Abstract
Comorbidities present considerable challenges to cancer treatment and care. However, little is known about the effect of comorbidity on cancer treatment decisions across a wide range of cancer types and treatment modalities. Harnessing a cohort of 280,543 patients spanning 19 site-specific cancers, we explored pan-cancer frequencies of 109 comorbidities. Multinomial logistic regression was used to analyse the relationship between comorbidities and cancer treatment types, while binomial logistic regression examined the association between comorbidities and chemotherapy drug types, adjusting for demographic and clinical factors. Patients with comorbidity exhibited lower odds of receiving chemotherapy and multimodality treatment. End-stage renal disease was significantly associated with a decreased odds of receiving chemotherapy and surgery. Patients with prostate cancer who have comorbid non-acute cystitis, obstructive and reflux uropathy, urolithiasis, or hypertension were less likely to receive chemotherapy. Among patients with breast cancer, dementia, left bundle branch block, peripheral arterial disease, epilepsy, Barrett’s oesophagus, ischaemic stroke, unstable angina and asthma were associated with lower odds of receiving multimodal chemotherapy, radiotherapy and surgery. Comorbidity is also consistently associated with the lower odds of receiving chemotherapy when comparing across 10 drug classes. Patients with comorbid dementia, intracerebral haemorrhage, subarachnoid haemorrhage, oesophageal varices, liver fibrosis sclerosis and cirrhosis and secondary pulmonary hypertension were less likely to receive antimetabolites. Comorbidity can influence the effectiveness and tolerability of cancer treatment and ultimately, prognosis. Multi-specialty collaborative care is essential for the management of comorbidity during cancer treatment, including prophylactic measures to manage toxicities.
Similar content being viewed by others
Introduction
Patients with cancer do not usually have cancer alone. Comorbidities present challenges to cancer treatment because they influence treatment decisions, prognosis and the overall trajectory of care. Yet, the influence of specific comorbidities at various points of the cancer care pathway is not well understood. Certain comorbidities might not directly influence survival but could affect patients’ tolerability to treatment. Cancer therapy could also exacerbate pre-existing conditions or give rise to new comorbidities due to treatment-related toxicities. In the UK, the National Institute for Health and Care Excellence (NICE) guidelines for cancer management have limited information on comorbidities. The guideline for lung cancer briefly mentioned that radiotherapy with curative intent should consider the presence of comorbidities1. The NICE breast cancer guideline stated that patients with invasive breast cancer should be treated with surgery and systemic therapy, irrespective of age unless significant comorbidity precludes surgery2. There is no mention of what significant comorbidity entails. The guideline also recommends offering trastuzumab to patients with invasive breast cancer and to consider comorbidities but did not specify which comorbidities to consider nor did it mention how comorbidities should be managed during cancer treatment. Similarly, the prostate cancer guidelines stated that docetaxel chemotherapy should only be offered to patients who do not have significant comorbidities but did not mention specific comorbidities3. Guidelines emphasise the importance of considering comorbidities in treatment planning, yet provide little information on how comorbidities should be handled.
Randomised controlled trials (RCTs) are essential for the investigation of the safety and efficacy of new cancer drugs while offering patients the opportunity to access experimental therapeutics. The number of RCTs has steadily increased over the years, however, inclusion and exclusion criteria have mostly remained unchanged. RCTs often exclude large segments of cancer patients with comorbidities4. The presence of one or more comorbidities is associated with a decreased odds of trial participation and trial offers5. An inevitable consequence is that RCTs create study cohorts that are divergent from real-world populations and produce limited data on the tolerability of therapies in people with comorbidities.
Due to limitations in clinical guidelines and trial evidence, relatively little is known about the influence of comorbidity on the choice of cancer treatment. Harnessing real-world cancer populations, we aim to address this gap by characterising real-world treatment patterns in patients with comorbid conditions. We performed a population cohort study using linked electronic health records (EHRs) from primary care, secondary care and the cancer registry. We explored 7 treatment categories and 10 chemotherapy drug classes across 19 adult cancers. Specific objectives are (i) to estimate the frequencies of 109 comorbidities diagnosed within the 5-year period before cancer diagnosis across 19 cancer types, (ii) to examine patterns of multimorbidity across cancer types, (iii) to estimate the associations between comorbidity and cancer treatment decisions and (iv) to estimate the associations between comorbidity and chemotherapy use across 10 chemotherapy drug classes. As cancer survivorship increases, the generation of a new knowledge base demonstrating the impact of comorbidity on cancer treatment could inform multidisciplinary team meetings to improve overall safety and optimise care.
Methods
Data sources
We employed linked electronic health records from primary care, secondary care and the cancer registry in England. Primary care data were recorded using Read and SNOMED codes. Secondary care Hospital Episode Statistics data were recorded in ICD-10. Detailed information on cancer, tumour stage, grade and tumour count were obtained from the cancer registry National Cancer Registration and Analysis Service. Partnering with organisations such as Cancer Research UK, Macmillan and the Public Health England’s National Cancer Intelligence Network, the registry collects data on cancer cases involving people living in England and the data is used to support research and public health initiatives6. Data sent to the registry come from multiple sources such as hospitals, GPs, radiotherapy departments, screening services, death certificates, hospices and histopathology services. The registry also links their data to other types of health information collected as part of routine healthcare in the NHS. Treatment information and chemotherapy drug details were obtained from the registry’s systemic anti-cancer treatment and radiotherapy datasets. Socioeconomic deprivation statuses were obtained from the Office for National Statistics. Socioeconomic deprivation was recorded using Index of Multiple Deprivation which is a measure of relative deprivation based on 37 indicators and seven domains to identify the most and least deprived areas7. Information governance approval was received from the Medicines and Healthcare products Regulatory Agency.
Study design and electronic health record phenotypes
Patients with incident site-specific cancer aged 18 years or older were identified during the study period of 01-01-1998 to 31-10-2020. We considered 19 site-specific cancers: (i) bladder, (ii) brain, (iii) breast, (iv) cervix, (v) colon and rectum, (vi) gallbladder and biliary tract, (vii) kidney, (viii) liver and intrahepatic bile duct, (ix) lung, (x) melanoma, (xi) oesophagus, (xii) oropharynx, (xiii) ovary, (xiv) pancreas, (xv) prostate, (xvi) stomach, (xvii) testis, (xviii) thyroid and (xix) uterus. We included patients who had received at least one form of active treatment modality. Our aim was to investigate how the presence of comorbidities influences the choice among active treatment options, rather than analysing the decision not to treat as such an analysis may require a different approach to account for the multifactorial nature of treatment abstention. We considered 7 treatment categories: (i) surgery alone, (ii) chemotherapy alone, (iii) radiotherapy alone, (iv) chemotherapy and surgery, (v) radiotherapy and surgery, (vi) chemotherapy and radiotherapy and (vii) chemotherapy, radiotherapy and surgery. We analysed ten chemotherapy drug categories: (i) alkylating agents, (ii) anthracyclines, (iii) antimetabolites, (iv) biological response modifiers including immunotherapy, (v) hormonal agents, (vi) kinase inhibitors, (vii) non-anthracycline antitumour antibiotics, (viii) plant alkaloids excluding vinca alkaloids, (ix) platinum agents and (x) vinca alkaloids.
Phenotypes were obtained from an open-access library8 and have been previously validated9. We considered 109 non-cancer comorbidities categorised into nine organ systems: cardiovascular (33 conditions), endocrine (5 conditions), gastrointestinal (18 conditions), haematological (8 conditions), immunology and infection (19 conditions), musculoskeletal (8 conditions), neurological (10 conditions), pulmonary (4 conditions) and renal (4 conditions). We considered comorbidities that were diagnosed in the 5-year period before cancer diagnosis to rule out historical diagnoses that may have been cured.
Statistical analyses
The primary outcomes were cancer treatment and chemotherapy drug type, given a specific comorbidity. Treatment outcomes were identified from the registry dataset. The proportions of patients with specific comorbidities were estimated by cancer type. The number of comorbidities (0, 1, 2, 3, 4, 5 and 6+) was also summarised by cancer type. As the dependent variable (treatment type) had 7 categories, multinomial logistic regression was employed to ascertain the association between comorbidity and cancer treatment, adjusting for age, sex, socioeconomic status, tumour grade, tumour stage, tumour count (according to the cancer registry’s data dictionary, this is the count of every tumour for a patient) and multimorbidity count. Comorbidity (independent variable) was defined as the presence or absence of a particular condition (i.e., with heart failure vs. no heart failure). Odds ratios (ORs) and 95% confidence intervals (CIs) for the odds of receiving a particular treatment given a comorbidity were calculated. Multinomial logistic regression results for treatment decisions were presented relative to surgery alone as the baseline choice of treatment.
For the association between comorbid conditions and chemotherapy type, as the dependent variable (chemotherapy type) had two categories (e.g., received or did not receive a particular chemotherapy drug), binomial logistic regression models were fitted. Binomial logistic regression models were adjusted for cancer type, age, sex, socioeconomic status, tumour grade, tumour stage, tumour count and multimorbidity count. For binomial regression, comorbidity was used as the independent variable and was defined as the presence or absence of a particular condition.
Variance inflation factors (VIFs) were calculated to test for multicollinearity of the independent variables in regression models. Variables with VIF greater than five were removed from the model. CIs for proportions were calculated using the bootstrap percentile method10. All analyses were performed according to the STROBE guidelines. All analyses were performed in accordance with the relevant guidelines and regulations. All analyses were performed using R 3.6.3 with the following packages: tidyverse (1.3.1), data.table (1.14.2), tableone (0.12.0), nnet (7.3.13), questionr (0.7.5) and performance (0.6.1).
Ethics approval and consent to participate
This study was approved by the Medicines and Healthcare products Regulatory Agency (19222). According to the data custodian, as patient identifiers are not available, the GP practice does not need to seek a patient’s consent to share data. All data are fully anonymised.
Results
We identified 280,543 patients having an incident cancer diagnosis with cancer treatment information (Table S1). Of these, 131,528 (46.9%) were male. Patients within each age group were as follows: age 18–34 (5,127; 1.8%), age 35–50 (29,553; 10.5%), age 51–65 (89,511, 31.9%), age 66–80 (120,080; 42.8%) and age ≥ 81 (36,272; 12.9%). Patients were categorised into seven treatment groups: surgery alone (117,007; 41.7%), chemotherapy alone (30,007; 10.7%), radiotherapy alone (36,100; 12.9%), chemotherapy and radiotherapy (18,326; 6.5%), chemotherapy and surgery (24,396; 8.7%), radiotherapy and surgery (35,121; 12.5%) and chemotherapy, radiotherapy and surgery (19,586; 7.0%). 57,384 patients had information on the types of chemotherapy that were prescribed (Table S2). The following chemotherapy types were considered: alkylating agents (8224; 14.3%), anthracyclines (8960; 15.6%), antimetabolites (21,224; 37.0%), biological response modifiers including immunotherapy (7638; 13.3%), hormonal agents (30,795; 53.7%), kinase inhibitors (1785; 3.1%), non-anthracycline antitumour antibiotics (791; 1.4%), plant alkaloids excluding vinca alkaloids (12,819; 22.3%), platinum agents (18,490; 32.2%) and vinca alkaloids (2147; 3.7%). The proportions of patients receiving each of the seven forms of therapy and each of the ten chemotherapy drug types for across cancer types are shown in Tables S3 and S4, respectively.
Patterns of recently diagnosed conditions across 19 adult cancers
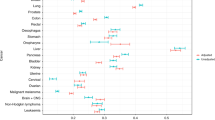
We analysed the proportions of 109 conditions (grouped into 9 organ systems) that were diagnosed in the 5-year period before cancer diagnosis. The full list of conditions is provided in Table S5. We observed a high variability in the diagnosis of comorbid conditions across cancer types. Among patients with brain cancer, the top three comorbidities were intracranial hypertension (14.03% [95% CI 13.01–15.05]), hypertension (13.65% [12.64–14.66]) and epilepsy (10.96% [10.04–11.88]) (Fig. 1, Table S6). The top three comorbidities for colorectal cancer were peritonitis (15.25% [14.92–15.59]), hypertension (13.51% [13.19–13.83]) and anaemia (12.31% [12.01–12.62]). For gallbladder and biliary tract cancer, the top comorbidities were cholangitis (49.96% [47.20–52.72]), cholelithiasis (30.95% [28.39–33.50]) and cholecystitis (30.71% [28.16–33.26]). For liver and intrahepatic bile duct cancer, the top comorbidities were hepatic failure (20.12% [18.26–21.99]), fatty liver (19.51% [17.66–21.35]), cholangitis (19.28% [17.45–21.11]) and portal hypertension (16.64% [14.91–18.37]). Common comorbidities in patients with lung cancer include lower respiratory tract infections (18.75% [18.31–19.18]), chronic obstructive pulmonary disease (15.78% [15.37–16.18]) and hypertension (15.28% [14.88–15.68]). For oesophageal cancer, the top comorbidities were oesophagitis and oesophageal ulcer (18.14% [17.30–18.97]), hypertension (13.31% [12.57–14.04]) and Barrett’s oesophagus (12.92% [12.19–13.64]). The full results for all cancers are available in Table S6 and graphically represented in Fig. 1 and Fig. S1.
Non-cancer conditions diagnosed before cancer. Boxplots show the proportion of patients having a first diagnosis of any of the 109 non-cancer conditions (grouped into 9 organ systems) in the 5-year period before cancer diagnosis. Boxplots for 8 cancer types are shown in this figure. Conditions with proportions ≥ 5% are annotated on the plots. Boxplots for the remaining 11 cancer types are shown in Fig. S1. Full data and confidence intervals are presented in Table S6.
Multimorbidity burden in patients with cancer
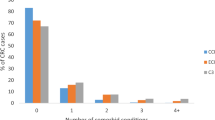
Pan-cancer analyses of the number of comorbidities revealed that patients with certain cancers experienced a high proportion of pre-existing conditions. Patients with liver, gallbladder, pancreatic or lung cancers had the highest number of comorbidities when considering 109 conditions (Fig. 2, Table S7). The proportion of patients with six or more co-morbid conditions were as follows: liver cancer (63.07% [60.83–65.31]), gallbladder cancer (53.22% [50.46–55.98]), pancreatic cancer (47.46% [46.03–48.90]) and lung cancer (41.69% [41.14–42.24]) (Table S7). By contrast, 45.43% [43.54–47.32] of patients with testicular cancer had no comorbid conditions. A reasonable number of patients with cervical cancer (27.66% [26.17–29.15]) or melanoma (20.96% [20.34–21.59]) also had zero comorbidities. See Table S7 for the full results.
Multimorbidity patterns across 19 adult cancers. Boxplot depicts the proportion of patients, within each cancer type, having 0, 1, 2, 3, 4, 5 or ≥ comorbidities. Cancers with proportions ≥ 20% are annotated on the plot. Full data and confidence intervals are presented in Table S7.
Associations between comorbidity and cancer treatment decisions
We examined the relationship between comorbid conditions and the type of cancer treatment using multinomial logistic regression, with ‘surgery alone’ as the baseline choice of treatment. Adjusted odds ratios (ORs) for the odds of receiving a particular treatment combination given a specific comorbidity were calculated (Table S8). ORs with P value < 0.05 are graphically represented in Fig. 3 and Figs. S2–S9; all results are presented in Table S8. Patients with cardiovascular conditions had lower odds of receiving (i) chemotherapy alone, (ii) chemotherapy and radiotherapy, (iii) chemotherapy, radiotherapy and surgery, (iv) chemotherapy and surgery and (v) radiotherapy and surgery (Fig. 3, Table S8). For example, patients with colorectal cancer having comorbid atrial fibrillation were less likely to receive chemotherapy, radiotherapy and surgery (OR 0.55 [CI 0.40–0.75]), chemotherapy and radiotherapy (0.63 [0.47–0.83]), chemotherapy and surgery (0.69 [0.58–0.81]), chemotherapy alone (0.69 [0.57–0.82]) and radiotherapy and surgery (0.80 [0.66–0.96]) (Fig. 3, Table S8). Patients with heart failure were also less likely to receive chemotherapy and radiotherapy; ORs for breast (0.47 [0.32–0.68]), oesophageal (0.48 [0.27–0.85]), lung (0.63 [0.50–0.79]), colorectal (0.63 [0.46–0.87]) and prostate (0.74 [0.57–0.95]) cancers. Similarly, patients with colorectal (0.42 [0.28–0.64]), breast (0.51 [0.39–0.67]) and prostate (0.57 [0.40–0.79]) cancers who also have heart failure were less likely to receive chemotherapy, radiotherapy and surgery.
Multinomial logistic regression investigating the associations between cardiovascular conditions and cancer treatment decisions across adult cancers. Forest plots show odds ratios for a particular treatment type, adjusted for age, sex, socioeconomic status, tumour grade, tumour stage, tumour count and multimorbidity count. Seven treatment categories were considered (i) surgery alone, (ii) chemotherapy alone, (iii) radiotherapy alone, (iv) chemotherapy and radiotherapy, (v) chemotherapy and surgery, (vi) radiotherapy and surgery, and (vii) chemotherapy, radiotherapy and surgery. Multinomial logistic regression models were fitted using surgery alone as the baseline choice of treatment for each cancer type (colour-coded). Only cardiovascular conditions are shown in this figure. Plots for the remaining 8 organ systems are shown in Figs. S2–S9. Only results with P < 0.05 are shown in the figure. P values are annotated on the plots. Full data and confidence intervals are presented in Table S8.
When considering gastrointestinal conditions, patients with cholelithiasis were less likely to receive chemotherapy alone (relative to surgery alone); ORs for gallbladder (0.44 [0.30–0.67]), liver (0.50 [0.35–0.72]) and prostate (0.61 [0.44–0.85]) cancers (Fig. S3, Table S8). Additionally, patients with kidney (0.44 [0.25–0.78]), colorectal (0.62 [0.52–0.74]), lung (0.83 [0.71–0.97]) or prostate (0.86 [0.74–0.99]) cancers with comorbid diverticular disease of the intestine were less likely to receive radiotherapy alone. When considering renal comorbidities, end-stage renal disease was significantly associated with a decreased odds of receiving chemotherapy and surgery; ORs for oesophageal (0.36 [0.19–0.69]), colorectal (0.48 [0.37–0.64]), lung (0.50 [0.32–0.77]) and bladder (0.62 [0.42–0.89]) cancers (Fig. S9, Table S8). Similarly, patients with lung (0.43 [0.26–0.70]), colorectal (0.47 [0.28–0.80]), prostate (0.50 [0.34–0.74]) or breast (0.70 [0.51–0.97]) cancers who have comorbid end-stage renal disease were less likely to receive all three forms of treatment (i.e., chemotherapy, radiotherapy and surgery).
When exploring specific cancer types, patients with prostate cancer who have comorbid non-acute cystitis (OR 0.30 [0.16–0.54]), obstructive and reflux uropathy (OR 0.45 [0.34–0.59]), urolithiasis (OR 0.48, [0.38–0.60]) or hypertension (OR 0.84 [0.75–0.95]) were less likely to receive chemotherapy alone, relative to surgery alone (Table S8). For patients with breast cancer, dementia (OR 0.25 [0.15–0.44]), left bundle branch block (OR 0.30 [0.16–0.57]), peripheral arterial disease (OR 0.50 [0.34–0.73]), epilepsy (OR 0.51 [0.29–0.92]), Barrett’s oesophagus (OR 0.58 [0.40–0.85]), ischaemic stroke (OR 0.60 [0.40–0.91]), unstable angina (OR 0.65 [0.51–0.82]) and asthma (OR 0.74 [0.63–0.87]) were associated with lower odds of receiving multimodal chemotherapy, radiotherapy and surgery (Table S8).
Associations between comorbidity and chemotherapy decisions
We next investigated the odds of receiving a particular chemotherapy drug given a specific comorbidity using binomial logistic regression. Adjusted ORs for all comorbidities are shown in Table S9. ORs with P value < 0.05 are graphically represented in Fig. 4 and Fig. S10; all results are presented in Table S9. Cancer patients with comorbidities compared with those without comorbidities were less likely to receive antimetabolites (Fig. 4, Table S9). The top 10 conditions were dementia (OR 0.19 [0.14–0.25]), intracerebral haemorrhage (0.31 [0.22–0.42]), subarachnoid haemorrhage (0.31 [0.21–0.43]), oesophageal varices (0.35 [0.22–0.52]), liver fibrosis sclerosis and cirrhosis (0.40 [0.31–0.51]), secondary pulmonary hypertension (0.41 [0.26–0.61]), primary pulmonary hypertension (0.41 [0.27–0.61]), ischaemic stroke (0.42 [0.35–0.51]), other interstitial pulmonary diseases with fibrosis (0.51 [0.37–0.67]) and venous thromboembolic disease (0.51 [0.46–0.57]). Similarly, patients with comorbidities were less likely to receive platinum-based chemotherapy: dementia (0.15 [0.11–0.21]), primary pulmonary hypertension (0.31 [0.19–0.47]), secondary pulmonary hypertension (0.31 [0.19–0.48]), intracerebral haemorrhage (0.38 [0.28–0.50]), oesophageal varices (0.38 [0.23–0.58]), subarachnoid haemorrhage (0.41 [0.30–0.55]), liver fibrosis sclerosis and cirrhosis (0.43 [0.33–0.55]), ischaemic stroke (0.47 [0.39–0.56]), glomerulonephritis (0.48 [0.37–0.63]) and scoliosis (0.51 [0.33–0.75]). Patients with comorbidities were also less likely to receive kinase-targeted therapy: ischaemic stroke (0.42 [0.22–0.70]), stroke (not otherwise specified) (0.45 [0.27–0.71]), obstructive and reflux uropathy (0.48 [0.25–0.81]), lower respiratory tract infections (0.53 [0.45–0.63]), peripheral arterial disease (0.54 [0.35–0.79]), venous thromboembolic disease (0.55 [0.40–0.73]), Raynaud’s syndrome (0.56 [0.35–0.85]), end stage renal disease (0.61 [0.45–0.80]), heart failure (0.63 [0.47–0.81]) and myocardial infarction (0.63 [0.49–0.81]) (Fig. S10, Table S9).
Binomial logistic regression investigating the associations between comorbidity and chemotherapy decisions. Forest plots show odds ratios for a particular chemotherapy type, adjusted for cancer type, age, sex, socioeconomic status, tumour grade, tumour stage, tumour count and multimorbidity count. Ten chemotherapy classes were considered—results for antimetabolites and platinum agents are shown in this figure. Plots for the remaining 8 chemotherapy classes are shown in Fig. S10. Binomial logistic regression models were fitted. Conditions were colour-coded according to the 9 organ systems. Only results with P < 0.05 are shown in the figure. P values are annotated on the plots. Full data and confidence intervals are presented in Table S9.
Discussion
This pan-cancer population-based study involving 19 adult cancers fills an evidence gap by investigating the associations between a wide range of comorbid conditions and cancer treatment patterns. For the first time, we investigated 109 comorbidities involving 9 organ systems and found that the type and magnitude of comorbidity burden were variable across cancers. For example, patients with liver cancer had a high burden of comorbidity involving a wide range of cardiovascular, infection, endocrine and gastrointestinal conditions. Given that the risk factors of gastrointestinal cancers include obesity, infection, alcohol abuse and smoking, a high burden of comorbidity was similarly observed in other gastrointestinal cancers (i.e., pancreatic, stomach, colorectal, gallbladder and oesophageal cancers). Pre-existing conditions were associated with a reduced likelihood of receiving multimodality therapy. Patients with comorbidities were less likely to receive chemotherapy; this pattern is consistent across the 10 chemotherapeutic agents we considered.
Comorbid conditions affect treatment choices in several ways. Our observations on the negative association between comorbid conditions and receipt of chemotherapy are consistent with other studies. A systematic review of 16 studies found 11 studies reporting that patients with comorbidities were less likely to be given chemotherapy11. Ten studies estimated the odds of chemotherapy use and except for a single study, they all reported decreased use of chemotherapy with ORs ranging from 0.25 to 0.99, regardless of cancer stage or tumour site11. Furthermore, a study on ovarian cancer demonstrated longer delays in initiating chemotherapy in patients with comorbidity12. A Canadian study on 20,689 patients with lung cancer found that pre-existing cardiovascular disease was associated with a lower likelihood of receiving chemotherapy (OR 0.53 [0.48–0.58])13. A Dutch study on stage III colon cancer reported that patients with comorbidity received chemotherapy less often (OR 0.6 [0.5–0.8])14. Functional limitations and geriatric syndromes were associated with a lower likelihood of receiving chemotherapy and surgery, but only if patients have two or more comorbidities15. Advanced age, presence of comorbidities and poor performance status were common reasons for withholding adjuvant chemotherapy in patients with stage III colorectal cancer16. Patients who were given chemotherapy were more likely to develop complications (52%) than those receiving surgery alone (41%), suggesting high toxicity rates due to interacting comorbidities16. Women with breast cancer and severe comorbidity were less likely to receive both chemotherapy and radiotherapy17. Women with severe comorbidity were also less likely to be offered the hormonal therapy tamoxifen (OR 0.88 [0.78–0.99])17. Breast-conserving surgery is less likely to be given to women with a high comorbidity burden (OR 0.63 [0.58–0.69])17. In patients with colorectal cancer, comorbidities were associated with a greater likelihood of receiving surgery alone15. Patients with stage III colon cancer with a Charlson Comorbidity Index score of ≥ 3 were less likely to be given chemotherapy18. Comorbidity, in patients with ovarian cancer, was associated with a lower likelihood of receiving standard combination chemotherapy (OR 0.03 [0.01–0.1])19. Patients with multimorbidity and those with functional limitations were 40% less likely to receive chemotherapy20. Age is a strong predictor of comorbidity and could, in part, explain the influence on cancer treatment choices. Elderly patients with colon cancer were less likely to receive antimetabolite chemotherapy (5-fluorouracil)21. Elderly patients with lung or head and neck cancer were also less likely to receive standard treatment22,23. Elderly patients with comorbidities were less likely to adhere to chemotherapy due to potential toxicities24. Indeed, our multinomial and binomial regression analyses that involved adjusting for age, and other relevant prognostic factors (tumour stage, grade, tumour count, sex and socioeconomic status) revealed notable associations between comorbid conditions and treatment decisions.
Previous studies investigating the impact of comorbidity on treatment choice are heterogeneous in design and methodology, limited to specific cancer types and treatment modalities. Index systems such as the Charlson Comorbidity Index (CCI) have been developed to generate an overall comorbidity score based on a list of conditions. CCI was created to predict the risk of death within 1 year of hospitalisation. However, CCI also includes solid tumours, leukaemia and lymphoma in its list of conditions, which might not add additional information if applied to patients with cancer. A cancer-specific Comorbidity Index was subsequently developed using prostate and breast cancer cases from the Surveillance, Epidemiology, and End Results Program dataset25. This index system uses conditions in CCI but excludes cancer conditions. Adult Comorbidity Evaluation 27 (ACE-27) is a 27-item comorbidity index designed using hospital-based cancer registries, which does not take into account information from primary care26. Validations of ACE-27 were also undertaken using data from a single medical site26. We demonstrated that the pattern of comorbidities is variable across cancer types. This means that each comorbidity might exert varying prognostic impact and the use of general index systems may result in limited specificity. We have taken a disease agnostic view to consider all comorbidities diagnosed in the preceding five years before cancer diagnosis. Most studies are limited by the sources of data used in the development of general or disease-specific comorbidity measures. To overcome this limitation, we have harnessed population health records across England rather than using a single disease population.
Challenges in considering the role of comorbidity in cancer treatment decisions
Decisions to offer a specific course of treatment and the suitability of the treatment for patients are often based on perceived benefits and risks. The presence of significant comorbidities or poor performance status are indicators for withholding therapy in patients with advanced cancer27. Over 50% of patients with advanced lung cancer receive no treatment, which is concerning. Among those receiving treatment, 94% of first-line therapy was platinum-based chemotherapy. Furthermore, an analysis on 600 RCTs revealed that 88% of the trials have specified a performance status restriction cut-off and among these trials, 40% had a strict inclusion criterion of ECOG ≤ 128. This suggests that the use of performance status in cancer trials is common and very few patients with poor performance status are enrolled. Nonetheless, patients with poor performance status experienced a significant survival benefit when treatment is provided, which contradicts the notion that reduced functional capacity should be a criterion for deciding against treatment27. While evidence suggests that patients with poor performance status can experience survival benefits from active treatment, we also recognise the value of early palliative care in demonstrating significant improvements in quality of life for patients with advanced cancer29. These findings underscore that treatment decisions should carefully weigh the potential benefits of active therapy against the advantages of palliative care. Performance status, while important, is nevertheless vulnerable to subjective evaluation with demonstrable observer biases30,31. The presence of specific comorbidities should be considered instead, and further evaluation of safety profiles should be conducted in specific patient populations.
Nephrotoxicity of chemotherapeutic agents has been well documented32. We observed that chemotherapy is less likely to be given to patients with end-stage renal disease. Cisplatin is primarily eliminated by the kidneys and it’s recommended that a lower dose should be used in patients with renal impairments33. Patients with end-stage renal disease receive dialysis, however, haemodialysis and plasmapheresis are ineffective in eliminating cisplatin to reverse overdose34. Lung resection provides the highest chance of cure in patients with lung cancer. However, patients with chronic obstructive pulmonary disease (COPD) and more severe airway obstruction were less likely to undergo thoracic surgery with curative intent (OR 0.025 [0.004–0.167])35. Additionally, the risks of postoperative pulmonary complications such as empyema and atelectasis are higher in patients with COPD36,37. Comorbidity can also exacerbate treatment-related complications. Patients with diabetes face a greater risk of complications from breast cancer treatment, including neuropathy, heart disease, poor wound healing, increased susceptibility to infection and nephropathy38. Diabetes is associated with ipsilateral upper arm dysfunction 5 years after mastectomy39. Diabetes is also linked to an increased risk of complications from radiotherapy. Patients with cervical cancer and comorbid diabetes experience bowel obstruction and rectovaginal fistula40. Over 60% of patients with ovarian cancer and diabetes who received paclitaxel or cisplatin chemotherapy experience worsening hyperglycaemia and progression of neurological symptoms41.
Management of comorbidity during and after cancer treatment
Radiotherapy and certain chemotherapy agents can adversely affect the vascular system and heart. Patients with pre-existing cardiovascular disease or cardiovascular risk factors are at an even greater risk of developing cardiac complications. Radiation can affect the valves, vascular structures and pericardium or myocardium42,43. Monitoring for cardiovascular function during anticancer therapy could reduce adverse effects. Radiation induces the fibrosis and calcification of valvular tissue. Valvular dysfunction induced by radiation has a median time to diagnosis of two decades after radiotherapy44. At the time of valvular disease diagnosis, most patients are no longer under the care of oncologists, thus the accuracy of detecting this condition becomes limited if cancer treatment is not captured in detail in the medical records45. For symptomatic patients, the American Society of Echocardiography and the European Association of Cardiovascular Imaging recommend yearly physical and clinical history examination of echocardiography46. For asymptomatic patients, transthoracic echocardiogram 10 years after radiotherapy is recommended.
Collaborative cardiac and cancer care is associated with improved outcomes and better cardioprotection. Consultations with cardiologists were associated with a higher frequency of heart failure medication prescription and better survival outcomes47. Chemotherapy-induced left ventricular systolic dysfunction is diagnosed, on average 2 years after cancer diagnosis, suggesting that regular echocardiography surveillance may help identify late-manifesting cardiac injury47. Baseline cardiovascular risk assessment (electrocardiogram, left ventricular ejection fraction [LVEF], cardiac biomarkers and lipid panel) before anticancer therapy could lessen the chances of developing cardiovascular complications48. Anthracyclines and certain targeted therapies (e.g., trastuzumab or sorafenib) are associated with a high risk of left ventricular dysfunction or heart failure. Patients with pre-existing cardiovascular disease who received trastuzumab, doxorubicin or both show sustained decline in LVEF over 3 years post-therapy49. Beta-blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers can reduce the risk of cardiotoxicity in these patients50.
Implications for practice
The role of comorbidity should be considered at different stages along the cancer care pathway. Due to the advancements in cancer treatment, more and more patients are surviving cancer and comorbidity may increase the risk of developing late effects51,52 during the survivorship period. Comorbidity may increase the risk of certain cancers and the risk of recurrence. Comorbid conditions may affect cancer diagnostic timeliness, participation in cancer screening programmes and consequently stage at diagnosis. Comorbidities exert varying effects on help-seeking behaviour. Patients with lung cancer who have COPD took longer to consult with symptoms of lung cancer53. Similarly, patients with dementia are less likely to seek help for cancer symptoms54. Comorbidity is associated with a longer diagnostic interval from the first presentation to cancer diagnosis and delayed diagnosis is often linked to a higher tumour stage at diagnosis55. Comorbidity influences treatment choice, adherence to treatment, surveillance post-treatment and overall prognosis. Comorbidity may influence the decision to offer adjuvant chemotherapy if there is a perceived risk for such treatment to affect the underlying condition. Comorbidity may increase treatment toxicity or might be a reason for withholding curative-intent or multimodality treatment, thus making it challenging to determine whether poor survival is due to the comorbidity or patients receiving less intensive therapy. Considering specific comorbidities individually rather than composite measures could be more useful to assess how they might affect cancer treatment, subsequent monitoring and overall outcome. It is also important to disentangle the impact of comorbidity on treatment decisions, i.e., clinicians not offering certain treatments for patients with certain comorbidities (physician factors) versus patients with a high degree of functional impairment refusing treatment due to previous health experience (patient factors). Age and other patient characteristics may also modify the relationship between comorbidity and cancer treatment decisions. To design an optimal treatment plan for patients, a data-informed systematic approach to assess the risks posed by individual comorbidities on specific treatment regimens is warranted. The definition of comorbidity is not consistent across studies, which does not allow for cross-comparisons and precludes meta-analysis. Some studies use disease-specific measures while others use index systems. Our study seeks to address this limitation by evaluating the impact of individual comorbidities, using a systematic and consistent methodology, across 19 cancer types.
Strengths and limitations
To our knowledge, this is the first population cohort study investigating the impact of 109 conditions on cancer treatment decisions across 19 adult cancers, employing a consistent methodology allowing for cross-comparison. We employed linked data from primary care, hospitals and cancer registry. The use of routinely collected health records means that this study is not affected by biases inherent to self-reported measures. Previous studies on comorbidities often rely on general measures (e.g., CCI), which assumes that they have a similar impact in different disease populations. We have taken a different approach to estimate the effects of single conditions on cancer treatment choice to improve prognostic utility. Other factors can influence preferences for treatment and treatment outcomes. We account for heterogeneity among patients by considering age, sex, socioeconomic status, tumour grade, tumour stage, tumour count and the presence of multiple comorbid conditions in regression models.
We acknowledge several limitations. Although this is a retrospective cohort study, it provides a real-world depiction of treatment patterns in patients across diverse cancer types. We do not have access to data on ECOG Performance Status and have attempted to mitigate this by utilising detailed clinical diagnosis data to infer patient health status, which may serve as a proxy for treatment suitability. We relied on diagnoses recorded in ICD-10, Read and SNOMED codes and have not quantified the severity of individual conditions. Nonetheless, despite this limitation, our study provides a comprehensive assessment of comorbidities, and the use of population-based cohorts helps ensure the generalisability of results. The data also reflects conditions that are managed in both community-based general practices and conditions requiring hospitalisation. However, conditions might vary in their compensated and decompensated states, and this is not captured in the data. Additionally, our analysis did not account for palliative care as a treatment category as we do not have access to palliative care data. As a result, we have focused on active treatment choices, while acknowledging that the inclusion of palliative care could have provided a more complete understanding of treatment decisions. We have not explored the impact of specific combinations of comorbidities on treatment decisions. We also acknowledge that including a ‘no treatment’ category could provide a broader understanding of treatment patterns. However, this aspect was beyond the scope of our analysis as it may warrant a distinct analytical approach to capture treatment-naïve patient populations. We opted for ‘surgery alone’ as the reference category in the regression models as surgery is frequently the initial and primary curative treatment across many cancer types, thereby providing a standardised baseline for comparison. We acknowledge, however, that the heterogeneity of cancers and their respective treatment paradigms present a challenge in selecting a one-size-fits-all reference category. Future research might employ disease-specific analyses to tailor the reference group more closely to the care pathway for each cancer type. We also did not explore the reciprocal effects of how cancer diagnosis or treatment could influence the management of comorbidities, which is beyond the scope of this study but may have implications on overall patient prognosis. Cancer stage was included as a covariate to uniformly control for its effects across a diverse cohort of cancers. The inclusion as a covariate was based on the broad, survey nature of our study, aiming to provide a generalised view of the impact of comorbidities on treatment decisions. We acknowledge, however, that the stage of cancer may indeed act as an effect modifier. While the current scope of this work did not extend to performing stratified analyses (primarily to maintain clarity, manageability of the data presented and to avoid the risk of obscuring the primary results), we recognise the value that this additional layer of analysis could bring to subsequent research. Our methodology utilised a 5-year period preceding cancer diagnosis to identify comorbidities. The broad definition of comorbidities is aimed at capturing the diverse health profiles of patients prior to their cancer diagnosis comprehensively. However, this could mean that certain conditions may be interpreted as comorbidities even though they could be related to cancer manifestation. While we have not implemented restriction windows to exclude comorbidities that are identified shortly before cancer diagnosis, our study’s initial objective of examining the associations between comorbidities and cancer treatment is still being met. In this context, including comorbidities identified close to cancer diagnosis may be pertinent in situations where these comorbidities might influence or be considered in the decision-making process for cancer treatment. As such, excluding these near-diagnosis comorbidities could potentially omit significant data that are relevant to understanding the real-world clinical decision-making processes. This scenario reflects clinical practice where decisions are often made based on the patient’s entire health profile at the time of diagnosis, rather than a segmented view based on timing restrictions. We also strived to mitigate this by including GP records to capture a broader spectrum of health conditions than those seen in hospitals, thus increasing the likelihood of capturing long-standing comorbidities that are managed in community settings. A future study could further explore the impact of implementing a restriction period to further refine the understanding of comorbidity impacts in cancer care, within the context of specific cancer types. It is important to mention that our study is epidemiological in nature. Our primary aim is to map out patterns of comorbidity in patients with cancer and to observe associations with treatment decisions. This approach, while not equipped to establish causal relationships, remains valuable in identifying trends and associations in large datasets that can guide future hypothesis-driven research. We further acknowledge that observational studies have inherent limitations in drawing causal inferences and that the conclusions of our work do not extend beyond what is shown in the data. However, they are crucial for generating hypotheses and understanding real-world scenarios, especially in areas where RCTs are not feasible or ethical. Our study adds to this body of knowledge by providing a comprehensive overview of comorbidity patterns in cancer care.
Conclusion
Comorbidity affects cancer treatment decisions. It can interact with cancer and influence the tolerability or effectiveness of cancer treatment. A thorough assessment of comorbid conditions, especially renal, liver and cardiac function, in every patient prior to starting cancer treatment would allow for dosage adjustment of anticancer drugs. Where nephrotoxic or cardiotoxic anticancer therapy is required, preventive measures such as hyper-hydration, diuresis or managing modifiable cardiovascular risk factors through drug prophylaxis may be warranted56,57. Patients with comorbidity require care by different physicians, which often occurs at different time points and involves different specialties and settings. To optimise care, effective information exchange and interprofessional or cross-sectoral collaboration are required. Patients may be offered a more active role in healthcare coordination. By keeping patients informed of their status, it could facilitate information flow between all providers of care58.
Data availability
This study employs routinely collected electronic health records (EHRs) in England. EHRs are classified as sensitive data by the UK Data Protection Act, thus information governance restrictions are in place to prevent public data sharing in order to protect patient confidentiality. Data are available on a successful application to the Medicines and Healthcare products Regulatory Agency and assessment by the Scientific Advisory Committee. Applications to access the cancer registry data independently can be submitted to Public Health England. All summarised data are available in the Supplementary Materials.
References
National Institute for Health and Care Excellence. Lung Cancer: Diagnosis and Management.
National Institute for Health and Care Excellence. Early and Locally Advanced Breast Cancer: Diagnosis and Management.
National Institute for Health and Care Excellence. Prostate Cancer: Diagnosis and Management.
Duma, N. et al. Characterization of comorbidities limiting the recruitment of patients in early phase clinical trials. Oncologist 24, 96–102 (2019).
Unger, J. M., Hershman, D. L., Fleury, M. E. & Vaidya, R. Association of patient comorbid conditions with cancer clinical trial participation. JAMA Oncol. 5, 326–333 (2019).
About the National Cancer Registration and Analysis Service. NCRAS. http://www.ncin.org.uk/about_ncin/.
GOV.UK. English Indices of Deprivation.
HDR UK. Phenotype Library. https://phenotypes.healthdatagateway.org/.
Kuan, V. et al. A chronological map of 308 physical and mental health conditions from 4 million individuals in the English National Health Service. Lancet Dig. Health 1, e63-77 (2019).
Good, P. I. Estimating population parameters. In Resampling Methods: A Practical Guide to Data Analysis (ed. Good, P. I.) (Springer, 2006).
Lee, L., Cheung, W. Y., Atkinson, E. & Krzyzanowska, M. K. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: A systematic review. J. Clin. Oncol. 29, 106–117 (2011).
Wright, J., Doan, T., McBride, R., Jacobson, J. & Hershman, D. Variability in chemotherapy delivery for elderly women with advanced stage ovarian cancer and its impact on survival. Br. J. Cancer 98, 1197–1203 (2008).
Batra, A., Sheka, D., Kong, S. & Cheung, W. Y. Impact of pre-existing cardiovascular disease on treatment patterns and survival outcomes in patients with lung cancer. BMC Cancer 20, 1–16 (2020).
Van Steenbergen, L. et al. Large age and hospital-dependent variation in administration of adjuvant chemotherapy for stage III colon cancer in southern Netherlands. Ann. Oncol. 21, 1273–1278 (2010).
Koroukian, S. M. et al. Comorbidities, functional limitations, and geriatric syndromes in relation to treatment and survival patterns among elders with colorectal cancer. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 65, 322–329 (2010).
Hoeben, K. et al. Treatment and complications in elderly stage III colon cancer patients in the Netherlands. Ann. Oncol. 24, 974–979 (2013).
Berglund, A. et al. Impact of comorbidity on management and mortality in women diagnosed with breast cancer. Breast Cancer Res. Treat. 135, 281–289 (2012).
Sarfati, D. et al. The effect of comorbidity on the use of adjuvant chemotherapy and survival from colon cancer: A retrospective cohort study. BMC Cancer 9, 1–10 (2009).
Jørgensen, T. L. et al. Significance of age and comorbidity on treatment modality, treatment adherence, and prognosis in elderly ovarian cancer patients. Gynecol. Oncol. 127, 367–374 (2012).
Koroukian, S. M. Assessment and interpretation of comorbidity burden in older adults with cancer. J. Am. Geriatr. Soc. 57, s275–s278 (2009).
Sundararajan, V., Grann, V. R., Jacobson, J. S., Ahsan, H. & Neugut, A. I. Variations in the use of adjuvant chemotherapy for node-positive colon cancer in the elderly: A population-based study. Cancer J. 7, 213–218 (2001).
Derks, W., De Leeuw, J., Hordijk, G. & Winnubst, J. Reasons for non-standard treatment in elderly patients with advanced head and neck cancer. Eur. Arch. Oto-Rhino-Laryngol. Head Neck 262, 21–26 (2005).
De Rijke, J. et al. Influence of age, comorbidity and performance status on the choice of treatment for patients with non-small cell lung cancer; results of a population-based study. Lung Cancer 46, 233–245 (2004).
Extermann, M. et al. Predicting the risk of chemotherapy toxicity in older patients: The chemotherapy risk assessment scale for high-age patients (CRASH) score. Cancer 118, 3377–3386 (2012).
Klabunde, C. N., Potosky, A. L., Legler, J. M. & Warren, J. L. Development of a comorbidity index using physician claims data. J. Clin. Epidemiol. 53, 1258–1267 (2000).
Piccirillo, J. F., Tierney, R. M., Costas, I., Grove, L. & Spitznagel, E. L. Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 291, 2441–2447 (2004).
Tabchi, S., Kassouf, E., Florescu, M., Tehfe, M. & Blais, N. Factors influencing treatment selection and survival in advanced lung cancer. Curr. Oncol. 24, 115–122 (2017).
Abi Jaoude, J. et al. Performance status restriction in phase III cancer clinical trials. J. Natl. Compr. Cancer Netw. 18, 1322–1326 (2020).
Temel, J. S. et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N. Engl. J. Med. 363, 733–742 (2010).
Ando, M. et al. Prognostic value of performance status assessed by patients themselves, nurses, and oncologists in advanced non-small cell lung cancer. Br. J. Cancer 85, 1634–1639 (2001).
Dajczman, E. et al. Should patient-rated performance status affect treatment decisions in advanced lung cancer? J. Thorac. Oncol. 3, 1133–1136 (2008).
Chiruvella, V., Annamaraju, P. & Guddati, A. K. Management of nephrotoxicity of chemotherapy and targeted agents: 2020. Am. J. Cancer Res. 10, 4151 (2020).
Bennis, Y. et al. Cisplatin dose adjustment in patients with renal impairment, which recommendations should we follow? Int. J. Clin. Pharm. 36, 420–429 (2014).
Charlier, C., Kintz, P., Dubois, N. & Plomteux, G. Fatal overdosage with cisplatin. J. Anal. Toxicol. 28, 138–140 (2004).
Hashimoto, N. et al. Clinical impact of prevalence and severity of COPD on the decision-making process for therapeutic management of lung cancer patients. BMC Pulm. Med. 14, 1–9 (2014).
Sekine, Y., Suzuki, H., Yamada, Y., Koh, E. & Yoshino, I. Severity of chronic obstructive pulmonary disease and its relationship to lung cancer prognosis after surgical resection. Thorac. Cardiovasc. Surg. 61, 124–130 (2013).
Kim, E. S. et al. Prevalence of and risk factors for postoperative pulmonary complications after lung cancer surgery in patients with early-stage COPD. Int. J. Chron. Obstruct. Pulm. Dis. 11, 1317 (2016).
Wolf, I., Sadetzki, S., Catane, R., Karasik, A. & Kaufman, B. Diabetes mellitus and breast cancer. Lancet Oncol. 6, 103–111 (2005).
Engel, J., Kerr, J., Schlesinger-Raab, A., Sauer, H. & Hölzel, D. Quality of life following breast-conserving therapy or mastectomy: Results of a 5-year prospective study. Breast J. 10, 223–231 (2004).
Chon, B. H. & Loeffler, J. S. The effect of nonmalignant systemic disease on tolerance to radiation therapy. The Oncologist 7, 136–143 (2002).
Gogas, H. et al. The impact of diabetes mellitus on the toxicity of therapy for advanced ovarian cancer. Gynecol. Oncol. 61, 22–26 (1996).
Darby, S. C. et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 368, 987–998 (2013).
Taylor, C. et al. Estimating the risks of breast cancer radiotherapy: Evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J. Clin. Oncol. 35, 1641 (2017).
Hull, M. C., Morris, C. G., Pepine, C. J. & Mendenhall, N. P. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA 290, 2831–2837 (2003).
Heidenreich, P. A., Hancock, S. L., Lee, B. K., Mariscal, C. S. & Schnittger, I. Asymptomatic cardiac disease following mediastinal irradiation. J. Am. Coll. Cardiol. 42, 743–749 (2003).
Lancellotti, P. et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: A report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 14, 721–740 (2013).
Ammon, M. et al. Cardiovascular management of cancer patients with chemotherapy-associated left ventricular systolic dysfunction in real-world clinical practice. J. Card. Fail. 19, 629–634 (2013).
Curigliano, G. et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 31, 171–190 (2020).
Narayan, H. K. et al. Detailed echocardiographic phenotyping in breast cancer patients: Associations with ejection fraction decline, recovery, and heart failure symptoms over 3 years of follow-up. Circulation 135, 1397–1412 (2017).
Cardinale, D. et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 114, 2474–2481 (2006).
Chang, W. H. et al. Late effects of cancer in children, teenagers and young adults: population-based study on the burden of 183 conditions, in-patient and critical care admissions and years of life lost. Lancet Reg. Health Eur. https://doi.org/10.1016/j.lanepe.2021.100248 (2021).
Chung, S.-C. et al. Multimorbidity patterns and risk of hospitalisation in children: A population cohort study of 3.6 million children in England, with illustrative examples from childhood cancer survivors. Lancet Reg. Health Eur. 20, 100433 (2022).
Smith, S. M. et al. Factors contributing to the time taken to consult with symptoms of lung cancer: A cross-sectional study. Thorax 64, 523–531 (2009).
Iritani, S., Tohgi, M., Miyata, H. & Ohi, G. Impact of dementia on cancer discovery and pain. Psychogeriatrics 11, 6–13 (2011).
Friese, C. R. et al. Timeliness and quality of diagnostic care for medicare recipients with chronic lymphocytic leukemia. Cancer 117, 1470–1477 (2011).
Alexandre, J. et al. Cardiovascular toxicity related to cancer treatment: A pragmatic approach to the American and European cardio-oncology guidelines. J. Am. Heart Assoc. 9, e018403 (2020).
Cosmai, L. et al. Preventive strategies for acute kidney injury in cancer patients. Clin. Kidney J. 14, 70–83 (2021).
Bossert, J. et al. What patients with lung cancer with comorbidity tell us about interprofessional collaborative care across healthcare sectors: Qualitative interview study. BMJ Open 10, e036495 (2020).
Acknowledgements
The authors are grateful to Eduardo Franco for his thorough and constructive comments.
Funding
AGL is supported by funding from the Wellcome Trust (204841/Z/16/Z), National Institute for Health Research (NIHR) University College London Hospitals Biomedical Research Centre (BRC714/HI/RW/101440), NIHR Great Ormond Street Hospital Biomedical Research Centre (19RX02) and Academy of Medical Sciences (SBF006\1084).
Author information
Authors and Affiliations
Contributions
W.H.C. and A.G.L. conceived and designed the study. W.H.C. undertook literature search. W.H.C. and A.G.L. analysed the data and interpreted the results. A.G.L. supervised the research. W.H.C. wrote the initial manuscript draft. A.G.L. revised the manuscript draft. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chang, W.H., Lai, A.G. Pan-cancer analyses of the associations between 109 pre-existing conditions and cancer treatment patterns across 19 adult cancers. Sci Rep 14, 464 (2024). https://doi.org/10.1038/s41598-024-51161-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-51161-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.