Abstract
Ticks are blood-sucking ectoparasites and can transmit various pathogens of medical and veterinary relevance. The life cycle of ticks can be completed under laboratory conditions on experimental animals, but the artificial feeding of ticks has attracted increased interest as an alternative method. This study represents the first report on the successful in vitro feeding of all life stages of two-host tick species, Hyalomma scupense and Hyalomma excavatum, and the three-host tick Hyalomma dromedarii. The attachment and engorgement rates of adults were 84% (21/25) and 76% (19/25) for H. scupense females. For adult H. excavatum and H. dromedarii, 70% (21/30) and 34.4% (11/32) of the females attached and all attached females successfully fed to repletion. The oviposition rates of the artificially fed females were 36.4%, 57.1% and 63.1% for H. dromedarii, H. excavatum and H. scupense, respectively, with a reproductive efficiency index varying between 44.3 and 60.7%. For the larvae, the attachment and engorgement rates were 44.2% (313/708) and 42.8% (303/708) for H. dromedarii, 70.5% (129/183) and 56.8% (104/183) for H. excavatum and 92.6% (113/122) and 55.7% (68/122) for H. scupense. The attachment and engorgement rates for the nymphs were 90.2% (129/143) and 47.6% (68/143) for H. dromedarii, 66.7% (34/51) and 41.2% (21/51) for H. excavatum, and 44.1% (30/68) and 36.8% (25/68) for H. scupense. Molting rates of the immature stages varied between 71.3% (216/303) and 100% (68/68) for the larvae and between 61.9% (13/21) and 96% (24/25) for the nymphs. The successful in vitro feeding of all stages of the three Hyalomma species makes this method a valuable tool for tick research, with potential applications in studies on the pathogens transmitted by these tick species such as Theileria annulata.
Similar content being viewed by others
Introduction
Ticks are hematophagous ectoparasites that may act as vectors for a variety of pathogens infecting humans and animals worldwide. This includes several species of the Hyalomma genus that are associated with the transmission of Crimean-Congo Hemorrhagic Fever virus in humans1,2 and Theileria annulata, the causal agent of tropical theileriosis in livestock3.
The duration of tick life cycle is strongly affected by environmental factors, e.g. temperature and relative humidity and by the availability of hosts4. Hyalomma scupense, H. dromedarii and H. excavatum have different life cycles. In Tunisia, H. scupense was found to behave as a two-host tick species with peak activity of the adults feeding on cattle in summer5, whereas in other regions, a cold-adapted ecotype of H. scupense was found to have a one-host life cycle6. Hyalomma scupense collected in China were reported to behave as one- and two-host tick under laboratory conditions7. Hyalomma excavatum can have a two-or three host life cycle tick depending on the availability of hosts. In North Africa, it is active during the whole year with a peak in spring. Larvae and nymphs of this species feed on rodents and the adults infest various hosts such as cattle, small ruminants and equids8. Hyalomma dromedarii can behave as a one-, two-, or three-host tick8,9,10, whereby the immature stages can feed on camels, but also on rodents and birds. The activity of this ticks species does not exhibit a seasonal variation and it can be found throughout the year on animals11,12.
Studies on the biology of these ticks frequently require laboratory tick colonies that are reared and maintained on animals29. In recent years, artificial tick feeding systems (ATFS) have been developed and adapted for different ixodid ticks such as Amblyomma hebraeum, Dermacentor reticulatus, Ixodes ricinus, Rhipicephalus appendiculatus as well as nymphs and adult Hyalomma species such as H. dromedarii and H. anatolicum13,14,15,16,17,18,19. The development and optimization of methods to feed hard ticks artificially in the laboratory not only contributes to the 3Rs principle (to Reduce, Replace and Refine animal experiments) for humane animal research20, but also provides researchers with a versatile tool to study various aspects of tick biology, pathogen transmission and drug discovery under controlled laboratory conditions13,21.
However, extensive optimization of the artificial feeding method is still required. There is for instance a lack of studies examining the artificial feeding of juvenile stages of one- and two-host tick species where ticks molt on the host to the subsequent life stage. In addition, the success rate reported for the in vitro feeding of ixodid ticks varies considerably and the addition of antibiotics to the blood meal to prevent microbial contamination may exert a negative effect on tick endosymbionts and tick fecundity22,23. In this study, we targeted the first point by feeding all life stages of H. dromedarii, H. excavatum and H. scupense in vitro.
Results
Feeding of Hyalomma adults
In vitro feeding
All life stages of H. excavatum, H. dromedarii and H. scupense were successfully fed in vitro (Fig. 1). Results of the in vitro feeding of adult Hyalomma ticks are summarized in Table 1. Briefly, H. excavatum females (fed in winter, January) achieved a 70% (21/30) attachment rate where all of the attached ticks (21/21) engorged. The mean weight of engorged females was 539.8 ± 163 mg. Twelve of the 21 engorged females (57.1%) laid eggs (6665 ± 2009 eggs), with a mean REI of 51.8 ± 11.9%, where 11 of the females (91.6%) produced viable larvae. The percentage of hatched larvae varied between 90 and 100% (except for one tick where the hatching rate was 30%) resulting in a mean of 6217 ± 1031 larvae (Table 1).
Hyalomma dromedarii fed in winter (January) reached an engorgement rate of 34.4% (11/32 females). The mean weight of engorged detached females was 276 ± 105 mg. Only four out of 11 females (36.4%) produced eggs, with a mean number of 4036 ± 2967 with a REI of 60%. Most of the eggs hatched successfully (99.5%) (Table 1).
As adult H. scupense ticks are under natural conditions in Tunisia (from where the tick colony originates) active in summer, we tried to examine this seasonal behaviour in vitro by conducting one feeding experiment in winter (January) and one in summer (June). This seasonal difference was not evaluated for the other two tick species as their activity does not exhibit seasonal variation. Hyalomma scupense adults that fed in winter showed lower engorgement rates compared to those fed in summer. Despite the high attachment rate observed in winter (18 out of 20 ticks, 90%), only five ticks (5/20, 25%) partially engorged and detached with a low mean detachment weight of 36.8 ± 22.5 mg. Whereas in summer, the attachment and engorgement rates were 84% (21/25) and 76% (19/25), respectively, with a mean detachment weight of 298.0 ± 65.4 mg. None of the females fed during the first experiment laid eggs, whereas females from the second in vitro experiment had an oviposition rate of 63.2% (12/19) and produced a mean number of eggs of 2656 ± 902 with a mean REI of 44.3 ± 7.3% (Table 1). Out of the 12 females laid eggs, nine were able to produce viable larvae (75%) with a hatching rate estimated to 54%.
In vivo feeding results and comparison with in vitro feeding of Hyalomma adults
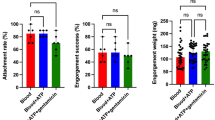
The engorgement rates of H. excavatum and H. dromedarii females fed on rabbits (“in vivo”, fed in winter and in summer, respectively) were 84% (10/12) and 70% (14/20), higher than their counterparts fed in vitro (Z-test, P = 0.35 for H. excavatum and P = 0.01 for H. dromedarii). For H. scupense fed in winter, the engorgement rate was low for both conditions but still higher in vitro 25% (5/20) than on calf 10% (1/10) (Z-test, P = 0.33) (Fig. 2a), but none of the in vitro fed ticks laid eggs whereas the one female that fed to repletion on a calf did. Hyalomma scupense fed in summer had engorgement rates of 76% (19/25) in vitro and 86.7% (13/15) when fed on a calf (Z-test, P = 0.4), respectively. The oviposition rate was with 92.3% (12/13) also higher in vivo than in vitro (63.2%, 12/19; Z-test, P = 0.06) (Fig. 2b).
Engorgement (a) and oviposition (b) rates of adult Hyalomma fed in vivo and in vitro. Asterisks indicate level of significance using Z-test (P < 0.01 (**), n.s = non-significant, # for Hyalomma scupense fed in winter, only one engorged tick laid eggs and the oviposition rate was not calculated. H. scupense S = Hyalomma scupense fed in summer (S); H. scupense W = Hyalomma scupense fed in winter (W). Engorgement rate (%) = (number of engorged females/numbers of initially placed ticks) *100; Oviposition rate (%) = (number of females laying eggs/number of engorged females) *100.
Adults of H. dromedarii and H. excavatum took longer to engorge on a rabbit (10.4 ± 1.8 days and 9.6 ± 2.5 days, respectively) than in vitro (9.1 ± 1.6 days and 7.6 ± 1.6 days, respectively) (Mann Whitney test, P = 0.03 for both species), whereas H. scupense females fed longer in vitro (11.5 ± 2.2 days) compared to H. scupense females fed on a calf (9.1 ± 0.95 days) (Mann Whitney test, P = 0.001; Fig. 3).
The weight of engorged females was significantly higher for all Hyalomma ticks fed on animals compared to those fed in vitro (One-way ANOVA and Tukey test, P < 0.0001). The highest weight of engorged females was found for H. excavatum fed on an animal (903 ± 114 mg) and in vitro (540 ± 167 mg) (Fig. 4a). There were no significant differences in egg mass produced between ticks fed in vivo and in vitro for any of the species (Fig. 4c). The REI was higher in vitro than in vivo for H. dromedarii (60.7 and 49.5%, respectively) and H. excavatum (51.8 and 44.6%), but not for H. scupense (in vivo 52.1%, in vitro 44.3%).
The longest preoviposition period was observed for H. excavatum fed in vivo (86 ± 41 days) and in vitro (58 ± 29 days) with no significant difference between the two feeding conditions (Mann Whitney test, P = 0.1). The shortest preoviposition duration was recorded for H. scupense fed in vitro (7.3 ± 1.4 days) and in vivo (7.8 ± 1.2 days, Mann Whitney test, P = 0.2). Hyalomma dromedarii females fed in vivo (8 ± 1.4 days) had significantly shorter preoviposition period than H. dromedarii fed in vitro (12.6 ± 2.5 days) (Mann Whitney test, P = 0.01) (Fig. 4b).
The mean oviposition-hatching duration (the period from the start of oviposition until the hatching of the first larvae) was relatively similar between the feeding conditions (Fig. 4d).
Feeding of larvae and nymphs
In vitro feeding of Hyalomma immatures
Results of the in vitro feeding of immature Hyalomma species are shown in Table 2. Hyalomma scupense acted as a two-host tick in vitro, i.e., the larvae molted to nymphs on the membrane (Supplementary Fig. 1) similar to ticks fed on rabbits. This contrasted with H. dromedarii, that acted as a three-host tick in vitro, but as a two-host tick when fed on rabbits. Hyalomma excavatum behaved as two-host tick when fed in vitro or on a rabbit, but as a three-host tick when the larvae were fed on a gerbil.
A high attachment rate was observed for H. scupense larvae, with 113 out of 122 attached ticks (92.6%). Sixty-eight larvae out of 122 (55.7%) engorged. All engorged larvae successfully molted to the nymphal stage on the membrane. Out of these 68 nymphs, 25 fed to repletion (36.8%). Twenty-four nymphs (96%) molted to the adult stage (11 males and 13 females) in a mean of 34.8 ± 6.1 days. The mean weight of engorged nymphs was 13.4 ± 3.6 mg, whereas the mean weight of unfed adults was 6.7 ± 1.7 mg.
For H. excavatum, 129 out of 183 larvae attached (70.5%) and 104 engorged (56.8%). Ninety-four larvae molted (90.4%) on the membrane to the nymphal stage. An attachment rate of 66.7% (34/51), engorgement rate of 41.2% (21/51) with a mean detachment weight of 10.4 ± 2.8 mg was recorded for the nymphs. Out of 21 engorged nymphs, 13 (61.9%) molted to the adult stage (five females and eight males) in 17.9 ± 1.9 days. The average weight of unfed adults was 6 ± 2 mg.
For H. dromedarii, an attachment and engorgement rate of 44.2% (313/708) and 42.8% (303/708) was recorded for the larvae. Of the engorged larvae, 71.3% (216/303) molted to the nymphal stage. For the nymphs, the attachment rate was 90.2% (129/143) and the engorgement rate was 47.6% (68/143). The nymphal weight varied between 6.1 and 19.9 mg. Fifty-four (79.4%) of the nymphs molted to adults (22 females and 32 males) in a mean of 29.7 ± 7.5 days (Table 2). The attachment, engorgement and molting proportions varied significantly between the three species for larvae and nymphs (Chi-square test, P < 0.0001).
In vivo feeding results and comparison with in vitro feeding of immatures of Hyalomma ticks
During the in vitro feeding, H. excavatum and H. scupense behaved as two-host ticks and H. dromedarii acted as a three-host tick. When fed on rabbits, all three Hyalomma species acted as two-host ticks. Hyalomma excavatum acted as a three-host when larvae were fed on gerbils.
Due to the different feeding behaviour, the only possible comparison that could be done was on the duration of feeding for H. dromedarii larvae, which was not significantly different between both conditions (Mann Whitney test, P = 0.06) (Fig. 5a). For the nymphs, the feeding duration varied significantly between the two feeding conditions for H. excavatum (Mann Whitney test, P < 0.01) and H. scupense (Mann Whitney test, P < 0.0001), but no significant difference was observed for H. dromedarii (Mann Whitney test, P = 0.2) (Fig. 5b).
Mean feeding duration of Hyalomma larvae (a) and nymphs (b) fed in vitro and in vivo. NA: For Hyalomma excavatum and H. scupense larvae, statistics between in vivo and in vitro feeding condition was not conducted as ticks acted different (H. excavatum and H. scupense engorged larvae molt to nymphs while attached to the membrane, so the feeding and molting durations were given together), therefore we indicated as not available (NA). Asterisks indicate level of significance using Mann Whitney test (P < 0.05 (*), P < 0.0001 (***), n.s = non-significant.
As for the females, the weight of engorged nymphs fed on animals was significantly higher than that of nymphs fed in vitro for all the three Hyalomma species (One-way ANOVA followed by Tukey test and confirmed by Mann Whitney test, P < 0.0001) (Fig. 6).
There was a significant positive correlation between engorged H. scupense nymphs in vitro and the weight of their counterpart unfed adults (Pearson r = 0.73, P < 0.0001) (Fig. 7a). The same but stronger correlation was observed for unfed adult H. excavatum fed in vitro (Pearson r = 0.92, P < 0.0001) (Fig. 7b).
The weight of unfed H. scupense adults obtained from in vivo feeding was significantly higher than those fed in vitro (One-way ANOVA and Tukey test, P < 0.0001) (Fig. 8a). The same trend was also reported for unfed H. excavatum adults (Mann Whitney test, P < 0.0001) (Fig. 8b). For both species fed in the two feeding conditions, unfed females had higher weight than unfed males (One-way ANOVA and Tukey test, P < 0.0001) except for H. scupense fed in vitro where the difference was not significant (Mann Whitney test, P = 0.06).
Discussion
The use of artificial feeding systems to feed ticks in a laboratory setting instead of relying on vertebrate hosts has been used to study various aspects of tick biology, including host-tick interactions, tick feeding behaviour, and the transmission of tick-borne pathogens24,25,26. Feeding all life stages on membranes was previously reported for the three-host ticks A. hebraeum15, A. variegatum27 and I. ricinus16.
A number of studies describing the in vitro feeding of Hyalomma ticks have been previously published. This includes H. dromedarii, H. anatolicum17,18, H. lusitanicum28, H. excavatum and H. marginatum29. However, these studies were limited to the nymphal or adult stage. In this paper, we present the first successful in vitro feeding of all life stages of H. dromedarii, H. scupense and H. excavatum on silicone membranes.
Feeding of Hyalomma adults
The highest engorgement rate was observed for adult H. scupense (76%) followed by H. excavatum (70%) and H. dromedarii (34.4%) with a mean feeding duration of 9, 7.6 and 11.5 days. These results were comparable to previous reports on the artificial feeding of H. lusitanicum which had an engorgement rate of 40.2% in approximately 11 days28. Similar results were previously reported by Tajeri and Razmi, (2011) for H. dromedarii (55%) and H. anatolicum (75%) with a feeding duration that varied between 11 and 16 days. The mean feeding duration found in this study for H. dromedarii (9.1 days) and H. excavatum (7.6 days) was shorter than the feeding durations of their counterparts fed on rabbits, which lasted 10.4 ± 1.8 days for H. dromedarii and 9.6 ± 2.5 days for H. excavatum. This is surprising as in vitro feeding usually lasts longer than the feeding on animals16,30,31. This could perhaps be attributed to the final feeding phase, which might have been quicker in vitro due to mechanical limitations caused by the confined space of the feeding units in combination with ticks clustering on the membrane in the feeding units, forcing the ticks detach earlier25,31 or perhaps a feeding stimulant factor(s) that was missing in the in vitro system. It would be interesting to observe how these ticks would behave in feeding units with a larger diameter or lower tick density.
A recent publication on the in vitro feeding H. excavatum adults, which originated from the same tick colony as used for this study, and H. marginatum showed much lower engorgement rates of 8.3 and 7.9%, respectively, with a longer feeding period of over 20 days29. Several factors might have led to this variation in feeding success such as tick fitness at time of feeding, feeding preferences and behaviour, membrane composition and thickness and specific environmental conditions such as temperature, relative humidity, CO2 levels, attachment stimuli, and dark/light condition22,32.
It has previously been suggested for H. lusitanicum that season could affect the tick feeding success (31.6%)28. Although it was limited to two experiments only, we did make similar observations for adult H. scupense, where we observed a higher engorgement rate in summer (June) (76%) compared to ticks fed in winter (January) (25%). This corroborates with the H. scupense adult activity in North Africa as these are mainly found on animals in summer5,33. However, more extensive experiments should be conducted to confirm whether the in vitro feeding success of H. scupense is truly season dependent.
Another important factor that can influence tick feeding efficacy is the feeding system itself. In a feeding system recently used for R. appendiculatus, the chambers were inverted (upside down) based on the natural tendency of ticks to crawl up towards a host34,35. Such an inverted system where the blood is placed above the membrane might also be useful for studies working on the artificial infection of ticks with intra-erythrocytic pathogens such as Babesia and Theileria spp.32.
In the present study, the highest mean detachment weight was recorded for H. excavatum (539.8 ± 167 mg) followed by H. dromedarii (305 ± 102 mg) and H. scupense S (298.01 ± 65.4 mg). The average weight of H. excavatum in this study is higher than the weight previously reported for H. excavatum fed in vitro (260.6 mg), where tick feeding did not take place inside an incubator but in a water bath to maintain a constant blood temperature. In general, the average weight of the engorged Hyalomma females was significantly higher for ticks fed in vivo than those fed in vitro (P < 0.0001), also leading to higher egg mass production. This finding is in line with previous report for H. lusitanicum fed in vivo36 and in vitro28 where the average weight were 543 mg and 274.65 mg, respectively. Higher average weights were also recorded for I. ricinus fed in vivo (231 ± 72.3 mg) compared to the ones fed in vitro (136 ± 44.9 mg)16. The lower engorgement weights of all fed life stages, resulting in smaller ticks and decreased egg production, is a consistent finding in the in vitro feeding of ixodid ticks when compared to feeding on natural hosts. This, in combination with the necessity to use antibiotics with potential negative effects on tick endosymbionts and tick fecundity remains a major stumbling block for the complete maintenance of tick laboratory colonies using artificial feeding alone. It will be essential to conduct long-term studies to examine whether Hyalomma tick colonies could also be maintained by in vitro feeding over several generations without detrimental side effects on the tick biology and physiology.
Despite the lower detachment weight, the percentage of engorged females that laid eggs was 50, 57.1 and 63.1% for H. dromedarii, H. excavatum and H. scupense, respectively. This was lower than their equivalents fed on animals, where 100%, 50% and 92.3% of the females laid eggs. The low oviposition rates observed in artificial feeding in comparison to in vivo feeding might be due to the elimination of endosymbionts or antibiotic toxicity. In general, the mean REI was around 50% for all three species under both feeding conditions (Table 1), except for H. dromedarii fed in vitro where the mean RE was 60%. This is higher than its counterparts fed on rabbit (49.5%) but in line with previous reports on H. dromedarii where high REI of up to 72% were reported4. This variation could be explained by host-differences, it was for instance shown that H. anatolicum had a higher REI when fed on a rabbit compared to sheep and goats37, but also by the tick strain, quantity of blood ingested, humidity, temperature and possible disturbance during oviposition38.
Feeding of immature stages
The engorgement rate of larvae in vitro was 42.8%, 56.8% and 55.7% for H. dromedarii, H. excavatum and H. scupense, respectively. These proportions are in line with those obtained for I. ricinus fed in vitro (55%) and in vivo (41%)16. An engorgement rate of 71% was obtained for R. australis larvae using a different feeding system, where most of the larvae attached after 48 h which is similar to the observations made in this study39. The encouraging engorgement rate in our study may be associated to the presence of attachment stimuli (hair extract and rabbit hair). The thickness and type of membrane appear to be crucial factors for the larval and nymphal feeding success. As Hyalomma juvenile ticks have a relatively short hypostome, varying from 54 to 119 μm for the larvae and 156–268 μm for the nymphs9,29, they require membranes thin enough to pierce through and feed while avoiding leakage. This problem might explain why immatures stages of other Hyalomma species could reportedly not feed or fed with low attachment rates28,29. In addition, the age of the ticks and the season in which they are fed were also suggested to influence the efficacy and the success of the tick feeding16.
Hyalomma anatolicum nymphs were previously fed on mouse skin membrane with an engorgement rate reached 89% with a feeding duration varied from 7 to 8 days in a water bath set to 37 °C17. The high engorgement rate reported in that study is related to the use of animal skins, which do not require external stimuli to simulate attachment and more closely mimic natural feeding conditions. However, the use of animal skin may cause increased contamination when maintained at 37 °C, especially when used for one- or two-host tick species which have a longer feeding duration, as well as bioethical concerns21,32.
In our study, the feeding-molting duration was between 9.3 ± 2.5 and 16.5 ± 3.5 days for H. excavatum and H. scupense, respectively. The longer feeding duration, especially when the tick are not attached synchronously (Fig. 3) as previously reported18,28, increase the risk of contamination inside the feeding unit. For H. scupense, partially engorged larvae were moved manually to another feeding unit where not all of them reattached, which explains why only 55.7% of the initially placed larvae engorged. The engorged larvae molted to nymphs on the membrane (Supplementary Fig. 1). The freshly molted nymphs were transferred to a new feeding unit where the engorgement rate was only 36.8% (Table 2). The fungal contamination inside the chamber was the main factor that affected the engorgement rate of the immature stage of both two-host ticks (H. excavatum and H. scupense). To prevent this from happening, we tried using nystatin cream to clean the inner side of the membrane and the addition of Whatman filter paper and bags with silica gel to absorb the humidity inside the feeding unit. These precautions appeared to help in delaying the contamination until most of the nymphs detached, but did not completely prevent contamination from occurring. In general, the transfer of immature ticks to clean feeding units and other precautions to limit contamination makes the feeding laborious, and future studies may focus on optimising these steps in order to improve the engorgement rate for one- and two-host tick species in vitro.
The average weight of nymphs fed in vitro was 12.7 ± 3.1, 10.4 ± 2.8 and 13.4 ± 3.6 mg for H. dromedarii, H. excavatum and H. scupense, respectively. As for the females, the nymphal weight was significantly higher for ticks fed on animals compared to in vitro fed ticks and showed a significant difference between the three Hyalomma species (Mann Whitney test, P < 0.0001). Lower detachment weights of all stages fed in vitro were also recorded for I. ricinus16. Although the weights were lower, larvae and nymphs fed in vitro had weights that allowed them to successfully molt to the next stage. We also observed that incomplete feeding of nymphs lead to their death or resulted in smaller adults, similar to previous reports40,41. We note a significant positive correlation between engorged H. scupense and H. excavatum nymphs fed in vitro and the weight of their counterpart unfed adults where the weight of unfed female is higher than males. These observations were in line with previous reports on I. ricinus, I. scapularis and Dermacentor variabilis16,42 which showed that weight of nymphs that become female imbibe more blood than those became male.
The reported in vitro feeding method was successfully used to feed all H. excavatum, H. dromedarii and H. scupense life stages. While there are some similarities in their response to in vitro feeding, some variations specific to the species existed which require more investigation and understanding these differences will improve our knowledge of tick biology, tick-borne disease transmission, and improve the development of control measures. The ATFS could also contribute to the 3Rs by reducing the use of laboratory animals required to maintain tick colonies, although the impact of in vitro feeding on tick microbiota due to the use of antibiotics requires further investigation.
Materials and methods
Maintenance of tick colonies
Colonies of Hyalomma scupense and H. dromedarii used in this study were originally obtained from the National School of Veterinary Medicine of Sidi Thabet, Tunisia and the H. excavatum ticks originated from a colony of the Aydın Adnan Menderes University, Türkiye. The colonies were maintained at the Institute of Parasitology and Tropical Veterinary Medicine of the Freie Universität Berlin, whereby the juvenile life stages were fed on gerbils or the ears of rabbits. The adults were fed on the ears of rabbits (for H. dromedarii and H. excavatum) or calves (for H. scupense) in linen bags. Ethical approval for the feeding of ticks on experimental animals was granted by the Landesamt für Gesundheit und Soziales, Berlin, Germany (LAGeSo) under Registration Number H0387/17 and 0144/22.
Collected ticks were stored in an exsiccator at 27 °C or at room temperature with approx. 90% relative humidity (RH). Data from the feeding of ticks on experimental animals for tick colony maintenance were recorded for comparison purpose with in vitro feeding results.
In vitro feeding of Hyalomma ticks
The methods for the in vitro feeding of all Hyalomma life stages were adapted from previously published protocols16,28. Ticks were fed on a silicone membrane based on a matrix of goldbeater’s skin (for larvae and nymphs) or lens cleaning paper (for adults) with thicknesses of 40–50 μm for larvae, 50–70 μm for nymphs and 80–120 μm for adults. The feeding units were placed in a 6-, or 12-well cell culture plate, depending on the unit's diameter and placed in an incubator (ICH110C, Memmert, Schwabach, Germany) at 27 °C, 70% RH and 5% CO2 in complete darkness. The culture plates were placed on a heating plate (hot plate 062, Labotect, Göttingen, Germany) set at 42 °C to keep the blood warm. Bovine hair extract and rabbit hair was added as an attachment stimulus on top of the silicone membranes approx. 45 min before ticks were introduced into the units. Bovine hair extract was prepared as described by Militzer et al.16. Briefly, approximately 50 g of fresh bovine hair was immersed three times for two hours each in different ratios of chloroform–methanol mixture (2:1, 1:1 and 1:2). The first two immersions took place at room temperature, the final step at 45 °C. Extracts from each immersion step were collected and combined, vacuum filtered and concentrated by roto-evaporation. Finally, the bovine hair extract was dissolved in a 1:2 chloroform–methanol mixture, aliquoted to small working solutions and stored at − 20 °C until use. One hundred μl of bovine hair extract was used for feeding units with a diameter of 30 mm and 50 μL for feeding units with a diameter of 20 mm. The feeding units were closed using a mite plug made from polyurethane foam (K-TK e.K., Retzstadt, Germany) to allow for gas exchange while preventing the ticks from escaping. Defibrinated aseptic bovine blood was purchased from Xebios Diagnostic (Düsseldorf, Germany) and supplemented with 0.1 M adenosine triphosphate (ATP, Carl Roth, Karlsruhe, Germany), vitamin B43, 2 mg/mL glucose and 5 μg/mL gentamicin prior to each blood change, which took place at 14:10 h intervals. The vitamin B was added based on previous observations by Militzer et al.16 suggesting that this may result in better engorgement and fecundity rates. To avoid contamination of the feeding unit, the underside of the membrane was washed with 1% nystatin diluted in PBS, followed by 0.9% NaCl at each blood change.
Once ticks were attached, the rabbit hair used to stimulate attachment was removed, as well as any dead ticks and faeces produced by the ticks during feeding to keep the feeding unit as clean as possible. The inner side of the feeding unit was initially cleaned with 1% nystatin in PBS when fungal contamination was observed. As this increased the humidity inside the feeding units and caused droplets to form that wetted the membrane and made it unsuitable for ticks to feed, we subsequently switched to the treatment of fungal contamination with a nystatin cream (Nystalocal, Pierre Fabre, Freiburg) and also added a Whatman filter paper to cover the attached ticks and bags with silica gel to absorb excess humidity to the feeding unit. In cases where this treatment was not successful, ticks were manually removed, washed in 0.9% NaCl and dried with tissue paper before being transferred to a newly prepared feeding unit. In each ø 30 mm feeding unit, ten male and ten to 12 female ticks were placed. For the in vitro feeding of the immature stages, a maximum of approx. 75 nymphs or 150 larvae were placed in each ø 30 mm unit. One group was fed for each life stage, with the exception of the H. scupense adults for which one group was fed in summer and one in winter.
Biological parameters
The rates of attached and engorged ticks were recorded for all life stages, as well as additional parameters such as detachment weight, molting rates and reproductive parameters including oviposition duration, weight of egg mass and oviposition-hatching duration. The number of eggs for each species was estimated by dividing the weight of the egg batch with the weight of a single egg, whereby the average egg weight was 0.058 ± 0.009 mg for H. dromedarii, 0.057 ± 0.001 mg for H. scupense and 0.053 ± 0.008 mg for H. excavatum as measured on an analytic scale. The percentage of hatched larvae was estimated visually under binocular stereoscope by two persons44. The following parameters were calculated:
Statistical analyses
Statistical analyses and graphs were generated using GraphPad Prism 5 windows (GraphPad Software, San Diego California, United States). Chi-square test was used to compare the feeding proportions between the three species and Z-test was used to compare two proportions using an online version of epitools (http://epitools.ausvet.com.au). One-way ANOVA followed by Tukey-test was used to assess the difference in feeding parameters such as feeding duration, weight, pre-oviposition and oviposition-hatching duration between the tick species in vivo and in vitro (between more than two groups). The Mann–Whitney test was used to analyse the variation of some parameters between two groups and to confirm the results of one-way ANOVA test. Pearson correlation and linear regression were performed to assess the relationship between the following parameters: female weight and egg mass produced, engorged nymphs' weight and their corresponding unfed adult weight. For all test used, a 5% threshold value was considered as statistically significant.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Abdullah, H. H. A. M., El-Shanawany, E. E., Abdel-Shafy, S., Abou-Zeina, H. A. A. & Abdel-Rahman, E. H. Molecular and immunological characterization of Hyalomma dromedarii and Hyalomma excavatum (Acari: Ixodidae) vectors of Q fever in camels. Vet. World 11, 1109–1119 (2018).
Bouaicha, F. et al. Epidemiological investigation of Crimean-Congo haemorrhagic fever virus infection among the one-humped camels (Camelus dromedarius) in southern Tunisia. Ticks Tick-Borne Dis. 12, 101601 (2021).
Gharbi, M. et al. Current status of tropical theileriosis in Northern Africa: A review of recent epidemiological investigations and implications for control. Transbound. Emerg. Dis. 67, 8–25 (2020).
ELGhali, A. & Hassan, S. M. Life cycle of the camel tick Hyalomma dromedarii (Acari: Ixodidae) under field conditions in Northern Sudan. Vet. Parasitol. 174, 305–312 (2010).
Gharbi, M. & Darghouth, M. A. A review of Hyalomma scupense (Acari, Ixodidae) in the Maghreb region: From biology to control. Parasite 21, 2 (2014).
Akyildiz, G. et al. Monthly dynamics of the cold-adapted one-host biological north form of Hyalomma scupense under the influence of the warm summer subtype of the Mediterranean climate in Turkey. Parasitol. Int. 85, 102427 (2021).
Yang, X. et al. The life cycle of Hyalomma scupense (Acari: Ixodidae) under laboratory conditions. Ticks Tick-Borne Dis. 13, 102019 (2022).
Walker, A. R. et al. Ticks of Domestic Animals in Africa: A Guide to Identification of Species (2014).
Apanaskevich, D. A., Schuster, A. L. & Horak, I. G. The genus Hyalomma: VII. Redescription of all parasitic stages of H. (Euhyalomma) dromedarii and H. (E.) schulzei (Acari: Ixodidae). J. Med. Entomol. 45, 817–831 (2008).
Salimi Bejestani, M. R., Changizi, E. & Darvishi, M. M. Abnormal life cycle of Hyalomma dromedarii (Acari: Ixodidae) on single-humped camels in Semnan, North-East of Iran. Arch. Razi Inst. 71, 195–198 (2016).
Elati, K. et al. Phenology and phylogeny of Hyalomma spp. ticks infesting one-humped camels (Camelus dromedarius) in the Tunisian Saharan bioclimatic zone. Parasite 28, 44 (2021).
Gharbi, M. et al. Population dynamics of ticks infesting the one-humped camel (Camelus dromedarius) in central Tunisia. Ticks Tick-Borne Dis. 4, 488–491 (2013).
Kröber, T. & Guerin, P. M. An in vitro feeding assay to test acaricides for control of hard ticks. Pest Manag. Sci. 63, 17–22 (2007).
Krull, C., Böhme, B., Clausen, P.-H. & Nijhof, A. M. Optimization of an artificial tick feeding assay for Dermacentor reticulatus. Parasit. Vectors 10, 60 (2017).
Kuhnert, F., Diehl, P. A. & Guerin, P. M. The life-cycle of the bont tick Amblyomma hebraeum in vitro. Int. J. Parasitol. 25, 887–896 (1995).
Militzer, N., Bartel, A., Clausen, P.-H., Hoffmann-Köhler, P. & Nijhof, A. M. Artificial feeding of all consecutive life stages of Ixodes ricinus. Vaccines 9, 385 (2021).
Tajeri, S., Razmi, G. & Haghparast, A. Establishment of an artificial tick feeding system to study Theileria lestoquardi Infection. PLoS ONE 11, e0169053 (2016).
Tajeri, S. & Razmi, G. R. Hyalomma anatolicum anatolicum and Hyalomma dromedarii (Acari: Ixodidae) imbibe bovine blood in vitro by utilizing an artificial feeding system. Vet. Parasitol. 180, 332–335 (2011).
Waladde, S. M., Ochieng’, S. A. & Gichuhi, P. M. Artificial-membrane feeding of the ixodid tick, Rhipicephalus appendiculatus, to repletion. Exp. Appl. Acarol. 11, 297–306 (1991).
Richmond, J. The 3Rs: Past, present and future. Scand. J. Lab. Anim. Sci. 27, 84–92 (2000).
Nijhof, A. M. & Tyson, K. R. In vitro feeding methods for hematophagous arthropods and their application in drug discovery. In Ectoparasites (eds Meng, C. Q. & Sluder, A. E.) 187–204 (Wiley, 2018).
Garcia Guizzo, M. et al. Optimizing tick artificial membrane feeding for Ixodes scapularis. Sci. Rep. 13, 16170 (2023).
Militzer, N., Pinecki Socias, S. & Nijhof, A. M. Changes in the Ixodes ricinus microbiome associated with artificial tick feeding. Front. Microbiol. 13, 1050063 (2023).
Joyner, L. P. & Purnell, R. E. The feeding behaviour on rabbits and in vitro of the Ixodid tick Rhipicephalus appendiculatus Neumann, 1901. Parasitology 58, 715–723 (1968).
Kröber, T. & Guerin, P. M. In vitro feeding assays for hard ticks. Trends Parasitol. 23, 445–449 (2007).
Kuhnert, F. Feeding of hard ticks in vitro: New perspectives for rearing and for the identification of systemic acaricides. ALTEX 13, 76–87 (1996).
Voigt, W. P. et al. In vitro feeding of instars of the ixodid tick Amblyomma variegatum on skin membranes and its application to the transmission of Theileria mutans and Cowdria ruminatium. Parasitology 107(Pt 3), 257–263 (1993).
González, J., Valcárcel, F., Aguilar, A. & Olmeda, A. S. In vitro feeding of Hyalomma lusitanicum ticks on artificial membranes. Exp. Appl. Acarol. 72, 449–459 (2017).
Bilgiç, H. B. et al. In vitro feeding of Hyalomma excavatum and Hyalomma marginatum tick species. Parasitol. Res. https://doi.org/10.1007/s00436-023-07867-7 (2023).
Andrade, J. J., Xu, G. & Rich, S. M. A silicone membrane for in vitro feeding of Ixodes scapularis (Ixodida: Ixodidae). J. Med. Entomol. 51, 878–879 (2014).
Böhme, B., Krull, C., Clausen, P.-H. & Nijhof, A. M. Evaluation of a semi-automated in vitro feeding system for Dermacentor reticulatus and Ixodes ricinus adults. Parasitol. Res. 117, 565–570 (2018).
Bonnet, S. & Liu, X. Y. Laboratory artificial infection of hard ticks: A tool for the analysis of tick-borne pathogen transmission. Acarologia 52, 453–464 (2012).
Gharbi, M., Hayouni, M. E., Sassi, L., Dridi, W. & Darghouth, M. A. Hyalomma scupense (Acari, Ixodidae) in northeast Tunisia: Seasonal population dynamics of nymphs and adults on field cattle. Parasite 20, 12 (2013).
Asri, B. et al. Assessment of an in vitro tick feeding system for the successful feeding of adult Rhipicephalus appendiculatus Ticks. Parasitologia 3, 101–108 (2023).
Vimonish, R. et al. Quantitative analysis of Anaplasma marginale acquisition and transmission by Dermacentor andersoni fed in vitro. Sci. Rep. 10, 470 (2020).
Ouhelli, H. & Pandey, V. S. Development of Hyalomma lusitanicum under laboratory conditions. Vet. Parasitol. 15, 57–66 (1984).
Ahmed, B. M., Taha, K. M. & El Hussein, A. M. Life cycle of Hyalomma anatolicum Koch, 1844 (Acari: Ixodidae) fed on rabbits, sheep and goats. Vet. Parasitol. 177, 353–358 (2011).
Hadi, U. K. Fecundity, oviposition and egg incubation period of female Rhipicephalus sanguineus Latreille (Acari: Ixodidae) ticks in Indonesia. J. Vet. Med. Res. 2, 1–4 (2015).
Trentelman, J. J. A., Kleuskens, J. A. G. M., van de Crommert, J. & Schetters, T. P. M. A new method for in vitro feeding of Rhipicephalus australis (formerly Rhipicephalus microplus) larvae: A valuable tool for tick vaccine development. Parasit. Vectors 10, 153 (2017).
Koch, H. G. Development of the lone star tick, Amblyomma americanum (Acari: Ixodidae), from immatures of different engorgement weights. J. Kans. Entomol. Soc. 59, 309–313 (1986).
Koch, H. G. & Hair, J. A. The effect of host species on the engorgement, molting success, and molted weight of the Gulf Coast tick, Amblyomma maculatum Koch (Acarina: Ixodidae). J. Med. Entomol. 12, 213–219 (1975).
Hu, R. & Rowley, W. A. Relationship between weights of the engorged nymphal stage and resultant sexes in Ixodes scapularis and Dermacentor variabilis (Acari: Ixodidae) ticks. J. Med. Entomol. 37, 198–200 (2000).
Duron, O. et al. Tick-bacteria mutualism depends on B vitamin synthesis pathways. Curr. Biol. CB 28, 1896-1902.e5 (2018).
Szabó, M. P. J. & Bechara, G. H. Immunisation of dogs and guinea pigs against Rhipicephalus sanguineus ticks using gut extract. Vet. Parasitol. 68, 283–294 (1997).
Acknowledgements
Khawla Elati was supported by a doctoral scholarship from the German Academic Exchange Service (DAAD). Ard Nijhof was funded by the German Federal Ministry of Education and Research (BMBF, Grant Number 01KI1720) as part of the Junior Research Group “Tick-borne Zoonoses.” The authors thank Dr Shahin Tajeri, Nina Militzer and Sophia Pinecki for their technical assistance. We acknowledge support by the Open Access Publication initiative of Freie Universität Berlin.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
K.E.: conceptualization, methodology, investigation, formal analysis, visualization, writing—original draft preparation. H.B., M.M., S.B., H.B.B., T.K.: resources, review and editing. P.H.K., K.F.: investigation. M.A.D.: conceptualization, supervision, review and editing. A.M.N.: conceptualization, supervision, project administration, writing—review and editing. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elati, K., Benyedem, H., Fukatsu, K. et al. In vitro feeding of all life stages of two-host Hyalomma excavatum and Hyalomma scupense and three-host Hyalomma dromedarii ticks. Sci Rep 14, 444 (2024). https://doi.org/10.1038/s41598-023-51052-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-51052-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.