Abstract
Pesticides are ubiquitous in the catchments of the Great Barrier Reef (GBR) and regularly discharge into the nearshore waters. Effective management of pesticides requires suitable water quality guideline values (WQGVs), and further ecotoxicological data for many pesticides are needed to improve the reliability of environmental risk assessments. To help address this issue, toxicity thresholds were determined to two species of tropical marine microalgae Tisochrysis lutea and Tetraselmis sp. for a suite of herbicides detected in the GBR. Photosystem II (PSII) herbicides significantly reduced growth with no effect concentration (NEC) and 10% effect concentration (EC10) values spanning two orders of magnitude from 0.60 µg L−1 for diuron to 60 µg L−1 for simazine across both species. However, growth was insensitive to the non-PSII herbicides. The NEC/EC10 thresholds for most herbicide-microalgae combinations were greater than recent WQGVs intended to protect 99% of species (PC99); however, metribuzin was toxic to T. lutea at concentrations lower than the current PC99 value, which may have to be revisited. The toxicity thresholds for alternative herbicides derived here further inform the development of national and GBR-specific WQGVs, but more toxicity data is needed to develop WQGVs for the > 50 additional pesticides detected in catchments of the GBR.
Similar content being viewed by others
Introduction
Globally, around two million tonnes of pesticides are applied on an annual basis to agricultural crops in order to enhance crop yield and to ensure food security for an ever-growing human population1, with Australia using over 60,000 tonnes of pesticides in 20202. Pesticides, including herbicides, insecticides, and fungicides are designed to control pest species; however, their unintentional contamination of aquatic systems has the potential to harm non-target species, potentially leading to deleterious ecological effects1,3. Previous research has shown that herbicide pollution, through agricultural runoff, is a potential threat to the health of the world’s largest tropical reef system, the Great Barrier Reef (GBR)4,5. Thirty-five rivers discharge into the GBR with large parts of these catchments downstream of areas with extensive agricultural activities where pesticides are heavily used (e.g. beef cattle grazing and sugarcane cultivation)6,7. This results in the year-round detection of pesticides in the Great Barrier Reef catchment area (GBRCA) with the highest concentrations usually detected in the wet season following heavy rain falls and associated runoff8,9. Recently, pesticides (mostly herbicides) were detected in 99.8% of over 2600 water samples collected over a five-year period from 15 waterways that discharge into the GBR lagoon9.
Established in 2005, the GBR Marine Monitoring Program (MMP) surveys and monitors marine water quality of the GBR and contributes data to assess risks as part of the Reef 2050 Water Quality Improvement Plan10. Initially, MMP pesticide monitoring focused on Photosystem II (PSII) herbicides11, which are extensively applied in agriculture along the Queensland coast12. More recently, MMP monitored 15 PSII herbicides (including breakdown products) in passive samplers at 11 fixed marine sites over the 12-month monitoring period, with herbicides diuron, atrazine and hexazinone being the most frequently detected at maximum concentrations of 250, 176 and 58 ng L−1, respectively13. A recent study reported significant increases in the concentrations of the five priority PSII herbicides (ametryn, atrazine, diuron, hexazinone, tebuthiuron) at some inshore GBR sites over the 14 years of MMP monitoring14. In a simulation exercise coupling end-of-system concentrations with a 3D hydrodynamic model across the entire GBR lagoon, it was indicated that diuron concentrations often exceeded 75 ng L−1 over 1000 km2 of GBR marine ecosystems during flood events15. The high usage of PSII herbicides, in conjunction with a high persistence in seawater, (i.e. half-lives > 100 days in seawater; Mercurio et al.16) illustrates why this class of herbicide represents the most frequently detected pesticides in monitored waterways13.
PSII herbicides inhibit photosynthesis by binding to the QB-binding site of PSII which interrupts the electron transport chain. This results in photooxidative stress and disruption of ATP synthesis, and ultimately leads to plant death17. The PSII complex is highly conserved in oxygenic photosynthetic organisms; thus, PSII herbicides can have negative effects on a wide range of non-target marine phototrophs. For example, PSII herbicides have been shown to inhibit photosynthesis in seagrass18,19,20, coral21,22, symbiotic foraminifera23, crustose coralline algae24, and growth in microalgae25,26,27,28 and in free living coral symbionts (Symbiodiniaceae)29. Due to potential risk of negative effects of PSII herbicides to key reef organisms, legislation10,30 and a voluntary shift in best farming practices has led to the substitution from the priority PSII herbicides (ametryn, atrazine, diuron, hexazinone, tebuthiuron) to alternative herbicides and triggered the expansion of the MMP to monitor over 40 pesticides in recent years13,31,32. Alternative PSII herbicides detected in inshore waters of the GBR include bromacil, metribuzin, simazine, propazine and prometryn, as well as a range of non-PSII herbicides13,31,33. The latter include synthetic auxins, such as 2,4‐dichlorophenoxyacetic acid (2,4-D), 2-methyl-4-chlorophenoxyacetic acid (MCPA) and fluroxypyr which mimic the plant growth hormone, resulting in abnormal plant growth, senescence, and plant death in dicots34. Other non-PSII herbicides detected include haloxyfop, a post-emergence herbicide, which specifically inhibits acetyl-coA carboxylase (ACCase), an enzyme critical in fatty acid synthesis35, and imazapic, a broad-spectrum herbicide that inhibits the acetohydroxyacid synthase enzyme (AHAS), which catalyses the first step in the synthesis of branched-chain amino acids such as valine, leucine and isoleucine36.
To assess environmental risks that herbicides pose to the GBR, detected herbicide concentrations have been compared against national WQGVs37, or more recently to merged default guideline values (DGVs) derived specifically for assessing pesticide risk to freshwater and marine ecosystems of the GBR38. In Australia, the preferred method to derive WQGVs for toxicants is to collate chronic toxicity threshold data for individual species into species sensitivity distributions (SSDs)39. SSDs are cumulative distributions of species’ responses to a given toxicant and are used to estimate concentrations that protect a certain proportion of the species community (PCx), such as 99, 95, 90 and 80% of all species (PC99, PC95, PC90 and PC80, respectively). According to the Australian and New Zealand Guidelines for Fresh and Marine Water Quality, SSDs require toxicity data for at least five species that belong to at least four taxonomic groups37. However, using toxicity data from at least eight species is strongly encouraged, and data for more than 15 species is considered optimal39.
Currently, there are national WQGVs for only 17 pesticides37 of the > 80 pesticides and their transformation products detected in the GBRCA40,41, which includes freshwater and marine WQGVs. In addition, there are GBR-specific WQGVs for 11 pesticides42. However, 10 of these WQGVs are characterised as being of low reliability (due to lack of chronic toxicity thresholds data for marine species). Moreover, there are no WQGVs for most alternative herbicides detected in the GBRCA. New freshwater and marine WQGVs have been proposed for 27 pesticides detected in the GBRCA43,44,45; however, many of the proposed guideline values remain at low or even very low reliability due to lack of appropriate toxicity data for tropical marine species. The reliability of marine WQGVs for use in a pesticide risk metric (PRM) has been partially addressed by applying merged fresh/marine SSDs to derive WQGVs for 22 pesticides, including the 15 that contribute to 99% of the risk to freshwater and marine GBR ecosystems40. Nevertheless, WQGVs for many alternative herbicides detected in the GBR remain of low to moderate reliability and/or have been derived from toxicity datasets that include few tropical marine species37.
Marine microalgae are widely used for routine chronic ecotoxicological testing due to their ecological importance as primary producers, short generation time, and relative ease of culturing in the laboratory e.g.46,47,48. Using two marine microalgal species from two different phyla, the haptophyte Tisochrysis lutea (formerly known as Isochrysis galbana) and the chlorophyte Tetraselmis sp., this study aimed to identify toxicity thresholds for a range of PSII and non-PSII herbicides to improve WQGVs for tropical marine environments. Specifically, the no-effect concentration (NEC) and the effect concentrations at 10% (EC10) and 50% (EC50) of population growth inhibition were derived for eleven and eight herbicides for T. lutea and Tetraselmis sp., respectively. Herbicides tested here were chosen according to data gaps identified in consultation with the Queensland Department of Environment and Science and included six PSII herbicides (simazine, tebuthiuron, bromacil, metribuzin, propazine and diuron as the reference herbicide26,27,29) and five non-PSII herbicides, the ACCase inhibitor haloxyfop-p-methyl, the acetohydroxyacid synthase inhibitor imazapic, and three auxin mimics 2,4-D, MCPA and fluroxypyr.
Results
Assay performance
Water quality parameters across all assays were within acceptable test limits49 (Table 1). Notably, the range of dissolved oxygen levels was greater likely due to measurements performed later in the day after microalgae were exposed to an extended period of light. More detailed information of water quality parameters for each treatment can be found in the online Supplementary Tables 1 and 2.
Analyses of herbicide concentrations did not detect herbicide contamination in any of the seawater controls. The average change in herbicide concentrations in test solutions between start (0 h) and end of test (72 h) measurements were within 12% for all herbicides, except for bromacil and haloxyfop, where a loss of 30% and 50% was detected, respectively. All nominal and measured concentrations of the herbicides tested can be found in the online Supplementary Tables 1 and 2.
Specific growth rates for all control samples were ≥ 0.92 day−1 with a coefficient of variation (CV) between control replicates ≤ 10% indicating test acceptability49. The control growth rate of T. lutea was 1.42 ± 0.23 day−1 (mean ± SD) with a CV of 3 ± 2% between replicates, whereas control growth rate of Tetraselmis sp. was 1.02 ± 0.066 day−1 with a mean CV of 2 ± 2% between replicates. There was no difference (p > 0.05) in SGR between the seawater controls and controls containing acetone and DMSO.
Toxicity of PSII herbicides
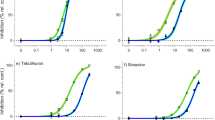
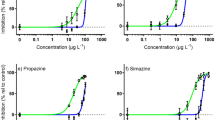
The inhibition of SGR of T. lutea and Tetraselmis sp. increased with increasing herbicide concentrations (Figs. 1, 2). Out of the suite of tested herbicides, PSII herbicides exhibited the highest toxicity to growth in both algal species with EC50 values of 3.1–206 µg L−1 for T. lutea and 5.2–154 µg L−1 for Tetraselmis sp. (Tables 2, 3). T. lutea and Tetraselmis sp. showed similar sensitivities to diuron and bromacil exhibiting similar EC50 toxicity thresholds (Tables 2, 3). The order of toxicity of T. lutea to PSII herbicides based on EC50 values was: metribuzin > diuron > bromacil > propazine > tebuthiuron > simazine (Table 2), while for Tetraselmis sp. diuron was most toxic and simazine remained the least toxic (Table 3). The EC50 values for all tested PSII herbicides (including the reference herbicide diuron) fell within the range of estimated EC50 values from previous research except for bromacil, with EC50 values of 6.8 and 6.7 µg L−1 for T. lutea and Tetraselmis sp., respectively, compared to 19–28 µg L−1 EC50 values for other microalgae (Table 4).
Concentration–response curves for ECx derivation. Concentration–response curves for Tisochrysis lutea showing the relative percent inhibition of 72 h specific growth rate (open white circles, mean ± SE) following herbicide exposure to: (a) bromacil, (b) diuron, (c) metribuzin, (d) propazine, (e) simazine, (f) tebuthiuron, (g) 2,4-D, (h) MCPA, (i) fluroxypyr, (j) haloxyfop and (k) imazapic. Closed black circles represent individual treatment replicates. The solid black line is the fitted regression model and the shaded areas represent the model’s 95% confidence limits. Best-fitting models (based on Akaike Information Criterion) were Weibull type II 3-parameter (bromacil, diuron, tebuthiuron), 4-parameter log-logistic (2,4-D, metribuzin), Weibull type I 3-parameter (propazine), Weibull type I 4-parameter (haloxyfop, imazapic) and Weibull type II 4-parameter (MCPA, simazine). All concentrations are reported in µg L−1. Note the dissimilar scaling on the horizontal axis.
Concentration–response curves for ECx derivation. Concentration–response curves for Tetraselmis sp. showing the relative percent inhibition of 72 h specific growth rate (open white circles, mean ± SE) following herbicide exposure to (a) bromacil, (b) diuron, (c) metribuzin, (d) propazine, (e) simazine, (f) tebuthiuron, (g) haloxyfop, and (h) imazapic. Closed black circles represent individual treatment replicates. The solid black line is the fitted regression model and the shaded areas represent the model’s 95% confidence limits. Best-fitting models (based on Akaike Information Criterion) were Weibull type II 3-parameter (bromacil, metribuzin, propazine, simazine, tebuthiuron), 3-parameter log-logistic (diuron) and Weibull type I 4-parameter (haloxyfop). All concentrations are reported in µg L−1. Note the dissimilar scaling on the horizontal axis.
Toxicity of non-PSII herbicides
For both algal species, the EC50 values of non-PSII herbicides were at least an order of magnitude higher compared to the PSII herbicides (Tables 2, 3). While the acetyl-CoA carboxylase inhibitor haloxyfop and the acetohydroxyacid synthase (AHAS) inhibitor imazapic exhibited similar EC50s of around 4300 µg L−1 for T. lutea (Table 2), the auxin mimics 2,4 D, MCPA and fluroxypyr hardly affected growth at the highest concentrations tested and EC50 concentrations could not be estimated for MCPA and fluroxypyr within the tested concentration range (Fig. 1, Table 2). Similarly, haloxyfop was > 1000 times less toxic to the growth of Tetraselmis sp. than the most toxic PSII herbicide diuron and the EC50 could not be estimated for imazapic within the tested concentration range (Fig. 2, Table 3). The EC50 values that could be estimated were within the same concentration range as reported in previous studies (Table 4).
Discussion
This study extends toxicity data for a suite of PSII herbicides and non-PSII herbicides commonly detected in the Great Barrier Reef catchment area (GBRCA) for two relevant marine microalgae species, Tisochrysis lutea and Tetraselmis sp. to further inform the development of national water quality guideline values (WQGVs) and GBR-specific default guideline values (DGVs) and associated ecological risk assessments for pesticide mixtures. Consistent with previous studies, PSII herbicides were at least an order of magnitude more toxic to marine microalgae than non-PSII herbicides. The NEC/EC10 thresholds for most herbicide-microalgae combinations were greater than recent PC99 DGVs, indicating adequate protection; however, the PSII herbicide metribuzin was toxic to T. lutea at concentrations lower than the most recent PC99 values, which may have to be revisited. The reproducibility of control growth rates and toxicity estimates identified for these two microalgal species reinforces their suitability for routine ecotoxicity testing46,47,48,64.
Toxicity of PSII herbicides to T. lutea and Tetraselmis sp.
Chronic exposure to the six different PSII herbicides significantly reduced growth in both test species, T. lutea and Tetraselmis sp. Despite their shared mode of action, there is a significant difference in toxicity among the PSII herbicides, with bromacil, diuron, and metribuzin displaying a much greater toxicity in both species compared to the other PSII herbicides propazine, simazine and tebuthiuron. Interestingly, the differences in toxicity do not seem to be related to the chemical class of these herbicides. For instance, diuron and tebuthiuron belong to the same phenylurea class, yet diuron was 28- and 13-fold more toxic than tebuthiuron to T. lutea and Tetraselmis sp., respectively. Such a disparity in toxicity between these two herbicides aligns with other studies whereby diuron was 6–150-fold more toxic than tebuthiuron to other marine microalgae, including Rhodomonas salina, Chaetoceros muelleri, Cladocopium proliferum and Skeletonema costatum. Marzonie et al.29 reported no correlation between toxicity and the octanol–water partition coefficient among these herbicides, implying that the ability to cross algal cell walls and membranes or accumulate within cells does not significantly influence their relative toxicity. Instead, factors including steric compatibility (the spatial arrangement of atoms or groups) and specific affinity of each herbicide for the QB binding site on the D1 protein in PSII are likely to be the key determinants of relative toxicity65. Drifts in pH during the experiments (less than 1.5 pH units) were unlikely to have affected the solubility and bioavailability of any of the herbicides (pKa values > 2 from the experimental pH range), with the potential exception of bromacil which has a pKa of 9.3. Bromacil would become more soluble as the pH increased, but any effects on toxicity are unknown.
Toxicity of non-PSII herbicides to T. lutea and Tetraselmis sp.
T. lutea and Tetraselmis sp. were insensitive to the non-PSII herbicides tested here with either relatively high or no EC50 values estimated for all non-PSII herbicides. Auxin regulators, including 2, 4-D, MCPA and fluroxypyr, are primarily used as selective herbicides for controlling dicotyledons (i.e. broadleaves) but not most monocotyledons (i.e. rice, wheat, maize) by mimicking the action of the plant hormone auxin resulting in uncontrolled growth and eventually plant death. Though microalgae have been observed to produce phytohormones, including auxins, they are present at very low concentrations66, likely explaining the low sensitivity of T. lutea to these herbicides. Haloxyfop inhibits acetyl-CoA carboxylase (ACCase), a key enzyme involved in the biosynthesis of fatty acids. This enzyme comes in two isoforms, the prokaryotic (heteromeric) form and eukaryotic (homomeric) form. The heteromeric form of this enzyme is found in the plastids of plants and algae while the homomeric ACCase is found in the cytosol of plants and algae67,68. ACCase inhibitors, including haloxyfop, bind to and block the eukaryote-type homomeric ACCase enzyme45. Studies have found that the microalgae Chlorella variabilis and T. lutea contain the heteromeric form of ACCase68,69, likely explaining the lack of response of haloxyfop to T. lutea and Tetraselmis sp. Additionally, the cryptophyte Rhodomonas salina, diatom Chaetoceros muelleri, and dinoflagellate Cladocopium proliferum were insensitive to haloxyfop26,27,29 suggesting this mode of action is unlikely to have a deleterious effect on the population growth of microalgae.
Imidazolinone herbicides, such as imazapic, act by inhibiting the acetohydroxyacid synthase (AHAS), an enzyme important in the synthesis of three branched-chain aliphatic amino acids leucine, isoleucine, and valine in plants, fungi and microorganisms70. AHAS-inhibiting herbicides have been commercially applied to crops for several decades and since then 197 site-of-action resistance isolates have been identified in weeds71. Lonhienne et al.71 demonstrated that these mutations reduced the binding affinity of these herbicides and prohibited time-dependent accumulative inhibition. Time-dependent accumulative inhibition is the prolonged effect of the inactivation of AHAS long after the inhibitor (herbicide) has left the catalytic site. It is feasible that T. lutea and Tetraselmis sp. also possess resistance isolates explaining their lack of sensitivity to imazapic. Additionally, Thomas et al.26 hypothesized that the structure of imazapic may affect its bioavailability in seawater. Imazapic has a carboxylic acid group which may complex with Mg and Ca ions in seawater reducing the availability of the herbicide to surrounding organisms.
Implications of water quality guideline development
Although > 80 pesticides and their transformation products have been detected in the GBRCA40,41, only 17 have national water quality guideline values, often of low reliability, due to lack of appropriate marine toxicity data37. Of the herbicides tested here, only bromacil, diuron, simazine, tebuthiuron, 2,4-D and MCPA have existing WQGVs, with no current WQGVs for metribuzin, propazine, haloxyfop, imazapic, and fluroxypyr. A comparison of the existing national marine WQGVs37, proposed marine guideline values44,45 and the more recent merged default guideline values38 against growth toxicity thresholds (NEC/EC10, the lower of the two) for T. lutea and Tetraselmis sp. is presented in Table 5. The PC99 WQGV for bromacil is inadequate to protect either of the microalgal species since the estimated toxicity thresholds for bromacil are two orders of magnitude lower than the current WQGV. However, the current PC99 WQGVs for diuron, simazine, 2,4-D, and MCPA are protective of T. lutea and Tetraselmis sp. The more recent PGVs and DGVs for all herbicides are all lower than the NEC/EC10 values for both T. lutea and Tetraselmis sp., indicating sufficient protection for these herbicide/algae species combinations except for metribuzin. The NEC (0.50 µg L−1) and EC10 (0.72 µg L−1) thresholds for metribuzin to T. lutea are lower than the PGV and DGV value of 2.0 µg L−1 (Table 5) which may have to be revisited.
The NEC and EC10 thresholds derived for these herbicides are higher than most concentrations reported in recent MMP surveys of pesticides from month-long passive sampler deployments13,31,32. However, based on a simulation exercise coupling end of system diuron concentrations with a 3D hydrodynamic model, orders of magnitude higher concentrations are expected over short durations (hours to days) in marine waters of the GBR15, often exceeding WQGVs and the NEC/EC10 thresholds for diuron reported here. Importantly, the vast majority (> 90%) of marine samples taken as part of the MMP in the three most recent surveys comprise mixtures of more than one pesticide13,32, and assessments of risk should always consider the contributions of all pesticides detected, rather than exceedances of individual WQGVs separately38. The ‘multisubstance-potentially affected fraction’ (ms-PAF) approach has recently been implemented in MMP reporting to help address this issue13,31,32. The ms-PAF method assesses the cumulative ecological risk of pesticide mixtures for species assemblages by deriving the percentage of species that would be affected by each pesticide alone and combining with an additivity model to predict the joint percent affected fraction72. Exceedances of PC99 in nearshore waters of the GBR are more frequent when accounting for the effect of pesticide mixtures using ms-PAF13,31,32. This study provides additional toxicity threshold data for alternative PSII and non-PSII herbicides for tropical marine species that can be incorporated in SSDs to improve WQGVs, including the merged DGVs applied to assess risk of pesticide mixtures to the GBR using ms-PAF methods38.
Conclusion
Growth toxicity thresholds for PSII and non-PSII herbicides for microalgae Tioschrysis lutea and Tetraselmis sp. were determined, and both species of microalgae were over an order of magnitude more sensitive to PSII herbicides than non-PSII herbicides. When herbicides were tested individually, current PC99 PGV and DGV are adequate to protect both microalgal species, except from the PSII herbicide metribuzin which was harmful to T. lutea at concentrations lower than the PC99 PGV/DGV. However, since herbicides are rarely detected in isolation, it is important to consider other approaches, such as the ms-PAF, to assesses the cumulative ecological risk of pesticide mixtures. This study extends toxicity data for a suite of alternative herbicides detected in the GBRCA to further inform the development of national WQGVs and GBR-specific DGVs and associated ecological risk assessments for pesticide mixtures. This study targeted the more frequently detected alternate PSII and non-PSII herbicides, where more data was required to improve DGVs. However, there are more than 50 pesticides detected in the GBRCA without DGVs and further toxicity data is required for these pesticides to be included in future risk assessments for pesticide mixtures to the GBR.
Methods
Laboratory procedures
All test equipment (glass, HDPE) were acid washed (5% v/v nitric acid; Univar) for at least 24 h before being rinsed thrice with deionized, reverse-osmosis water and allowed to dry thoroughly. Erlenmeyer flasks (borosilicate, 125 mL) that served as test chambers for algal growth rate inhibition assays were additionally silanized with 2% dimethyldichlorosilane in 1,1,1-trichloroethane (Coatasil; Ajax Finechem) prior to acid-washing to minimize sorption of herbicides and algae to the glass.
Microalgal culture conditions
Tisochrysis lutea (strain CS-177) and Tetraselmis sp. (strain CS-317) were obtained from the Australian National Algae Supply Service, Hobart (CSIRO). Tetraselmis sp. is a clonal strain described and tentatively named Tetraselmis moretonica sp. nov. in Mazid (2009)73, but further work is required to confirm this. Both species were cultivated as batch cultures using sterile 500 mL Erlenmeyer flasks that contained 300 mL of sterile EDTA-free Guillard’s f/2 marine medium74. Inoculum (5 mL) of 7-day-old cultures were aseptically transferred weekly to 300 mL of fresh sterile f/2 medium to maintain cultures in exponential growth. All culture flasks were swirled daily to resuspend and aerate algae. Cultures were maintained in plant growth chambers (Labec, model PG 36) at 28 ± 1 °C, 33 ± 1.5 psu, and 12:12 h light:dark cycle (80–100 μmol photons m–2 s–1, Sylvania Aquastar 39W).
Preparation of test solutions
The toxicity of eleven and eight herbicides were tested with T. lutea and Tetraselmis sp., respectively. Herbicides tested for both species included six PSII herbicides simazine (CAS 122-34-9), tebuthiuron (CAS 34014-18-1), bromacil (CAS 314-40-9), metribuzin (CAS 21087-64-9), propazine (CAS 139-40-2) and diuron (CAS 330-54-1) as well as the acetyl-CoA carboxylase inhibitor haloxyfop-p-methyl (CAS 72619-32-0) and the acetohydroxyacid synthase inhibitor imazapic (CAS 104098-48-8). In addition, toxicity of the three auxin mimics, 2,4-D (CAS 94-75-7), MCPA (94-74-6) and fluroxypyr (CAS 69377-81-7) were assessed for T. lutea. Herbicide stock solutions (100–1000 mg L−1) were prepared in milli-Q water from analytical grade products (purity ≥ 98%, Sigma Aldrich, Castle Hill, NSW, Australia). Either dimethyl sulfoxide (DMSO) or acetone was used as a solvent carrier with the maximum amount in exposure not exceeding 0.02% (v/v) for DMSO and 0.04% (v/v) for acetone, except for the highest test concentrations for 2,4 D (≤ 0.4% (v/v) acetone), imazapic (≤ 0.1% (v/v) acetone) and simazine (0.25% (v/v) DMSO).
Toxicity testing
Toxicity thresholds of the different herbicides for T. lutea and Tetraselmis sp. were determined by 72 h growth inhibition assays48,49. Test solutions for at least six different herbicide concentrations, including a seawater control (no herbicide), were prepared using filtered natural seawater (FSW, 0.5 µm), supplemented with quarter-strength EDTA-free f/2 media as a nutrient source48 (final concentration 1/8f). A separate experiment was performed to assess potential effects of the solvent carrier (DMSO or acetone) at the same concentration as used in the highest test treatment. Inoculum was taken from microalgae cultures in exponential growth phase (4–5 days old)48,75. Prior to inoculation, algae suspensions were centrifuged twice (T. lutea: 780×g for 7 min; Tetraselmis sp.: 240×g for 4 min), and algal pellets resuspended in FSW to remove any residual culture medium. Densities of the concentrated algal cultures were assessed via manual counts using a hemocytometer and test solutions were inoculated with a starting density of either 3 × 103 or 1 × 104 cells mL−1 for T. lutea and 2.5 × 103 cells mL−1 for Tetraselmis sp., respectively. Following inoculation, each test solution was aliquoted into two or three replicate 125 mL Erlenmeyer flasks (50 mL test solution per flask) that were capped with a cotton plug and placed in a plant growth chamber (Labec, model PG36) set to 28 ± 1 °C and a 12:12 h light:dark cycle (80–100 μmol photons m–2 s–1, Sylvania Aquastar 39W). Test flasks were randomized and swirled daily to ensure sufficient gas exchange.
Cell density measurements
Post 72 h exposure, subsamples (7 mL) were taken from each replicate flask and cell densities determined using a flow cytometer (BD Accuri C6, BD Biosciences, CA, USA) with a standard filter set as per Trenfield et al.48. The flow rate was set to 35 µL min−1, 16 µm core size and sample volume of 25 µL. Fixed gating was used around the viable (chlorophyll fluorescing) cells to avoid the counting of dead cells or non-microalgal particles. Samples were run in duplicates and the average count in the respective gated region was used to calculate the specific growth rate (SGR). SGR was expressed as the logarithmic increase in cell density over the exposure duration using the following Eq. (1):
where SGRα-β (day−1) is the specific growth rate between day α to β; Cα and Cβ (cells mL−1) are the cell densities at time tα and tβ (day), respectively49.
An assay was considered valid if the mean SGR of control samples was ≥ 0.92 day−1 and a coefficient of variation (CV) of mean SGR between controls was ≤ 10%49. At least two independent assays were performed for each species and herbicide combination.
Water quality and chemical analyses
Physicochemical water quality parameters, including dissolved oxygen (mg L−1 and % saturation), pH, salinity (PSU) and electrical conductivity (mS cm−1), of all test solutions were measured at start (0 h) and at end of test (72 h) using a portable multi meter (HQ40D, Hach). Temperature was logged in 10-min intervals throughout the duration of tests (HOBO, Onset). At the end of the test, treatment replicate flasks were pooled after subsamples were taken for flow cytometry. Analytical samples (2 mL) were taken from pooled solutions and transferred into 4 mL amber glass vials before water quality measurements were conducted. Analytical samples were stored at − 20 °C before being transported to the Queensland Alliance for Environmental Health Sciences (QAEHS) at the University of Queensland for analysis. Prior to analysis, samples were spiked with surrogate labelled internal standards (Supplementary Table 3) for each herbicide at a final concentration of 10 ng mL−1. Limits of detection (LOD) and spike recoveries can be found in Supplementary Table 3. Blank samples (milli-Q water) were used for every 10–12 samples per batch. Herbicide analysis was performed using HPLC–MS/MS (SCIEX Triple Quad 6500 QTRAP mass spectrometer coupled to Shimadzu Nexera X2 uHPLC system) following the methodology provided in Thomas et al.26. Herbicide concentrations of 2–3 treatments per assay (including seawater control) were measured from the start and end of the test. To derive ‘measured’ concentrations for all other treatments, the geometric mean was calculated using the initial and final measured concentrations (time-weighted average). The average loss or difference from these measured concentrations was applied to all nominal concentrations.
Statistical analyses
Specific growth rate for each treatment was expressed as percent inhibition relative to the control response. This allowed pooling of data from multiple assays that were performed for each herbicide and algae species combination. In order to generate concentration–response curves, measured herbicide concentrations were used and regression analyses were conducted following prescribed procedures76. The package DRC in R77,78 was used to model concentration–response relationships and estimate toxicity thresholds that inhibited 10% and 50% of the SGR relative to controls (EC10 and EC50, respectively). Regression models included log-logistic, Weibull and hormesis models of different levels of parametrization. Model comparisons were conducted using the Akaike Information Criterion (AIC) and the model that best described the data was applied to derive estimates of toxicity. The associated 95% confidence limits were estimated using the delta method.
A no effect concentration (NEC) value is the preferred measure of toxicity since NECs are more closely aligned with the objective of guideline values. The estimations of NEC values were performed using the Bayesian model fitting software JAGS79, via the R2jags80 and jagsNEC81 packages in R82. Proportional decline in SGR (1-inhibition) was modelled as a function of log measured concentration of each herbicide using a Bayesian non-linear gaussian model. This model has been specifically developed to derive NECs for a binomial response variable83 but can be more generally defined by the following Eq. (2):
where E[Yi|xi] is the mathematical expectation of Yi (the response, e.g. in this case the proportional decline in SGR) conditional on a given concentration xi. The model parameters for the generalised case are α (the response at zero or low concentrations, also called “top”), − β (the rate of decay in the response after the NEC) and γ (the NEC value). For a gaussian Y, as used here, the model has the additional parameters Δ (an offset or intercept) and δ (the random error variance in Y). We used uninformative priors for the model parameters, including: α ~ dnorm(0, 0.1), β ~ dgamma(0.0001, 0.0001), y ~ dnorm(0, 0.01), Δ ~ dnorm(0, 0.1), and δ ~ dunif(0, 29). Note that in jags dnorm is parameterised as a mean and precision (rather than mean and sd, as in R). Models were run with 10,000 Markov chain Monte Carlo (MCMC) iterations after an initial “burn-in” period of 20,000 iterations and for five separate chains. Model fits were evaluated using trace plots and were found to have relatively good mixing in all cases. Bayesian 95% credible intervals (uncertainty) were based on the upper 97.5th and lower 2.5th percentile of the posterior sample.
Data availability
The datasets generated during the study are available in the eAtlas data repository at: https://eatlas.org.au/data/uuid/91967f34-b24d-4352-b6b0-526e54ec052f and https://eatlas.org.au/data/uuid/4a8d5927-0619-4f7e-8894-2e3aaf8d3aed.
References
Sharma, A. et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 1, 1. https://doi.org/10.1007/s42452-019-1485-1 (2019).
Ritchie, H., Roser, M. & Rosado, P. Pesticides. https://ourworldindata.org/pesticides (2022).
Pimentel, D. Amounts of pesticides reaching target pests: Environmental impacts and ethics. J. Agric. Environ. Ethics 8, 17–29. https://doi.org/10.1007/BF02286399 (1995).
Haynes, D. & Michalek-Wagner, K. Water quality in the Great Barrier Reef World Heritage Area: Past perspectives, current issues and new research directions. Mar. Pollut. Bull. 41, 428–434 (2000).
Lewis, S. E. et al. Herbicides: A new threat to the Great Barrier Reef. Environ. Pollut. 157, 2470–2484 (2009).
Brodie, J. & Landos, M. Pesticides in Queensland and Great Barrier Reef waterways—Potential impacts on aquatic ecosystems and the failure of national management. Estuar. Coast. Shelf Sci. 230, 106447. https://doi.org/10.1016/j.ecss.2019.106447 (2019).
Devlin, M. et al. Advancing Our Understanding of the Source, Management, Transport and Impacts of Pesticides on the Great Barrier Reef. A Report for the Queensland Department of Environment and Heritage Protection (James Cook University, 2015).
Kennedy, K. et al. The influence of a season of extreme wet weather events on exposure of the World Heritage Area Great Barrier Reef to pesticides. Mar. Pollut. Bull. 64, 1495–1507. https://doi.org/10.1016/j.marpolbul.2012.05.014 (2012).
Warne, M. St. J., Smith, R. A. & Turner, R. D. R. Analysis of pesticide mixtures discharged to the lagoon of the Great Barrier Reef, Australia. Environ. Pollut. 265, 114088. https://doi.org/10.1016/j.envpol.2020.114088 (2020).
Reef 2050 WQIP. Reef 2050 Water Quality Improvement Plan 2017–2022. www.reefplan.qld.gov.au (2018).
Kennedy, K. et al. Final Report—Monitoring of Organic Chemicals in the Great Barrier Reef Marine Park Using Time Integrated Monitoring Tools (2009–2010) (The University of Queensland, 2010).
Kroon, F. J. et al. River loads of suspended solids, nitrogen, phosphorus and herbicides delivered to the Great Barrier Reef lagoon. Mar. Pollut. Bull. 65, 167–181. https://doi.org/10.1016/j.marpolbul.2011.10.018 (2012).
Thai, P. et al. Marine Monitoring Program: Annual Report for Inshore Pesticide Monitoring 2018–19. Report to the Great Barrier Reef Marine Park Authority (Great Barrier Reef Marine Park Authority, 2020).
Taucare, G. et al. Detecting long temporal trends of photosystem II herbicides (PSII) in the Great Barrier Reef lagoon. Mar. Pollut. Bull. 177, 113490. https://doi.org/10.1016/j.marpolbul.2022.113490 (2022).
Skerratt, J. et al. Dispersal of the pesticide diuron in the Great Barrier Reef. Sci. Total Environ. 879, 163041. https://doi.org/10.1016/j.scitotenv.2023.163041 (2023).
Mercurio, P., Mueller, J. F., Eaglesham, G., Flores, F. & Negri, A. P. Herbicide persistence in seawater simulation experiments. PLoS ONE 10, e0136391. https://doi.org/10.1371/journal.pone.0136391 (2015).
Oettmeier, W. Chapter 9—Herbicides of photosytem II. In The Photosystems (ed. Barber, J.) 349–408 (Elsevier, 1992).
Flores, F., Collier, C. J., Mercurio, P. & Negri, A. P. Phytotoxicity of four photosystem II herbicides to tropical seagrasses. PLoS ONE 8, e75798. https://doi.org/10.1371/journal.pone.0075798 (2013).
Wilkinson, A. D., Collier, C. J., Flores, F. & Negri, A. P. Acute and additive toxicity of ten photosystem-II herbicides to seagrass. Sci. Rep. 5, 17443. https://doi.org/10.1038/srep17443 (2015).
Haynes, D., Ralph, P., Prange, J. & Dennison, B. The impact of the herbicide diuron on photosynthesis in three species of tropical seagrass. Mar. Pollut. Bull. 41, 288–293. https://doi.org/10.1016/S0025-326X(00)00127-2 (2000).
Jones, R., Mueller, J., Haynes, D. & Schreiber, U. Effects of herbicides diuron and atrazine on corals of the Great Barrier Reef, Australia. Mar. Ecol. Prog. Ser. 251, 153–167 (2003).
Jones, R. & Kerswell, A. P. Phytotoxicity of photosystem II (PSII) herbicides to coral. Mar. Ecol. Prog. Ser. 261, 149–159 (2003).
van Dam, J. W., Negri, A. P., Mueller, J. F., Altenburger, R. & Uthicke, S. Additive pressures of elevated sea surface temperatures and herbicides on symbiont-bearing foraminifera. PLoS ONE 7, e33900. https://doi.org/10.1371/journal.pone.0033900 (2012).
Harrington, L., Fabricius, K., Eaglesham, G. & Negri, A. P. Synergistic effects of diuron and sedimentation on photosynthesis and survival of crustose coralline algae. Mar. Pollut. Bull. 51, 415–427. https://doi.org/10.1016/j.marpolbul.2004.10.042 (2005).
Magnusson, M., Heimann, K., Quayle, P. & Negri, A. P. Additive toxicity of herbicide mixtures and comparative sensitivity of tropical benthic microalgae. Mar. Pollut. Bull. 60, 1978–1987. https://doi.org/10.1016/j.marpolbul.2010.07.031 (2010).
Thomas, M. C., Flores, F., Kaserzon, S., Fisher, R. & Negri, A. P. Toxicity of ten herbicides to the tropical marine microalgae Rhodomonas salina. Sci. Rep. 10, 7612. https://doi.org/10.1038/s41598-020-64116-y (2020).
Thomas, M. C., Flores, F., Kaserzon, S., Reeks, T. A. & Negri, A. P. Toxicity of the herbicides diuron, propazine, tebuthiuron, and haloxyfop to the diatom Chaetoceros muelleri. Sci. Rep. 10, 19592–19592. https://doi.org/10.1038/s41598-020-76363-0 (2020).
Dupraz, V. et al. Demonstrating the need for chemical exposure characterisation in a microplate test system: Toxicity screening of sixteen pesticides on two marine microalgae. Chemosphere 221, 278–291 (2019).
Marzonie, M. et al. Toxicity thresholds of nine herbicides to coral symbionts (Symbiodiniaceae). Sci. Rep. 11, 21636. https://doi.org/10.1038/s41598-021-00921-3 (2021).
APVMA. Diuron Final Review Report: The Reconsideration of the Registrations of Selected Products Containing Diuron and Their Associated Labels (Australian Pesticides and Veterinary Medicines Authority, 2012).
Gallen, C. et al. Marine Monitoring Program: Annual Report for Inshore Pesticide Monitoring 2017–18. Report for the Great Barrier Reef Marine Park Authority 118 (Great Barrier Reef Marine Park Authority, 2019).
Grant, S. et al. Marine Monitoring Program: Annual Report for Inshore Pesticide Monitoring 2015–2016 128 (Great Barrier Reef Marine Park Authority, 2017).
Davis, A. M., Lewis, S. E., Brodie, J. E. & Benson, A. The potential benefits of herbicide regulation: A cautionary note for the Great Barrier Reef catchment area. Sci. Total Environ. 490, 81–92. https://doi.org/10.1016/j.scitotenv.2014.04.005 (2014).
Song, Y. Insight into the mode of action of 2,4-dichlorophenoxyacetic acid (2,4-D) as an herbicide. J. Integr. Plant Biol. 56, 106–113. https://doi.org/10.1111/jipb.12131 (2014).
Kaundun, S. S. Resistance to acetyl-CoA carboxylase-inhibiting herbicides. Pest Manag. Sci. 70, 1405–1417. https://doi.org/10.1002/ps.3790 (2014).
Maruyama, C. R. et al. Nanoparticles based on chitosan as carriers for the combined herbicides imazapic and imazapyr. Sci. Rep. 6, 19768. https://doi.org/10.1038/srep19768 (2016).
ANZG. Australian and New Zealand Guidelines for Fresh and Marine Water Quality (Australian and New Zealand Governments and Australian State and Territory Governments, 2018).
Warne, M. St. J. et al. Development of a Method for Estimating the Toxicity of Pesticide Mixtures and a Pesticide Risk Baseline for the Reef 2050 Water Quality Improvement Plan (2020).
Warne, M. St. J. et al. Revised Method for Deriving Australian and New Zealand Water Quality Guideline Values for Toxicants—Update of 2015 Version 48 (Australian and New Zealand Governments and Australian State and Territory Governments, 2018).
Warne, M. St. J. et al. Estimating the aquatic risk from exposure to up to twenty-two pesticide active ingredients in waterways discharging to the Great Barrier Reef. Sci. Total Environ. 892, 164632 (2023).
Water Quality & Investigations. Catchment Loads Monitoring Program Pesticide Reporting Portal. https://arcg.is/19rnf8 (2020).
GBRMPA. Water Quality Guidelines for the Great Barrier Reef Marine Park (Great Barrier Reef Marine Park Authority, Townsville, 2009).
Warne, M. St. J., King, O. & Smith, R. A. Ecotoxicity thresholds for ametryn, diuron, hexazinone and simazine in fresh and marine waters. Environ. Sci. Poll. Res. 25, 3151–3169. https://doi.org/10.1007/s11356-017-1097-5 (2018).
King, O. C., Smith, R. A., Mann, R. M. & Warne, M. St. J. Proposed Aquatic Ecosystem Protection Guideline Values for Pesticides Commonly Used in the Great Barrier Reef Catchment Area: Part 1 (amended)—2,4-D, Ametryn, Diuron, Glyphosate, Hexazinone, Imazapic, Imidacloprid, Isoxaflutole, Metolachlor, Metribuzin, Metsulfuron-Methyl, Simazine, Tebuthiuron 296 (Department of Environment and Science, 2017).
King, O. C., Smith, R. A., Warne, M. St. J., Frangos, J. S. & Mann, R. M. Proposed Aquatic Ecosystem Protection Guideline Values for Pesticides Commonly Used in the Great Barrier Reef Catchment Area: Part 2—Bromacil, Chlorothalonil, Fipronil, Fluometuron, Fluroxypyr, Haloxyfop, MCPA, Pendimethalin, Prometryn, Propazine, Propiconazole, Terbutryn, Triclopyr and Terbuthylazine 213 (Department of Environment and Science, 2017).
Levy, J. L. et al. Uptake and internalisation of copper by three marine microalgae: Comparison of copper-sensitive and copper-tolerant species. Aquat. Toxicol. 89, 82–93. https://doi.org/10.1016/j.aquatox.2008.06.003 (2008).
Tato, T. & Beiras, R. The use of the marine microalga Tisochrysis lutea (T-iso) in standard toxicity tests: Comparative sensitivity with other test species. Front. Mar. Sci. 6, 488. https://doi.org/10.3389/fmars.2019.00488 (2019).
Trenfield, M. A. et al. Aluminium, gallium, and molybdenum toxicity to the tropical marine microalga Isochrysis galbana. Environ. Toxicol. Chem. 34, 1833–1840. https://doi.org/10.1002/etc.2996 (2015).
OECD. Organisation for Economic Cooperation and Development (OECD) Guidelines for the Testing of Chemicals Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test (OECD Publishing, 2011).
USEPA. ECOTOX User Guide: ECOTOXicology Database System. Version 5.0. https://cfpub.epa.gov/ecotox (2023).
Dupraz, V., Menard, D., Akcha, F., Budzinski, H. & Stachowski-Haberkorn, S. Toxicity of binary mixtures of pesticides to the marine microalgae Tisochrysis lutea and Skeletonema marinoi: Substance interactions and physiological impacts. Aquat. Toxicol. 211, 148–162 (2019).
Dupraz, V. et al. Combined effects of antifouling biocides on the growth of three marine microalgal species. Chemosphere 209, 801–814 (2018).
DeLorenzo, M. E., Danese, L. E. & Baird, T. D. Influence of increasing temperature and salinity on herbicide toxicity in estuarine phytoplankton. Environ. Toxicol. 28, 359–371 (2013).
Magnusson, M., Heimann, K. & Negri, A. P. Comparative effects of herbicides on photosynthesis and growth of tropical estuarine microalgae. Mar. Pollut. Bull. 56, 1545–1552. https://doi.org/10.1016/j.marpolbul.2008.05.023 (2008).
Bao, V. W., Leung, K. M., Qui, J. W. & Lam, M. H. Acute toxicities of five commonly used antifouling booster biocides to selected subtropical and cosmopolitan marine species. Mar. Pollut. Bull. 62, 1147–1151. https://doi.org/10.1016/j.marpolbul.2011.02.041 (2011).
Gatidou, G., Thomaidis, N. S. & Zhou, J. L. Fate of Irgarol 1051, diuron and their main metabolites in two UK marine systems after restrictions in antifouling paints. Environ. Int. 33, 70–77 (2007).
Jung, S. M. et al. Acute toxicity of organic antifouling biocides to phytoplankton Nitzschia pungens and zooplankton Artemia larvae. Mar. Pollut. Bull. 124, 811–818. https://doi.org/10.1016/j.marpol.bul.2016.11.047 (2017).
Koutsaftis, A. & Aoyama, I. The interactive effects of binary mixtures of three antifouling biocides and three heavy metals against the marine algae Chaetoceros gracilis. Environ. Toxicol. 21, 432–439 (2006).
Devilla, R. A. et al. Impact of antifouling booster biocides on single microalgal species and on a natural marine phytoplankton community. Mar. Ecol. Prog. Ser. 286, 1–12 (2005).
Hook, S. E. et al. RNA-Seq analysis of the toxicant-induced transcriptome of the marine diatom, Ceratoneis closterium. Mar. Genom. 16, 45–53 (2014).
Osborn, H. L. & Hook, S. E. Using transcriptomic profiles in the diatom Phaeodactylum tricornutum to identify and prioritize stressors. Aquat. Toxicol. 138–139, 12–25 (2013).
Magnusson, M. Effects of Priority Herbicides and Their Breakdown Products on Tropical, Estuarine Microalgae of the Great Barrier Reef Lagoon. PhD thesis, James Cook University (2009).
His, E. & Seaman, M. Effects of twelve pesticides on larvae of oysters (Crassostrea gigas) and on two species of unicellular marine algae (Isochrysis galbana and Chaetoceros calcitrans). In Acte du Conseil International pour l'Exploration de la Mer E:22 (1993).
Naveira, C., Rodrigues, N., Santos, F. S., Santos, L. N. & Neves, R. A. F. Acute toxicity of bisphenol A (BPA) to tropical marine and estuarine species from different trophic groups. Environ. Pollut. 268, 115911. https://doi.org/10.1016/j.envpol.2020.115911 (2021).
Haworth, P. & Steinback, K. E. Interaction of herbicides and quinone with the Q(B)-protein of the diuron-resistant Chlamydomonas reinhardtii mutant Dr2. Plant Physiol. 83, 1027–1031 (1987).
Do, T. C. V., Tran, D. T., Le, T. G. & Nguyen, Q. T. Characterization of endogenous auxins and gibberellins produced by Chlorella sorokiniana TH01 under phototrophic and mixtrophic cultivation modes toward applications in microalgal biorefinery and crop research. J. Chem. 2020, 4910621. https://doi.org/10.1155/2020/4910621 (2020).
Sasaki, Y. & Nagano, Y. Plant acetyl-CoA carboxylase: Structure, biosynthesis, regulation, and gene manipulation for plant breeding. Biosci. Biotechnol. Biochem. 68, 1175–1184. https://doi.org/10.1271/bbb.68.1175 (2004).
Huerlimann, R., Zenger, K. R., Jerry, D. R. & Heimann, K. Phylogenetic analysis of nucleus-encoded acetyl-CoA carboxylases targeted at the cytosol and plastid of algae. PLoS ONE 10, e0131099. https://doi.org/10.1371/journal.pone.0131099 (2015).
Misra, N., Panda, P. K., Patra, M. C., Pradhan, S. K. & Mishra, B. K. Insights into molecular assembly of accase heteromeric complex in Chlorella variabilis—A homology modelling, docking and molecular dynamic simulation study. Appl. Biochem. Biotechnol. 170, 1437–1457. https://doi.org/10.1007/s12010-013-0277-0 (2013).
Liu, Y., Li, Y. & Wang, X. Acetohydroxyacid synthases: Evolution, structure, and function. Appl. Microbiol. Biotechnol. 100, 8633–8649. https://doi.org/10.1007/s00253-016-7809-9 (2016).
Lonhienne, T. et al. Structures of fungal and plant acetohydroxyacid synthases. Nature 586, 317–321. https://doi.org/10.1038/s41586-020-2514-3 (2020).
Traas, T. P. et al. The potentially affected fraction as a measure of ecological risk. In Species Sensitivity Distributions in Ecotoxicology (eds Posthuma, L. & Suter, G. W.) 315–344 (CRC Press, 2002).
Mazid, M. A. A Comparative Ultrastructure, Physiology and Phylogeny of Two Tetraselmis Species Isolated from Australian Waters. Student Thesis: Doctor of Philosophy (PhD). Charles Darwin University (2009).
Guillard, R. R. L. & Ryther, J. H. Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 8, 229–239 (1962).
Golding, L. A. et al. Derivation of a water quality guideline for aluminium in marine waters. Environ. Toxicol. Chem. 34, 141–151 (2014).
OECD. Current approaches in the statistical analysis of ecotoxicity data: A guidance to application. In OECD Series on Testing and Assessment, No. 54 (OECD Publishing, 2006).
Ritz, C. & Streibig, J. C. Bioassay analysis using R. J. Stat. Softw. 12, 1–22. https://doi.org/10.18637/jss.v012.i05 (2005).
R Development Core Team. R: A Language and Environment for Statistical Computing. http://www.R-project.org (R Foundation for Statistical Computing, 2015).
Plummer, M. JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling. In Proc. 3rd International Workshop on Distributed Statistical Computing (eds. S. Laskaridis, S. et al.) 1–10 (2003).
Su, Y.-S. & Yajima, M. Package "R2jags" (Version 003-08) [Computer Software]. https://CRAN.R-project.org/package=R2jags (2015).
Fisher, R., Ricardo, G. & Fox, D. R. Bayesian Concentration-Response Modelling Using jagsNEC. https://zenodo.org/records/3966864 (2020).
Fisher, R., Ricardo, G. F. & Fox, D. R. jagsNEC: A Bayesian No Effect Concentration (NEC) Package. R Package Version 1.0. https://github.com/AIMS/NEC-estimation (2019).
Fox, D. R. A Bayesian approach for determining the no effect concentration and hazardous concentration in ecotoxicology. Ecotoxicol. Environ. Saf. 73, 123–131 (2010).
Acknowledgements
This research was supported by the Australian Government’s National Environmental Science Program (NESP) Tropical Water Quality Hub Project 3.1.3 Ecotoxicology of pesticides on the Great Barrier Reef for guideline development and risk assessments. We acknowledge the Larrakia, Bindal and Wulgurukaba people as the Traditional Owners of the land where this work took place. We pay our respects to their Elders past, present and emerging.
Funding
The funding was provided by the Australian Government’s National Environmental Science Programme (NESP Project TWQ 3.1.5 Ecotoxicology of pesticides on the Great Barrier Reef for guideline development and risk assessments) and also by the Australian Institute of Marine Science.
Author information
Authors and Affiliations
Contributions
J.v.D. and L.S.S. designed and performed the experiments. J.v.D., R.F., L.S.S., F.F. analysed the data. S.K. performed herbicide analyses. F.F. wrote the manuscript with input from A.P.N., L.S.S., J.v.D., R.F., and S.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Flores, F., Stapp, L.S., van Dam, J. et al. Toxicity of herbicides to the marine microalgae Tisochrysis lutea and Tetraselmis sp.. Sci Rep 14, 1727 (2024). https://doi.org/10.1038/s41598-024-51401-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-51401-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.