2004).
Godoy, R. G. et al. Bacterial community composition and potential pathogens along the Pinheiros River in the southeast of Brazil. Sci. Rep. 10, 9331. https://doi.org/10.1038/s41598-020-66386-y (2020).
Rastogi, R. P., Madamwar, D. & Incharoensakdi, A. Bloom dynamics of cyanobacteria and their toxins: Environmental health impacts and mitigation strategies. Front. Microbial. 6, 1254. https://doi.org/10.3389/fmicb.2015.01254 (2015).
dos Anjos, F. M. et al. Detection of harmful cyanobacteria and their toxins by both PCR amplification and LC-MS during a bloom event. Toxicon Off. J. Int. Soc. Toxinol. 48, 239–245. https://doi.org/10.1016/j.toxicon.2006.05.006 (2006).
Wengrat, S. & Bicudo, D. C. Spatial evaluation of water quality in an urban reservoir (billings complex, southeastern Brazil). Acta Limnol. Bras. 23, 200–216. https://doi.org/10.1590/S2179-975X2011000200010 (2011).
Petrere, M. Jr., Walter, T. & Minte-Vera, C. V. Income evaluation of small - scale fishers in two Brazilian urban reservoirs: Represa Billings (SP) and Lago Paranoa (DF). Braz. J. Biol. 66, 817–828. https://doi.org/10.1590/s1519-69842006000500007 (2006).
Rezende, K. F., Santos, R. M., Borges, J. C., Salvo, L. M. & da Silva, J. R. Histopathological and genotoxic effects of pollution on Nile Tilapia (Oreochromis niloticus, Linnaeus, 1758) in the Billings Reservoir (Brazil). Toxicol. Mech. Methods 24, 404–411. https://doi.org/10.3109/15376516.2014.925020 (2014).
Poupeau, F. et al. Water Conflicts and Hydrocracy in The Americas. Coalitions, networks, Policies, São Paulo: IEE-USP (2018).
Fracalanza, A. P. & Freire, T. M. Crise da água na região metropolitana de São Paulo: injustiça ambiental, privatização e mercantilização de um bem comum. Geousp – Espaço e Tempo 19, 464–478 (2015). doi: https://doi.org/10.11606/issn.2179-0892.geousp.2015.103064
Gemelgo, M. C., Mucci, J. L. & Navas-Pereira, D. Population dynamics: Seasonal variation of phytoplankton functional groups in brazilian reservoirs (Billings and Guarapiranga, Sao Paulo). Braz. J. Biol. 69, 1001–1013. https://doi.org/10.1590/s1519-69842009000500004 (2009).
Herlemann, D. P. et al. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 5, 1571–1579. https://doi.org/10.1038/ismej.2011.41 (2011).
Pereira da Fonseca, T. A., Pessoa, R. & Sanabani, S. S. Molecular investigation of bacterial communities: Data from two frequently used surfaces in the Sao Paulo Institute of Tropical Medicine. Data Brief 8, 399–403. https://doi.org/10.1016/j.dib.2016.05.064 (2016).
Pereira da Fonseca, T. A., Pessoa, R. & Sanabani, S. S. Molecular analysis of bacterial microbiota on Brazilian currency note surfaces. Int. J. Environ. Res. Public Health 12, 13276–13288. https://doi.org/10.3390/ijerph121013276 (2015).
Pereira da Fonseca, T. A., Pessoa, R., Felix, A. C. & Sanabani, S. S. Diversity of bacterial communities on four frequently used surfaces in a large Brazilian Teaching Hospital. Int. J. Environ. Res. Public Health 13, 152. https://doi.org/10.3390/ijerph13020152 (2016).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. https://doi.org/10.1093/bioinformatics/btu170 (2014).
Rognes, T., Flouri, T., Nichols, B., Quince, C. & Mahe, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 4, e2584. https://doi.org/10.7717/peerj.2584 (2016).
Myers, E. W. & Miller, W. Optimal alignments in linear space. Comput. Appl. Biosci. CABIOS 4, 11–17. https://doi.org/10.1093/bioinformatics/4.1.11 (1988).
Wheeler, T. J. & Eddy, S. R. nhmmer: DNA homology search with profile HMMs. Bioinformatics 29, 2487–2489. https://doi.org/10.1093/bioinformatics/btt403 (2013).
Yoon, S. H. et al. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evolut. Microbial. 67, 1613–1617. https://doi.org/10.1099/ijsem.0.001755 (2017).
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C. & Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200. https://doi.org/10.1093/bioinformatics/btr381 (2011).
Chao, A. & Lee, S.-M. Estimating the number of classes via sample coverage. J. Am. Stat. Assoc. 87, 210–217. https://doi.org/10.1080/01621459.1992.10475194 (1992).
Chao, A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics 43, 783–791 (1987).
Burnham, K. P. & Overton, W. S. Robust estimation of population size when capture probabilities vary among animals. Ecology 60, 927–936. https://doi.org/10.2307/1936861 (1979).
Magurran, A. E. Measuring biological diversity (Malden, MA, John Wiley & Sons). https://scholar.google.com/scholar_lookup?hl=en&publication_year=2013&author=A.+E.+Magurran&title=Measuring+biological+diversity (2013).
Chao, A. & Shen, T.-J. Nonparametric estimation of Shannon’s index of diversity when there are unseen species in sample. Environ. Ecol. Stat. 10, 429–443. https://doi.org/10.1023/A:1026096204727 (2003).
Gil, M., Ramil, F. & AgIs, J. A. Hydroids (Cnidaria, Hydrozoa) from mauritanian coral mounds. Zootaxa https://doi.org/10.11646/zootaxa.4878.3.2 (2020).
Heck, K. L., van Belle, G. & Simberloff, D. Explicit calculation of the rarefaction diversity measurement and the determination of sufficient sample size. Ecology 56, 1459–1461. https://doi.org/10.2307/1934716 (1975).
Whittaker, R. H. Dominance and diversity in land plant communities: Numerical relations of species express the importance of competition in community function and evolution. Science 147, 250–260. https://doi.org/10.1126/science.147.3655.250 (1965).
Lin, J. Divergence measures based on the Shannon entropy. IEEE Trans. Inf. Theory 37, 145–151. https://doi.org/10.1109/18.61115 (1991).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60. https://doi.org/10.1186/gb-2011-12-6-r60 (2011).
Kruskal, W. H. & Wallis, W. A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 47, 583–621. https://doi.org/10.2307/2280779 (1952).
McGarigal, K., Cushman, S. & Stafford, S. Multivariate Statistics for Wildlife & Ecology Research (Springer, 2000).
Kelly, J. J., Minalt, N., Culotti, A., Pryor, M. & Packman, A. Temporal variations in the abundance and composition of biofilm communities colonizing drinking water distribution pipes. PloS one 9, e98542. https://doi.org/10.1371/journal.pone.0098542 (2014).
Hao, C. et al. Significant seasonal variations of microbial community in an acid mine drainage lake in Anhui Province, China. Environ. Pollut. 223, 507–516. https://doi.org/10.1016/j.envpol.2017.01.052 (2017).
Shahraki, A. H., Chaganti, S. R. & Heath, D. Spatio-temporal dynamics of bacterial communities in the shoreline of Laurentian great Lake Erie and Lake St. Clair’s large freshwater ecosystems. BMC Microbiol. 21, 253. https://doi.org/10.1186/s12866-021-02306-y (2021).
Zhu, C. et al. Seasonal succession and spatial distribution of bacterial community structure in a eutrophic freshwater Lake, Lake Taihu. Sci. Total Environ. 669, 29–40. https://doi.org/10.1016/j.scitotenv.2019.03.087 (2019).
Zhang, W. et al. Toward understanding the dynamics of microbial communities in an estuarine system. PloS One 9, e94449. https://doi.org/10.1371/journal.pone.0094449 (2014).
Liu, L. et al. Relationship between bacterial community and its environmental in Zuohai Lake, China. Wei sheng wu xue bao = Acta microbiologica Sinica 55, 1177–1189 (2015).
Cai, W. et al. Revealing the relationship between microbial community structure in natural biofilms and the pollution level in urban rivers: A case study in the Qinhuai River basin, Yangtze River Delta. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 74, 1163–1176. https://doi.org/10.2166/wst.2016.224 (2016).
Sutela, T. & Vehanen, T. Effects of water-level regulation on the nearshore fish community in boreal lakes. Hydrobiologia 613, 13–20. https://doi.org/10.1007/s10750-008-9468-z (2008).
Mankiewicz-Boczek, J. et al. Cyanophages infection of Microcystis bloom in lowland dam reservoir of Sulejow, Poland. Microb. Ecol. 71, 315–325. https://doi.org/10.1007/s00248-015-0677-5 (2016).
Timothy, W. D., Dianna, L. B., Gregory, L. B. & Christopher, J. G. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 8, 715–725. https://doi.org/10.1016/j.hal.2009.02.004 (2009).
Yoshida, M., Yoshida, T., Takashima, Y., Hosoda, N. & Hiroishi, S. Dynamics of microcystin-producing and non-microcystin-producing Microcystis populations is correlated with nitrate concentration in a Japanese lake. FEMS Microbiol. Lett. 266, 49–53. https://doi.org/10.1111/j.1574-6968.2006.00496.x (2007).
Montserrat, A., Rodrigo, D. I. I., Anthony, D. B. & Osvaldo, U. Oxygen modulates bacterial community composition in the coastal upwelling waters off central Chile. Deep Sea Res. Part II Top. Stud. Oceanogr. 156, 68–79. https://doi.org/10.1016/j.dsr2.2018.02.001 (2018).
Pierangeli, G. M. F. et al. Spatial variation and environmental parameters affecting the abundant and rare communities of bacteria and archaea in the sediments of tropical urban reservoirs. Microb. Ecol. https://doi.org/10.1007/s00248-022-02047-z (2022).
Zhang, X. et al. Seasonal variations of soil bacterial communities in Suaeda wetland of Shuangtaizi River estuary, Northeast China. J. Environ. Sci. 97, 45–53. https://doi.org/10.1016/j.jes.2020.04.012 (2020).
Aknaf, A. et al. Study of the spatial and temporal variation of physical-chemical parameters characterizing the quality of surface waters of the lagoon Marchica–North-East Morocco. JMES 8, 3216–3225 (2017).
da Costa, M. R. A., Attayde, J. L. & Becker, V. Effects of water level reduction on the dynamics of phytoplankton functional groups in tropical semi-arid shallow lakes. Hydrobiologia 778, 75–89. https://doi.org/10.1007/s10750-015-2593-6 (2016).
Chellappa, N. T., Borba, J. M. & Rocha, O. Phytoplankton community and physical-chemical characteristics of water in the public reservoir of Cruzeta, RN, Brazil. Braz. J. Biol. 68, 477–494. https://doi.org/10.1590/s1519-69842008000300004 (2008).
Braga, G. G. & Becker, V. Influence of water volume reduction on the phytoplankton dynamics in a semi-arid man-made lake: A comparison of two morphofunctional approaches. Anais Acad. Bras. Cienc. 92, e20181102. https://doi.org/10.1590/0001-3765202020181102 (2020).
Mendes, C. F. et al. The reduction in water volume favors filamentous cyanobacteria and heterocyst production in semiarid tropical reservoirs without the influence of the N: P ratio. Sci. Total Environ. 816, 151584. https://doi.org/10.1016/j.scitotenv.2021.151584 (2022).
Lira, G. A., Araujo, E. L., Bittencourt-Oliveira Mdo, C. & Moura, A. N. Phytoplankton abundance, dominance and coexistence in an eutrophic reservoir in the state of Pernambuco, Northeast Brazil. Anais Acad. Bras. Cienc. 83, 1313–1326. https://doi.org/10.1590/s0001-37652011000400018 (2011).
Descy, J.-P. et al. Phytoplankton pigments and community composition in Lake Tanganyika. Freshw. Biol. 50, 668–684. https://doi.org/10.1111/j.1365-2427.2005.01358.x (2005).
Lung’Ayia, H. B. O., M’Harzi, A., Tackx, M., Gichuki, J. & Symoens, J. J. Phytoplankton community structure and environment in the Kenyan waters of Lake Victoria. Freshw. Biol. 43, 529–543. https://doi.org/10.1046/j.1365-2427.2000.t01-1-00525.x (2000).
Tian, C., Lu, X., Pei, H., Hu, W. & Xie, J. Seasonal dynamics of phytoplankton and its relationship with the environmental factors in Dongping Lake, China. Environ. Monit. Assess. 185, 2627–2645. https://doi.org/10.1007/s10661-012-2736-4 (2013).
Duong, T. T. et al. Seasonal variation of cyanobacteria and microcystins in the Nui Coc Reservoir, Northern Vietnam. J. Appl. Phycol. 25, 1065–1075. https://doi.org/10.1007/s10811-012-9919-9 (2013).
Briland, R. D., Stone, J. P., Manubolu, M., Lee, J. & Ludsin, S. A. Cyanobacterial blooms modify food web structure and interactions in western Lake Erie. Harmful Algae 92, 101586. https://doi.org/10.1016/j.hal.2019.03.004 (2020).
Loza, V., Perona, E. & Mateo, P. Specific responses to nitrogen and phosphorus enrichment in cyanobacteria: factors influencing changes in species dominance along eutrophic gradients. Water Res. 48, 622–631. https://doi.org/10.1016/j.watres.2013.10.014 (2014).
Aparicio Medrano, E., Uittenbogaard, R. E., van de Wiel, B. J., Dionisio Pires, L. M. & Clercx, H. J. An alternative explanation for cyanobacterial scum formation and persistence by oxygenic photosynthesis. Harmful Algae 60, 27–35. https://doi.org/10.1016/j.hal.2016.10.002 (2016).
Yang, C., Sun, J., Chen, Y., Wu, J. & Wang, Y. Linkage between water soluble organic matter and bacterial community in sediment from a shallow, eutrophic lake, Lake Chaohu, China. J. Environ. Sci. 98, 39–46. https://doi.org/10.1016/j.jes.2020.05.023 (2020).
Zhou, J. et al. Phycosphere microbial succession patterns and assembly mechanisms in a marine dinoflagellate bloom. Appl. Environ. Microbiol. https://doi.org/10.1128/AEM.00349-19 (2019).
Michael, A., Sánchez, F., Nandini, S. & Sarma, S. S. S. Zooplankton community structure in relation to microcystins in the eutrophic Lake Zumpango (State of Mexico). Fundam. Appl. Limnol. https://doi.org/10.1127/fal/2020/1256 (2020).
Zeng, Y. X., Yu, Y., Qiao, Z. Y., Jin, H. Y. & Li, H. R. Diversity of bacterioplankton in coastal seawaters of Fildes Peninsula, King George Island, Antarctica. Arch. Microbiol. 196, 137–147. https://doi.org/10.1007/s00203-013-0950-2 (2014).
Yang, S. et al. Impacts of agricultural topdressing practices on cyanobacterial bloom phenology in an early eutrophic plateau Lake, China. J. Hydrol. 594, 125952. https://doi.org/10.1016/j.jhydrol.2020.125952 (2021).
Postius, C. & Ernst, A. Mechanisms of dominance: Coexistence of picocyanobacterial genotypes in a freshwater ecosystem. Arch. Microbiol. 172, 69–75. https://doi.org/10.1007/s002030050742 (1999).
Callieri, C. & Stockner, J. G. Freshwater autotrophic picoplankton: A review. J. Limnol. 61, 1–14. https://doi.org/10.4081/jlimnol.2002.1 (2002).
Ouellette, A. J., Handy, S. M. & Wilhelm, S. W. Toxic Microcystis is widespread in Lake Erie: PCR detection of toxin genes and molecular characterization of associated cyanobacterial communities. Microb. Ecol. 51, 154–165. https://doi.org/10.1007/s00248-004-0146-z (2006).
Zhang, Q. et al. Cyanobacterial blooms contribute to the diversity of antibiotic-resistance genes in aquatic ecosystems. Commun. Biol. 3, 737. https://doi.org/10.1038/s42003-020-01468-1 (2020).
Zhou, Z., Tran, P. Q., Kieft, K. & Anantharaman, K. Genome diversification in globally distributed novel marine Proteobacteria is linked to environmental adaptation. ISME J. 14, 2060–2077. https://doi.org/10.1038/s41396-020-0669-4 (2020).
Wan, W. et al. Dredging alleviates cyanobacterial blooms by weakening diversity maintenance of bacterioplankton community. Water Res. 202, 117449. https://doi.org/10.1016/j.watres.2021.117449 (2021).
Author information
Authors and Affiliations
Contributions
M.A.M. data curation, visualization, investigation, methodology, R.P. formal analysis, visualization. A.J.D.S.D. writing, review and editing, visualization. P.B.C. formal analysis, visualization, writing, reviewing and editing; S.S.S. conceptualization, monitoring, project management, fundraising and writing the final manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marcondes, M.A., Pessôa, R., José da Silva Duarte, A. et al. Temporal patterns of bacterial communities in the Billings Reservoir system. Sci Rep 14, 2062 (2024). https://doi.org/10.1038/s41598-024-52432-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52432-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.