Abstract
The consumption of bovine milk and its derivatives is associated with inflammation, gastrointestinal disorders and the development of diseases in humans. Most studies related to milk effects are based on either clinal trials or experimental models such as mice and cell cultures. In this study we present the nematode Caenorhabditis elegans as an alternative model to evaluate the effects of milk on oxidative stress in other animal models. The toxicological effect of 20% milk exposure for 8 h on C. elegans was evaluated by progeny quantification, body size and pharyngeal pumping rate. Treating the worms with milk did not affect the worms brood size but interfered with their fecundity by delaying the average number of eggs in the first day of oviposition when compared to the control group. The size of worms treated with milk were significantly smaller compared to control. The pharyngeal pumping rate of milk-treated animals was not significantly different compared to untreated animals. Taking together, the results suggest that 20% milk treatment is not toxic for the worms but induces a minor delay achieving its adulthood and therefore its reproduction period. Milk exposure did not reduce the worms’ survival under stress conditions and increase endogenous ROS levels. This study contributes to characterize the effects of milk exposure on the C. elegans nematode.
Similar content being viewed by others
Introduction
Milk is considered a vital part of a healthy and balanced diet because of its nutrition value. Bovine milk and its derivatives have been the target of criticism, precisely because their consumption is associated with biomarkers of inflammation, gastrointestinal disorders and disease development1,2. On the other hand, studies have demonstrated that dairy intake produces significant and substantial suppression of oxidative and inflammatory stress1,3,4, and milk proteins are capable of attenuating the reactivity of reactive oxygen species (ROS) and increasing the antioxidant enzyme activities4,5. The administration of milk with high CLA (conjugated linoleic acid) to rats resulted in beneficial effects on lipid metabolism, inflammation and oxidative stress in the liver6.
Most studies related to milk effects are based on either clinical trials or experimental models such as mice and cell cultures. Here we present Caenorhabditis elegans as an alternative model to evaluate the effects of milk on oxidative stress in other animal models. C. elegans is an excellent model organism for experimental research involving metabolic disorders, as 60-80% of human genes have an ortholog in the C. elegans genome and 40% of known genes and associated with human diseases have orthologs in C. elegans7,8. In addition, due to the difficulty of using metabolic processes in humans in real time, studies are based on animal models which can contribute to clarifying the molecular pathways involved in human diseases at the metabolic or genomic level in vivo. As C. elegans has signaling pathways that are highly conserved to study oxidative stress, we sought to assess whether C. elegans could be used as a model to study the interference of milk in the levels of reactive oxygen species and in the resistance capacity against stress conditions. Therefore, the objective herein was to evaluate the effect of exposure to milk on the levels of reactive oxygen species and on the stress resistance of Caenorhabditis elegans.
Methods
Milk samples
Figure 1 illustrates the methodology of the study with C. elegans. Four samples of pasteurized cow milk from different herds were used (Table 1). The samples were stored in small aliquots at − 20 °C until used for experiments. This study was approved by the Animal Research Ethics Committee of the Federal University of Rio Grande do Norte, under protocol No. 022/2020.
Caenorhabditis elegans maintenance and treatment
Caenorhabditis elegans strain used in this work was N2 wildtype for all tests. Synchronized L1 larvae were obtained by alkaline lysis (10 M NaOH, 2% sodium hypochlorite) and grown on Nematode Growth Medium (NGM) plates (0.25% Tryptone, 0.3% NaCl, 1.5% Agar, 1 mM CaCl, 1 mM MgSO4, 25 Mm KPO4 pH 6.0, and 5 µg/mL Cholesterol) seeded with Escherichia coli OP50 for 40 h at 20 °C unil L3 stage9. The worms were then transferred to M9 solution (20 mM M KH2PO4, 40 mM Na2HPO4, 850 mM NaC and 1 mM MgSO4) containing 20% milk and E. coli OP50 for another 8 h at 20 °C under agitation until L4 stage. For all assays, the control group consisted of animals treated with M9 + E. coli OP50 without milk.
Toxicity assays in C. elegans
The toxicity to milk exposure was assessed by measuring brood size, growth and pharyngeal pumping. To determine total progeny, ten L4 worms, previously treated with 20% milk or M9 solution were placed on individual NGM plates. During the egg-laying period, nematodes were transferred onto new plates every 24 h for five days until the end of the reproductive period. The F1 progeny from each individual worm was counted. The total progeny numbers for each plate were calculated and divided by the number of animals. For growth, 30 worms from each group after treatment were photographed using the AmScope MU300 Digital Camera connected to an optical microscope (Axio Imager Z2, Zeiss, NY, USA). Body area and length was determined by using the ImageJ software. The average pharyngeal pumping rate was calculated by three 20-second intervals of ten worms per group. All experiments were conducted in triplicates.
Oxidative stress resistance assay
After milk treatment, worms were transferred to solid NGM containing 10 mM tert-butyl hydroperoxide (t-BOOH). This medium was poured into a 24-well plate. Each well was then seeded with E. coli OP50. For each condition, which included the control and the 20% milk condition, we utilized five wells, each containing ten nematodes. The survival fractions were scored after 15 hours. The experiment was carried out in three independent experiments.
Quantification of reactive oxygen species (ROS)
ROS levels were measured using H2DCF-DA (2′,7′-Dichlorodihydrofluorescein diacetate) as described previously10. After milk treatment, approximately forty L4 worms per group were transferred to PBS+ 1% Tween-20 on a 96-well microtiter plate, to which 50 μM H2DCF-DA was added. Measurements were performed in triplicate in a multilabel microplate reader GloMax®-Multi Detection System (Promega, Wisconsin, USA), with excitation at 490 nm and emission at 510–570 nm, and the mean values were calculated. Readings were performed every 30 min for 2 h.
Statistical analysis
Data analysis was performed using descriptive statistics using mean and standard deviation. All data obtained were submitted to the statistical program GraphPad Prism® version 6.01 (USA) for graphing and data analysis. Non-parametric data were analyzed by ANOVA to compare groups. The Student’s t-test was used for data with normal distribution. Significance was considered at 5%.
Results and discussion
Evaluation of toxicological effects of milk in C. elegans
Milk supplementation has been already employed in C. elegans as an alternative strategy to develop an axenic growth medium named C. elegans Habituation and Reproduction (CeHR) medium11. Interestingly, this protocol uses 20% of milk in the final volume of a liquid medium. Here, we also exposed C. elegans to 20% milk but differently from the procedures previously described for the axenic CeHR medium, we first grew L1 worms on regular solid NGM medium for 40 h and then submitted them at L3–L4 transition state for 8 h in order to evaluate the effect of exposure to milk on the levels of reactive oxygen species and on the stress resistance.
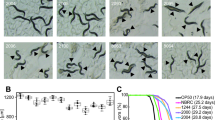
Since changing the worms from agar to liquid axenic media is associated with phenotypical alterations such as delayed development, and reduced fecundity12,13, we first evaluated the effect of milk ingestion on these two biological parameters. Treating the worms with 20% milk for 8 hours did not affect the worm's brood size (Fig. 2A). However, milk exposures interfered with their fecundity by delaying the average number of eggs on the first day of oviposition when compared to the control group (Fig. 2B). Using an approach based on bacterial ring assay to evaluate the effect of chemical exposure on C. elegans chemotactic behavior and reproduction over the three-day life cycle, Worku and Gerald11 observed that both colostrum and non-fat milk increased migration and reproduction. Interestingly, we also observed that the worms treated with milk were significantly smaller compared to control (Fig. 2C). These results also suggest that even a short period exposure of 8 hours incubation in milk-supplemented M9 is able to influence the worms’ development.
Effect of 20% milk treatment on C. elegans toxicological parameters. L3 wild-type animals were treated with 20% milk for 8 h until they reached L4 stage and then toxicity was assessed by measuring total brood size (A), number of embryos laid per day (B), body size (C) and pharyngeal pumping (D). (A) In terms of total number of eggs laid, there is no statistical difference between control and treated worms. (B) On the first day of oviposition, the number of eggs laid by the animals treated with milk was significantly reduced. ***p = 0.0006 by 2-Way-ANOVA. (C) Worms treated with 20% have body length significantly reduced. ****p < 0.0001 by Student’s t-test. (D) There is no statistical difference in pharyngeal pumping rate between control and treated worms.
We also measured the pharyngeal pumping rate in L4 animals in order to verify whether milk treatment alters feeding behavior. The pharyngeal pumping rate of milk-treated animals was not significantly different compared to untreated animals (Fig. 2D). Taking together, the results suggest that 20% milk treatment is not toxic for the worms but induces a minor delay achieving its adulthood and therefore its reproduction period.
Effect of milk treatment on oxidative stress resistance in C. elegans
Several studies have already highlighted the antioxidant potential and response to oxidative stress of milk and dairy protein intake1,3,4,5,14. However, few studies have explored these effects in the C. elegans animal model. Here, we evaluated how 20% milk treatment would affect C. elegans stress resistance.
The composition of bovine milk can vary according to several factors, such as genetics, age and physical condition, but it is mainly influenced by the animals' diet. Since milk composition can vary, we tested different samples (Table 1). Although the average survival fraction of milk-treated worms was reduced compared to control group, statistical analysis showed that there was no significant difference, expect for milk sample #3 (Fig. 3A).
Effect of 20% milk on ROS production and oxidative stress resistance of C. elegans. Stress resistance assay. L3 wild-type animals were treated with 20% milk for 8 h until they reached L4 stage and transferred to NGM supplemented with 10 mM t-BOOH to induce oxidative stress conditions. Animal survival was scored after 24 h. Assays were performed in triplicate and expressed as mean ± SEM. *(P < 0.05) Student’s t-test (A). ROS quantification. L3 wild-type animals were treated with 20% milk for 8 h and the production of ROS was measured using the H2DCF-DA probe. Assays were performed in triplicate and expressed as mean ± SEM. ***p < 0.001 by Student’s t-test (B). #1, #2, #3 and #4: milk samples with different compositions.
In order to associate these results with a possible oxidant effect induced by milk treatment, we measured worms’ endogenous ROS production. We observed that ROS production in milk-treated worms was not different when compared to the control group except for sample #3 (Fig. 3B). Interestingly, only worms treated with this sample showed significantly increased ROS levels, which is the same milk sample that causes reduced oxidative stress survival. Xing et al. observed that worms treated with lactose showed reduced lifespan, which was completely rescued by the co-administration of the antioxidant N-Acetyl-L-cysteine (NAC), indicating that redox stress could be, at least in part, responsible for the lactose-induced senescence15. Interestingly, the percentage of lactose in sample #3 is the lowest compared to other samples used in our work, which contradicts what was observed by Xing et al.15. Protein fraction present in milk is also able to interfere in ROS production as shown by an ex vivo study carried out by Albenzio16. The study demonstrates that lower ROS levels were found in cells incubated with α-lactalbumin and β-lactoglobulin, while the highest level was found after incubation with β-casein isolated from bovine milk when compared to goat and sheep species. Thus, the protein composition of the studied milk may influence the individual’s response to oxidative stress. When using the in vivo C. elegans model from the tested samples, the milk treatment generally does not affect resistance to oxidative stress and does not increase ROS levels under oxidative stress conditions. The main results of the study are illustrated in Fig. 4.
Although most of the studies cited in the literature claim reduced oxidative stress by ingestion of dairy products, the present study was not able to find a reduction in ROS for the evaluated samples. It is important to emphasize that as there are few works using milk in C. elegans, the comparisons which can be made are based on studies with other experimental models, which despite being widely used, have different metabolic responses.
Conclusion
Under the conditions of this study, exposure to 20% milk does not seem to decrease resistance under stress conditions and does not interfere with endogenous ROS levels in wild-type C. elegans.
Data availability
Data will be made available upon request to the corresponding author.
References
Zemel, M. B., Sun, X., Sobhani, T. & Wilson, B. Effects of dairy compared with soy on oxidative and inflammatory stress in overweight and obese subjects. Am. J. Clin. Nutr. 91(1), 16–22. https://doi.org/10.3945/ajcn.2009.28468 (2010).
Rangel, A. H. N. et al. Lactose intolerance and cow’s milk protein allergy. Food Sci. Technol. 36(2), 179–187. https://doi.org/10.1590/1678-457X.001910.1590/1678-457X.0019 (2016).
Stancliffe, R. A., Thorpe, T. & Zemel, M. B. Dairy attenuates oxidative and inflammatory stress in metabolic syndrome. Am. J. Clin. Nutr. 94(2), 422–430. https://doi.org/10.3945/ajcn.111.013342 (2011).
Khan, I. T. et al. The antioxidant components of milk and their role in processing, ripening, and storage: Functional food. Vet. World 12(1), 12–33. https://doi.org/10.14202/vetworld.2019.12-33 (2019).
Awad, S. et al. Antioxidant activity of milk protein hydrolysate in alloxan-induced diabetic rats. J. Dairy Sci. 99(11), 8499–8510. https://doi.org/10.3168/jds.2015-10626 (2016).
Trinchese, G. et al. Milk from cow fed with high forage/concentrate ratio diet: Beneficial effect on rat skeletal muscle inflammatory state and oxidative stress through modulation of mitochondrial functions and AMPK activity. Front. Physiol. https://doi.org/10.3389/fphys.2018.01969 (2019).
Culetto, E. & Sattelle, D. B. A role for Caenorhabditis elegans in understanding the function and interactions of human disease genes. Hum. Mol. Genet. 9, 869–877. https://doi.org/10.1093/hmg/9.6.869 (2000).
Kaletta, T. & Hengartner, M. O. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 5, 387–398. https://doi.org/10.1038/nrd2031 (2006).
Brenner, S. Genetics of caenorhabditis-elegans. Genetics 77(1), 71–94. https://doi.org/10.1093/genetics/77.1.71 (1974).
Caland, R. B. O., Cadavid, C. O. M., Carmona, L., Pena, L. & Oliveira, R. P. Pasteurized orange juice rich in carotenoids protects against oxidative stress and β-amyloid toxicity through direct and indirect mechanisms. Oxid. Med. Cell. Longev. 2019, 1–13. https://doi.org/10.1155/2019/5046280 (2019).
Worku, M. & Gerald, C. C. elegans chemotaxis and reproduction following environmental exposure. in Proceedings of the 2007 National Conference on Environmental Science and Technology (Springer, New York, 2009). Doi: https://doi.org/10.1007/978-0-387-88483-7_39
Çelen, I., Doh, J. H. & Sabanayagam, C. R. Genetic adaptation of C. elegans to environment changes I: Multigenerational analysis of the transcriptome. bioRxiv 194506. Doi: https://doi.org/10.1101/194506 (2017).
Flavel, M. R. et al. Growth of Caenorhabditis elegans in defined media is dependent on presence of particulate matter. Genes Genomes Genetics 8(2), 567–575. https://doi.org/10.1534/g3.117.300325 (2018).
Bruckbauer, A. & Zemel, M. Dietary calcium and dairy modulation of oxidative stress and mortality in aP2-agouti and wild-type mice. Nutrients 1(1), 50–70. https://doi.org/10.3390/nu1010050 (2009).
Xing, S. et al. Lactose induced redox-dependent senescence and activated Nrf2 pathway. Int. J. Clin. Exp. Pathol. 12(6), 2034–2045 (2019).
Albenzio, M. et al. Milk nutrition and childhood epilepsy: An ex vivo study on cytokines and oxidative stress in response to milk protein fractions. J. Dairy Sci. 101, 4842–4852. https://doi.org/10.3168/jds.2017-13104 (2018).
Acknowledgements
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). D.C. is a fellowship honored researcher Post doctorate of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação de Amparo e Promoção da Ciência, Tecnologia e Inovação do Rio Grande do Norte (FAPERN). Figures 1 and 4 were created in Biorender.com.
Author information
Authors and Affiliations
Contributions
Conceptualization and investigation, I.L., A.H., R.P., G.M. and C.O.; data curation, writing—original draft preparation, I.L.; writing—review and editing, A.H., R.P., G.M., C.O. and D.C.; supervision, A.H., R.P., G.M., C.O., D.C. and K.A; Figure 4 preparation, D.C.; Figures 1, 2 and 3 preparation, I.L. and R.P. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Oliveira, I.L.S., Silva, G.M.M., Cadavid, C.O.M. et al. Effect of milk exposure on the redox profile of Caenorhabditis elegans. Sci Rep 14, 1926 (2024). https://doi.org/10.1038/s41598-024-52254-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52254-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.