Abstract
Ixodes ricinus (Acari: Ixodida) is the main vector in Europe of Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Babesia microti. Wolinski National Park (WNP) is situated by the Baltic Sea and is frequently visited by tourists. The aim of the study was to determine the potential risk of exposure to tick borne infection with B. burgdorferi s.l., A. phagocytophilum and B. microti on the areas of WNP. In total, 394 I. ricinus were tested. The pathogens in ticks were detected by PCR, nested PCR, RFLP and sequencing. Altogether, pathogens were detected in 12.69% of the studied ticks. B. burgdorferi s.l., was shown in 0.25% of the studied I. ricinus, while A. phagocytophilum and B. microti were detected in 1.01% and 10.65% of studied ticks, respectively. Co-infection by A. phagocytophilum and B. microti was shown in only one I. ricinus nymph. Analysis of B. burgdorferi s.l., genospecies showed that 0.25% of the studied ticks were infected with Borrelia garinii. The obtained results show the potentially high human risk of exposure to tick-borne infection with B. microti, and the low potential risk of infection with B. garinii and A. phagocytophilum on the studied areas of WNP.
Similar content being viewed by others
Introduction
It is commonly known that in Europe, including Poland, Ixodes ricinus is a vector and/or reservoir of many pathogens including Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Babesia microti. These pathogens are etiological agents of dangerous tick borne diseases such as: Lyme borreliosis, anaplasmosis and human babesiosis1. Lyme borreliosis is a multisystemic disease caused by the spirochete B. burgdorferi s.l. Three stages may be distinguished in the development of this disease which are connected with the various clinical symptoms in humans2. Such genospecies as Borrelia afzelii, Borrelia garinii, Borrelia burgdorferi sensu stricto, Borrelia lusitaniae, Borrelia valaisiana, Borrelia spielmanii, Borrelia finlandensis, Borrelia bavariensis are considered as pathogenic to humans and are also responsible for causing the specific clinical symptoms of this disease3. This is particularly true for B. afzelii, B. garinii and B. burgdorferi s.s. These genospecies cause acrodermatitis chronica atrophicans (ACA), neuroborreliosis and chronic arthritis, respectively2. In turn, A. phagocytophilum cause human granulocytic ehrlichiosis (granulocytic anaplasmosis). Most cases of infection by this rickettsia manifest as flu-like symptoms, conjunctivitis and lymphadenopathy4, whereas B. miroti in humans causes babesiosis. The main symptoms of this disease are flu-like symptoms similar to those in anaplasmosis. Moreover, in extreme cases, infection with this parasite in humans may affect the kidneys, lungs, myocardium, spleen and liver5. The aim of the study was to determine the potential risk of human exposure to tick borne infection with B. burgdorferi s.l., A. phagocytophilum and B. microti on the areas of the Wolinski National Park (WNP).
Materials and method
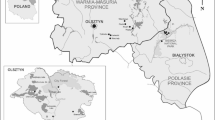
WNP is situated by the Baltic Sea in the mid-western part of Wolin Island 53o57′15″N and 14o29′20″E in Poland (Fig. 1). In the forests covering the area of WNP pine is the dominant species, while other tree species—beech, oak and a few other tree species—occur there with a lower percentage. WNP also has a rich and varied fauna. On the area of WNP occur many species of arthropods and birds, including rare species. The forest areas of this National Park, among others, are the numerous habitats of wild boars (Sus scrofa), deer (Cervus elaphus) and roe deer (Capreolus capreolus), foxes (Vulpes vulpes), martens (Martes martes) and badgers (Meles meles). A Bison bonasus demonstration farm is located on the area of WNP, and nearby there are many places which are popular summer holiday destinations for visitors, mainly from Poland and Europe, but also increasingly from other countries worldwide. Humans who spend their free time in or who are working on the areas of WNP can be exposed to infestation by ticks, and potential infection with one or more tick borne pathogen.
The location of the Wolinski National Park in Europe. (QGIS 3.10; https://qgis.org/pl/site/forusers/download.html).
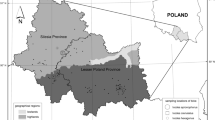
Ticks were collected from vegetation by the flagging method in four areas of the WNP: Area 1—53°55′53.8″N 14°28′14.6″E, Area 2—53°56′04.0″N 14°31′59.3″E, Area 3—53°55′05.5″N 14°29′03.4″E and Area 4—53°54′48.3″N 14°26′37.3″E (Fig. 2)6. In Areas 1 and 3, pine dominate while in Areas 2 and 4, beech and oak are the dominant species (Fig. 1). Ticks were identified to the species and developmental stage with the use of key by Nowak-Chmura1. DNA was isolated from 394 I. ricinus—15 females, 13 males, 266 nymphs and 100 larvae by the ammonia method7. The DNA was isolated from single adults and nymphs, while larvae were pooled in 10 individuals. Next, the concentration was measured in nanospectrophotometer PEARL (Implen, Germany) at 260/280 wave length. The samples were frozen at − 20 °C and stored for further analysis. The pathogens in ticks were detected by PCR and nested PCR. For preliminary screening for the presence of B. burgdorferi s.l., a pair of primers specific to the flagellin gene fragment was used8. In order to determine B. burgdorferi s.l. for the genospecies, the nested PCR–RFLP method was used. Next, B. burgdorferi s.l., positive samples were amplified with the use of two pairs of primers specific to the flagellin gene9. The nested PCR products were then cut with HpyF3I restriction enzyme10. In turn, to detect A. phagocytophilum and B. microti, the two pairs of primers specific to the 16S rRNA and 18S rRNA gene were used, respectively11,12. The amplicons were separated electrophoretically in 2% ethidium bromide stained agarose gels, whereas, the nested PCR–RFLP products were separated in 3.5% ethidium bromide stained agarose gels. Next, the samples were visualized under ultra violet light and photographed using an Omega 10 device (UltraLum, USA). The results were analyzed in the Total Lab computer programme (Total Lab, UK). The nested PCR product of B. burgdorferi s.l., was isolated from the gel by an Agarose DNA purification kit (EURx, Poland), according to the manufacture’s protocol and sequenced (Genomed, Poland). Statistical analysis was performed using CSS-Statistica for Windows 10. Statistical significance was declared at the p value of less than 0.05. Results were analyzed using Yates-corrected chi-square test (χ2).
Results
In total, pathogens were detected in 12.69% of the studied ticks and showed 47 mono-infections and three co-infections. Borrelia garinii was detected in 0.25% of the studied ticks. In turn, A. phagocytophilum and B. microti were shown in 1.01% and 10.65% of the studied I. ricinus, respectively. Co-infection of A. phagocytophilum and B. microti was shown in only 0.76% of the studied individuals (Table 1). It should be stressed that the difference between the frequency of ticks infected with B. microti and A. phagocytophilum was statistically significant (Yates-corrected χ2 = 11.48; p = 0.0007).
Borrelia garinii, was found in only one I. ricinus nymph collected in Area 3 (Tables 1 and 2), whereas, A. phagocytophilum was found in ticks collected in Areas 1 and 3. The I. ricinus infection level of this rickettsia in these two studied areas was similar—4.76% and 4.25%, respectively (Table 1). In turn, B. microti was detected in ticks collected in Areas 1, 3 and 4. The highest percentage of I. ricinus infected with this protozoan was showed in Area 4, while in the two other studied areas the percentage of ticks infected with B. microti was 2.38% and 6.38%, respectively (Table 1). This difference was statistically insignificant (Yates-corrected χ2 = 1.17; p = 0.2790).
Co-infection of A. phagocytophilum and B. microti was detected only in three nymphs collected in Area 4 (Tables 1 and 2), whereas in the three other studied areas co-infections with the studied pathogens were not found in the ticks.
The highest infection level of the studied tick borne pathogens was shown in I. ricinus nymphs, and in this developmental stage all three pathogens were detected. Babesia microti was shown in 15.41% of nymphs, while B. garinii, and A. phagocytophilum were detected in 0.25% and 1.12%, respectively. It should be stressed that all these differences were statistically significant (Yates-corrected χ2 = 14.13 and 11.48, p = 0.0002 and p = 0.0007, respectively; p < 0.001). Moreover, in 1.12% of studied nymphs the co-existence of A. phagocytophilum and B. microti was shown (Table 2). In turn, in the studied adults, among the I. ricinus ticks collected in the areas of WNP the presence of A. phagocytophilum and B. microti was shown in only one male and one female, respectively (Table 2). In turn, the presence of the studied pathogens were not found in the tested I. ricinus larvae. The differences in frequency of ticks infected with B. garinii, A. phagocytophilum or co-infected with A. phagocytophilum and B. microti between particular sites were statistically non-significant (Yates- corrected χ2 = 11.48; p > 0.05, in all cases). Ticks collected in all three areas were significantly less frequently infected with B. microti than in Area 4 (Yates-corrected χ2 = 12.5, 17.64 and 5.73; p = 0.0004; p ≤ 0.00001 and p = 0.0167, respectively). The difference in the prevalence of ticks infected with this protozoan between Ars 2 and 3 was also statistically significant (Yates-corrected χ2 = 4.30; p = 0.0382), whereas the remaining differences between sampling locations were statistically no-significant (Yates-corrected χ2 = 0.51; p = 0.4773).
Males were significantly more frequently infected with A. phagocytophilum than nymphs (Yates-corrected χ2 = 4.19; p = 0.041) or females and larvae (Yates-corrected χ2 = 6.38; p = 0.0115, in both cases) (Table 2). On the other hand, males and larvae were significantly less frequently infected with B. microti than nymphs (Yates-corrected χ2 = 14.13; p = 0.0002) and females (Yates- corrected χ2 = 5.33; p = 0.0210) (Table 2), whereas the difference in frequency of infected ticks between nymphs and females was statistically not significant (Yates-corrected χ2 = 2.50; p = 0.1137).
Discussion
Generally, in Poland and in the rest of Europe, the dominant genospecies of B. burgdorferi s.l. is B. afzelii13. A study conducted in the areas of western Poland showed the possibility of the existence of seven genospecies of this spirochete in I. ricinus: B. afzelii, B. burgdorferi s.s., B. valaisiana, B. bissettii, B. lusitaniae, B. miyamotoi, B. bavariensis10,14, while a study conducted on the areas of south-western Poland showed the presence in this tick species such genospecies as: B. afzelii, B. garinii, B. burgdorferi s.s. and B. valaisiana15. B. miyamotoi was also noted in these areas15. On the other hand, a study conducted in areas of the Silesian Province showed only B. afzelii, B. garinii and B. burgdorferi s.s. in ticks3,16,17. The percentage of ticks infected with B. garinii in Poland is varied and ranged from 1.3% in north-western Poland to 22.9% in the south3,18. In turn, a study conducted by Stańczak et al.12 in Polish woodlands showed this genospecies in 14.4% of studied I. ricinus ticks. The results presented in this work are significantly lower that those obtained in northern and southern Poland. These differences in the tick infection level may be caused, among others, by the type of biotope which may influence the level of infection of this ectoparasites by this spirochete19. Moreover, the variable prevalence may suggest that various B. burgdorferi s.l., genospecies have different competence towards reservoir, e.g. B. afzelii is associated more with forest rodents than B. garinii and B. valaisiana which are more associated with birds20,21,22,23. However, B. garinii is very heterogeneous and some strains may infect ticks via rodents24. The lower number of ticks infected with B. garinii than in other regions in Poland, and lack of other genospecies related with rodents in the studied areas, may be related with an insufficient number of reservoir animals and/or their uneven distribution in WNP. Furthermore, large numbers of the host species (some species of lizards and birds) may be present in this area which may suppress the effective function of vector in spreading this spirochete in the environment. In the ecology of this spirochete, a significant role is also played by red deer (Cervus elaphus). Studies conducted by Wodecka25 confirmed the inability to survive of B. burgdorferi s.l. in I. ricinus feeding on red deer blood. The presence of the red deer population in the studied areas may also be one of the reasons for the level of tick infection with this spirochete. As WNP is an island, it can be treated as an isolated area, a fact that could also have a potential effect on limiting the migration of rodents, the main reservoirs of this spirochete.
Human granulocytic ehrlichiosis (HGE) is caused by an Ehrlichia species closely related to obligate intracellular bacteria which cause granulocytic in sheep, cattle, and horses26,27. Anaplasma phagocytophilum occurs mainly in ruminants, but probably also in small mammals. In turn, E. equi, E. canis and E. chaffeensis occur in horses, dogs and Cervids, respectively28,29. The percentage of ticks infected with A. phagocytophilum in Europe ranges from 0.4–66.7%4, whereas in Poland the percentage of I. ricinus infected with A. phagocytophilum varies up to 2.6% in south-western Poland to even 76.7% in the some forest areas of the southern part of this country30,31. A study conducted in the various seaside areas of northern Poland showed that the number of I. ricinus ticks infected with A. phagocytophilum ranged from 0.9% in seaside areas of the Slowinski National Park (SNP) to 14% in the suburban and urban forests areas of the Tri-City agglomeration32,33. The results obtained in this study are similar to those obtained in the areas of SNP, and may confirm the low presence of this pathogen in I. ricinus ticks on the areas of north-western Poland. Moreover, the presence of this pathogen mainly in nymphs confirms their important role in the circulation of this rickettsia in the environment, while, the presence of A. phagocytophilum in I. ricinus male confirms that this pathogen has the ability of transstadial passage in the I. ricinus population.
Studies on the occurrence of B. microti in Poland showed that the percentage of I. ricinus infected with this protozoan in the northern areas varied by up to 2.3% in the suburban and urban forests areas of the Tri-City agglomeration, and by up to 15.2 the areas of SNP32,33. In turn, the percentage of ticks infected with this protozoan may be higher in other region of Poland and in some areas of southern Poland may amount to even 50%17,34. The percentage of ticks infected with B. microti on the areas of WNP is slightly lower than that showed by Asman et al.32 on the areas of SNP. In turn, this percentage is almost four times higher than that shown by Stańczak et al.33 on selected areas of Tri-City agglomeration. These results may indicate that the varied potential risk of human exposure to tick-borne infection of this protozoan in different parts of Poland.
Moreover, although the main reservoir of this pathogen are rodents, the lack of this pathogens in the studied ticks on Area 2 of the current and the domination of beech and oak trees may suggest that this pathogen occurs in a concentrated manner in the environment. The circulation of B. microti in the natural environment takes place mainly with the juvenile stage of the ticks (Siński, 1999). The obtained results confirm this fact. In turn, the presence of the I. ricinus female may suggest that it has the ability to transstadial passage in the tick population, similar to the other species—B. divergens.
The co-existence of A. phagocytophilum and B. microti occurs very often in ticks. This phenomenon is caused by the fact that many B. microti are also competent for this rickettsia and even for B. burgdorferi s.l.35. However, co-infection usually occurs in ticks in a lower percentage than mono-infection. The study by Sytykiewicz et al.36 in central-eastern Poland showed this co-existence in 1.8% of studied ticks and in 0.9% of studied nymphs. In turn, in the areas of northern Poland this co-existence in urban and suburban forests was shown to be 10.6%33. However, other studies conducted in southern and eastern Poland showed the presence of these both pathogens simultaneously in 0.6% and 1.05% of the studied ticks from these areas, respectively8,37. The obtained results are significantly lower than these obtained by Stańczak et al.33 and similar to those obtained by Asman et al.37 on selected areas of the Kraków-Częstochowa Upland. The results of this study confirmed the possibility of the co-existence both these pathogens in a single tick. Moreover, the demonstration of this co-existence only in nymphs may be caused both by the large number of studied individuals, as well as by the fact that the developmental stage plays a key role in both of these pathogens in the environment.
Conclusion
The obtained results show the potentially high risk of human exposure to tick-borne infection of B. microti and the low risk of tick-borne infection with B. garinii and A. phagocytophilum on the selected areas of WNP. In turn, demonstration of the co-existence of A. phagocytophilum and B. microti confirmed the possibility of the occurrence of more than one pathogen in a single tick. Moreover, the demonstration of the presence of the studied pathogens mainly in I. ricinus nymphs confirm that this developmental stage is very dangerous from the epidemiological point of view.
References
Nowak-Chmura, M. Fauna of ticks (Ixodida) of Central Europe (Wydawnictwo Naukowe Uniwersytetu Pedagogicznego, Kraków, 2013).
Balmelli, T. & Piffaretti, J. C. Association between different clinical manifestations of Lyme disease and different species of Borrelia burgdorferi sensu lato. Res. Microbiol. 146, 329–340 (1995).
Strzelczyk, J. K. et al. Prevalence of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected from southern Poland. Acta Parasitol. 60, 666–674 (2015).
Blanco, J. R. & Oteo, J. A. Human granulocytic ehrlichiosis in Europe. Clin. Microbiol. Infect. 8, 763–772 (2002).
Boustani, M. R. & Gelfand, J. A. Babesiosis. Clin. Infect. Dis. 22, 611–614 (1996).
Siuda, K. Kleszcze (Acari: Ixodida) Polski Część II Systematyka i Rozmieszczenie (Polskie Towarzystwo Parazytologiczne, Warsaw, 1993).
Guy, E. & Stanek, G. Detection of Borrelia burgdorferi in patients with Lyme disease by the polymerase chain reaction. J. Clin. Pathol. 29, 610–611 (1991).
Wójcik-Fatla, A., Szymańska, J., Wdowiak, L., Buczek, A. & Dutkiewicz, J. Coincidence of three pathogens (Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Babesia microti) in Ixodes ricinus ticks in the Lublin macroregion. Ann. Agric. Environ. Med. 16, 151–158 (2009).
Wodecka, B., Rymaszewska, A., Sawczuk, M. & Skotarczak, B. Detectability of tick-borne agents DNA in the blood of dogs, undergoing treatment for borreliosis. Ann. Agric. Environ. Med. 16, 9–14 (2009).
Wodecka, B. FlaB gene as a molecular marker for distinct identification of Borrelia species in environmental samples by the PCR-restriction fragment length polymorphism method. Appl. Environ. Microbiol. 77, 7088–7092 (2011).
Massung, R. F. et al. Nested PCR assay for detection of granulocytic ehrlichiae. J. Clin. Microbiol. 36, 1090–1095 (1998).
Persing, D. H. et al. Detection of Babesia microti by polymerase chain reaction. J. Clin. Microbiol. 30, 2097–2103 (1992).
Stańczak, J., Kubica-Biernat, B., Racewicz, M., Kruminis-Łozowska, W. & Kur, J. Detection of three genospecies of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in different regions of Poland. Int. J. Med. Microbiol. 290, 559–566 (2000).
Wodecka, B. & Skotarczak, B. First isolation of Borrelia lusitaniae DNA from Ixodes ricinus ticks in Poland. Scand. J. Infect. Dis. 37, 27–34 (2005).
Kiewra, D., Stańczak, J. & Richter, M. Ixodes ricinus ticks (Acari, Ixodidae) as a vector of Borrelia burgdorferi sensu lato and Borrelia miyamotoi in Lower Silesia. Tick Tick-borne Dis. 5, 892–897 (2014).
Asman, M. et al. Occupational risk of infections with Borrelia burgdorferi sensu lato, B. burgdorferi sensu stricto, B. garinii and B. afzelii in agricultural workers on the territory of Beskid Żywiecki. in Arthropods: Medical and Economical Significance (ed. Buczek, A. & Błaszak, Cz.) 163–170 (Akapit, 2012).
Asman, M., Witecka, J., Solarz, K., Zwonik, A. & Szilman, P. Occurrence of Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Babesia microti in Ixodes ricinus ticks collected from selected areas of Opolskie Province in south-west Poland. Ann. Agric. Environ. Med. 26, 544–547 (2019).
Wodecka, B. & Skotarczak, B. Genetic diversity of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in north-west Poland. Wiad Parazytol. 46, 475–485 (2000).
Bartosik, K., Lachowska-Kotowska, P., Szymańska, J., Pabis, A. & Buczek, A. Lyme borreliosis in south-eastern Poland: Relationships with environmental factors and medical attention standards. Ann. Agric. Environ. Med. 18, 131–137 (2011).
Hubálek, Z., Halouzka, J., Juricová, Z., Sikutová, S. & Rudolf, I. Effect of forest clearing on the abundance of Ixodes ricinus ticks and the prevalence of Borrelia burgdorferi s.l. Med. Vet. Entomol. 20, 166–172 (2006).
Hanincova, K. et al. Association of Borrelia afzelii with rodents in Europe. Parasitology 126, 11–20 (2003).
Hanincova, K. et al. Association of Borrelia garinii and B. valaisiana with songbirds in Slovakia. Appl. Environ. Microbiol. 69, 2825–2830 (2003).
Kurtenbach, K. et al. Differential transmission of the genospecies of Borrelia burgdorferi sensu lato by game birds and small rodents in England. Appl. Environ. Microbiol. 64, 1169–1174 (1998).
Rauter, C. & Hartung, T. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: A metaanalysis. Appl. Environ. Microbiol. 71, 7203–7216 (2005).
Wodecka, B. Significance of red deer (Cervus elaphus) in the ecology of Borrelia burgdorferi sensu lato. Wiad Parazytol. 53, 231–237 (2007).
Chen, S.-M., Dumler, J. S., Bakken, J. S. & Walker, D. H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 32, 589–595 (1994).
Zhang, Y., Cui, Y., Sun, Y., Jing, H. & Ning, Ch. Novel Anaplasma variants in small ruminants from central China. Front. Vet. Sci. 7, 1–7 (2020).
Petrovec, M. et al. Human disease in Europe caused by a granulocytic Ehrlichia species. J. Clin. Microbiol. 35, 1556–1559 (1997).
Siński, E. Enzoonotic reservoir for new Ixodes ricinus—Transmitted infections. Wiad Parazytol. 45, 135–142 (1999).
Kiewra, D., Zaleśny, G. & Czułowska, A. The risk of infection with Anaplasma phagocytophilum and Babesia microti in Lower Silesia, SW Poland. in Arthropods: Threat to Human and Animals Health (ed. Buczek, A. & Błaszak, Cz.) 103–110 (Koliber, 2014).
Asman, M. et al. The risk of exposure to Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, Babesia sp. and coinfections in Ixodes ricinus ticks on the territory of Niepołomice Forest (southern Poland). Ann. Parasitol. 59, 13–19 (2013).
Asman, M. et al. Anaplasma phagocytophilum, Babesia microti, Borrelia burgdorferi sensu lato, and Toxoplasma gondii in Ixodes ricinus (Acari, Ixodida) ticks collected from Slowinski National Park (Northern Poland). J. Vector Ecol. 42, 200–202 (2017).
Stańczak, J., Gabre, M. R., Kruminis-Łozowska, W., Racewicz, M. & Kubica-Biernat, B. Ixodes riciuns as a vector of Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Babesia microti in urban and suburban forests. Ann. Agric. Environ. Med. 11, 109–114 (2004).
Asman, M. et al. Detection of protozoans Babesia microti and Toxoplasma gondii and their co-existence in ticks (Acari: Ixodida) collected in Tarnogórski district (Upper Silesia, Poland). Ann. Agric. Environ. Med. 22, 80–83 (2015).
Yabsley, M. J. & Shock, B. C. Natural history of Zoonotic Babesia: Role of wildlife reservoirs. Int. J. Parasitol. Parasites Wildl. 2, 18–31 (2013).
Sytykiewicz, H. et al. Molecular evidence of Anaplasma phagocytophilum and Babesia microti co-infections in Ixodes ricinus ticks in central-eastern region of Poland. Ann. Agric. Environ. Med. 19, 45–49 (2012).
Asman, M. et al. The occurrence of three tick-borne pathogens in Ixodes ricinus ticks collected from the area of the Kraków—Czestochowa Upland (Southern Poland). Acarologia 58, 967–975 (2018).
Acknowledgements
The authors would like to thank the Directorate of WNP for issuing a permit to collect ticks for the study (Permit No. 40-07-1/19).
Author information
Authors and Affiliations
Contributions
M.A.—preparation of the manuscript, developing tables, identification of tick species and developmental stages, performance of some molecular studies. J.W.—performing some molecular research, assisting in methodology and manuscript discussions. J.K.—collected tick species, developing figures. K.S.—development of statistical surveys.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asman, M., Witecka, J., Korbecki, J. et al. The potential risk of exposure to Borrelia garinii, Anaplasma phagocytophilum and Babesia microti in the Wolinski National Park (north-western Poland). Sci Rep 11, 4860 (2021). https://doi.org/10.1038/s41598-021-84263-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-84263-0
This article is cited by
-
Disparate dynamics of pathogen prevalence in Ixodes ricinus and Dermacentor reticulatus ticks occurring sympatrically in diverse habitats
Scientific Reports (2023)
-
Metagenomics of the midgut microbiome of Rhipicephalus microplus from China
Parasites & Vectors (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.