Abstract
Ixodiphagus endoparasitoid wasps are natural tick enemies that can reduce their abundance. In this study, we investigated the presence of Ixodiphagus hookeri in Haemaphysalis concinna and Ixodes ricinus ticks in the Slovak Karst (southern Slovakia) and analysed the ecological and physiological relationships in the parasitoid-host system. Unfed H. concinna and I. ricinus nymphs harvested from vegetation were fed on rabbits. The engorged specimens were kept at 25 °C and 75% RH until the emergence of the adult wasps. For the first time in Europe, we found the presence of I. hookeri in two species of ticks collected in the same locality and compared their development in these tick species. The prevalence of wasps in H. concinna and I. ricinus during their spring activity was estimated at 10.64% and 27.78%, respectively. The presence of the wasps did not affect the duration of nymph feeding. Engorged wasp-infected ticks achieved higher body mass than non-infected specimens. In both tick species, there were no differences in the length of the development period and the number and sex ratio of adult I. hookeri. The analysed indicators and characteristics of the I. hookeri wasp-tick system can be used in research on tick control.
Similar content being viewed by others
Introduction
The increase in the number of tick-borne diseases in humans and animals noted worldwide over the last few decades has aroused considerable interest in the chemical and biological methods for limitation of the number of their vectors, i.e. ticks, and in elucidation of the mode of the spread and persistence of tick-borne pathogens in the environment.
The use of biological agents, e.g. parasitoid Ixodiphagus wasps, for tick control was supposed to be an alternative to acaricides, as numerous studies reported emerging resistance of some tick populations to chemical products1,2,3 and toxic effects of acaricides on animals and humans4,5,6,7. Since no fully satisfactory elimination or replacement of chemicals with parasitoids in tick control has been achieved in large areas, it has been assumed that the presence of endoparasitoid wasps in tick habitats may reduce the abundance of these arthropods8,9,10,11.
As shown by investigations conducted so far, tick parasitoids may contribute to the circulation of pathogens in tick populations12,13,14 and contribute to the persistence of foci of tick-borne diseases with high importance for public health. Additionally, Ixodiphagus wasps transmit some commensal and symbiotic microorganisms that may play an essential role in tick biology and physiology, e.g. nutrition, development, reproduction, immunity, and adaptation to environmental conditions, and influence the occurrence and distribution of pathogens in ticks13,14,15,16,17,18,19.
Eight species of Ixodiphagus wasps were detected in ixodid ticks on different continents, e.g. in Ixodes scapularis, Dermacentor variabilis, and Dermacentor andersoni in North America20,21,22,23,24,25, Amblyomma sp. in South America26, Hyalomma truncatum, Hyalomma rufipes, and Amblyomma variegatum in Africa27,28,29, Hyalomma anatolicum, Dermacentor silvarum, and Haemaphysalis concinna in Asia30,31, Ixodes holocyclus, Ixodes tasmani, Haemaphysalis bancrofti, and Haemaphysalis bremner in Australia32,33, and Ixodes uriae and Ixodes eudyptidis in Oceania34. Endoparasitoid wasps were also found in Rhipicephalus sanguineus s. l. ticks colonizing large areas from North and South America through Africa to Asia26,35,36,37,38,39,40,41,42.
In Europe, representatives of the hymenopteran wasp Ixodiphagus hookeri were identified in several species of ticks, e.g. Ixodes ricinus8,12,13,14,43,44,45,46,47,48, Ixodes persulcatus8,49, Dermacentor reticulatus (= Dermacentor pictus)8, Dermacentor marginatus50, Haemaphysalis concinna51,52,53, and R. sanguineus38. Another endoparasitoid wasp species, i.e. I. caucurtei, was found in I. ricinus and R. sanguineus54.
Although Ixodiphagus wasps were already detected in several tick species, the knowledge of the determinants of their spread, behaviour, and development is still unsatisfactory. The ecological and trophic relationships between encyrtid wasps and their tick hosts have been poorly elucidated as well.
In the present study, we investigated the presence of I. hookeri wasps infesting ticks collected in a habitat in the Slovak Karst (southern Slovakia) and analysed environmental conditions that may promote this phenomenon. To determine the physiological relationships between hymenopteran wasps and their hosts, we examined the development of I. hookeri in nymphs of two species of ticks: H. concinna and I. ricinus.
Results
The H. concinna and I. ricinus nymphs were collected in the Slovak Karst in a temperature range from 15.8 to 22.7°C and 47.8–89.9% humidity during their spring activity. The laboratory analyses showed that both tick species were infected by I. hookeri encyrtid wasps. In April, May, and June 2018, these parasitoids were detected in 25.0%, 5.0%, and 13.33% of H. concinna nymphs, respectively. Throughout the observation period, the prevalence of I. hookeri in the nymphs of this species was estimated at 10.64%. During the peak activity of I. ricinus nymphs in June, I. hookeri wasps were detected in as many as 27.78% of the tick specimens (Table 1).
Haemaphysalis concinna and I. ricinus nymphs infected with I. hookeri and specimens without the endoparasitoid wasps kept in laboratory conditions at a temperature of 20°C ± 2°C and 50% ± 5% humidity fed on the rabbits for 4.0 ± 1.5 days and 4.4 ± 1.3 days as well as 4.2 ± 0.4 and 3.7 ± 0.6 days, respectively. The Mann–Whitney U test showed no statistically significant differences in the feeding length between the parasitoid-infected and non-infected nymphs of both tick species (Table 2).
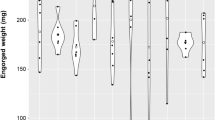
The body mass of the wasp-infected engorged nymphs and its increase were higher than the mass of the non-infected specimens in both tick species (Table 2). The mean body mass was 6.27 ± 1.40 mg in the group of the engorged H. concinna nymphs infested by the parasitoids and 6.53 ± 1.92 mg in the group of the I. ricinus nymphs. The Cochran-Cox test showed significant differences in the nymph engorgement mass between the infected and non-infected I. ricinus nymphs (df = 4.54; p = 0.0341).
The increase in nymph body mass in the engorged H. concinna and I. ricinus nymphs infected with I. hookeri wasps was 25.7 ± 5.7 and 40.2 ± 11.2, respectively. The Cochran-Cox test showed statistically significant differences in the INBM values between the infected and non-infected nymphs in I. ricinus (df = 4.54; 0.0341) (Table 2).
Similarly, the nymph feeding efficiency index in both tick species was higher in the case of the I. hookeri-infected nymphs vs. the non-infected specimens. The NFEI value of was 1.56 ± 0.47 mg/day in the endoparasitoid-infested I. ricinus nymphs and 1.78 ± 0.82 mg/day in the H. concinna nymphs (Table 2). The t-test showed a significant difference in the NFEI value between the infected and non-infected specimens only in the case of Ixodes ricinus (df = 16; p = 0.0340).
Adult I. hookeri emerged from the H. concinna and I. ricinus nymphs on average at days 35.0 ± 3.8 and 35.4 ± 2.9, respectively, after the onset of the feeding of hungry specimens on the host and at days 31.0 ± 5.1 and 31.2 ± 2.9, respectively, after cessation of feeding and detachment of the engorged specimens from the skin. The t-test showed no significant difference in the length of the development of the parasitoids in the H. concinna and I. ricinus nymphs (Table 3).
The number of I. hookeri adults that emerged from the I. ricinus nymphs ranged from 7 to 17 specimens and from 4 to 13 specimens in the case of the H. concinna nymphs (Table 1). Females and males were identified among the I. hookeri adults emerging from the cuticle of a majority of nymphs of both species. In one H. concinna nymph collected in April, only male wasps developed.
Before emergence from the tick cuticle, adult I. hookeri wasps pierced the back of their host's body with their mouthpieces. Our observations showed that the period between piercing the nymph cuticle and the emergence of the first wasp specimen was even up to 10 h long. After the emergence of the first adult wasp, the other specimens left the tick cuticle within a few seconds (Fig. 1).
During development, I. hookeri wasps digested all internal organs of tick nymphs, leaving only the sclerotised cuticle (Figs. 1 and 2). The body of the wasp-infected nymphs changed colour and became matt. At the piercing site, a depression appeared on the posterior edge of the idiosoma due to the thinning of the cuticle. The adult wasps pierced an area near the posterior edge of the idiosoma in all nymphs (Figs. 1 and 2).
At the temperature of 25 °C and 75% RH, the adult I. hookeri wasps died after emergence through the chitinous body covers of H. concinna and I. ricinus nymphs within 3–6 and 2–8 days, respectively.
Discussion
To the best of our knowledge, this is the first report on the presence of I. hookeri in I. ricinus and H. concinna nymphs from the same locality in Europe. The area near Hrhov (Slovak Karst) is a new locality of I. hookeri in Central Europe. In the area of former Czechoslovakia (now Slovakia and the Czech Republic), I. hookeri wasps were previously identified in I. ricinus in two other habitats: in Poteplí near Prague (50°05′16″N 14°25′16″E, Czech Republic)55 and near Bratislava (17°06′ E, 48°08′ N, SW Slovakia)12,14. The parasite was also detected in H. concinna in one locality near Šoporña (17°49’E, 48°15′N, SW Slovakia)53.
Besides the favourable geoclimatic conditions and the presence of numerous hosts of various tick developmental stages, the presence of the wasps in the Slovak Karst may be influenced by the species diversity of the tick hosts. The area is colonised by six tick species, i.e. H. concinna, I. ricinus, Ixodes trianguliceps, D. reticulatus, D. marginatus, and Haemaphysalis inermis56, which are characterised by allochronic or asychronic peak activity57,58,59,60,61,62,63,64,65,66. The different dynamics of the activity of tick larvae and nymphs present in the study area increases the opportunity for I. hookeri females to infest a host and lay eggs in its body.
During the study period from April to June and with the applied method for collecting ticks, we were not able to harvest juvenile stages (larvae and nymphs) of D. marginatus, D. reticulatus, and I. trianguliceps to determine whether these species present in the Slovak Karst locality are parasitized by encyrtid wasps as well.
Studies carried out to date indicate a correlation between the occurrence of I. hookeri wasps and the number of ticks and their hosts24,48. In forest habitats in the Netherlands, the relationship between the prevalence of I. hookeri in questing nymphs and the density of all developmental stages of I. ricinus and roe deer (Capreolus capreolus) was confirmed by Krawczyk et al.48. Similarly, Stafford et al.24 found that the degree of infestation of I. scapularis nymphs with these parasitoids in the USA was influenced by the density of ticks and white-tailed deer (Odocoileus virginianus). As suggested by these authors, the lower threshold limiting the incidence of wasps in I. scapularis is the density of 13–20 hosts per km2. It is still unclear whether the spread of endoparasitoid wasps in various regions is associated with the density of small mammals, i.e. hosts of tick larvae and nymphs. No relationship between the occurrence of endoparasitoid wasps and rodents was found in I. ricinus habitats in the northern part of Europe48.
As shown by Collatz et al.67, the questing behaviour of I. hookeri females is influenced by chemical substances emitted by tick-infested vertebrate animals. In turn, Takasu et al.68 confirmed experimentally that a hexane extract fraction from engorged nymphs stimulated ovipositor probing in I. hookeri females. Substances present in the faeces of engorged ticks may also be an attractant for female encyrtid wasps69. In Western Kenya, 30–45% of unfed A. variegatum nymphs and as many as 45–86% of engorged nymphs infesting cattle were infected by I. hookeri10.
The high percentage of I. ricinus tick nymphs (27.78%) infected with endoparasitoid wasps among the few randomly selected specimens collected in the Slovak Karst suggests their high prevalence in this habitat. Bohacsova et al.14 found I. hookeri in 13.8% of 50 I. ricinus nymphs collected in the area in Bratislava. In different research years, the number of I. hookeri-infected I. ricinus nymphs ranged in different years from 1.86% to 3.8% in Lower Saxony (northern Germany) and from 2.41 to 3.24% in the Baden-Württemberg region (south-west Germany)44. I. hookeri wasps were detected in 4–26% (average 9.5%) of I. ricinus nymphs in the south-west of the Netherlands in 201045 and in 0.1–16% in the north-west of the country (Buunderkamp and Amsterdamse Waterleidingduinen) in 201948.
A similar I. hookeri infection rate in I. ricinus nymphs was reported from the Mediterranean region. In France, I. hookeri parasitoids were found to infest from 3.2 to 12.5% of I. ricinus nymphs collected from roe deer in Chizé (Poitou–Charentes region in the west) and from 19.6 to 20% of nymphs collected from roe-deer and vegetation in Gardouch (Occitanie region in the south-west)13. In southern Italy, encyrtid wasps were detected in 8.2% of I. ricinus nymphs46. In turn, I. hookeri DNA was detected in only 0.4–2.3% of nymphs collected from plants in 2012–2014 in Finland47.
In the Slovak Karst locality, a high prevalence of I. hookeri parasitoid wasps was found in H. concinna nymphs throughout the spring activity period, with the highest value recorded for specimens collected in April. The literature does not provide information about the I. hookeri infection rate in H. concinna nymphs in other localities.
The length of the development of adult wasps and the short survival time of female wasps after emergence from the host body, also observed by other authors9,11,13, may indicate that the females of these insects laid eggs in the hungry H. concinna nymphs collected in April and May probably before the winter diapause of the ticks. The ability of non-embryonated I. hookeri eggs to survive during winter months inside hungry nymphs has been shown in I. ricinus70 and I. scapularis25. Another species of hymenopteran endoparasitoid wasps, i.e. Ixodiphagus texanus, can overwinter in engorged D. variabilis larvae and unfed nymphs71. In the I. ricinus and H. concinna nymphs collected in the Slovak Karst in June, I. hookeri eggs may have been laid before winter or during spring. It cannot be ruled out that the parasitoids infecting unfed or engorged larvae of these species were transferred transstadially to the nymphs, as observed in other tick species21,70,72,73,74,75.
The activity of H. concinna and I. ricinus larvae and nymphs in spring enables the ticks to find a host and ingest its blood, which is necessary to initiate the embryonic development of I. hookeri. In the Slovak Karst locality in spring, Apodemus flavicollis, Apodemus agrarius, Crocidura leucodon, Microtus arvalis, and Myodes glareolus were parasitized by H. concinna and I. ricinus nymphs and larvae56.
The presence of I. hookeri wasps in H. concinna and I. ricinus nymphs does not change the dynamics of tick feeding on the host but increases the body mass of engorged specimens and, consequently, elevates the values of indicators of the parasitic phase of the life cycle in comparison with these parameters in non-infected specimens. The body mass of the engorged H. concinna and I. ricinus nymphs, which was higher than 4.25 mg and 4.16 mg, respectively, may indicate I. hookeri infestation. As reported by Hu and Hyland76, the mean scutal index (ratio between body length and scutal length) in foraging I. scapularis nymphs increased with the increase in the diameter of I. hookeri eggs.
The embryonic development of wasps in I. scapularis nymphs ended 72 h after attachment to the host76. In the present study conducted at a temperature of 25 °C and 75% RH, the entire embryonic and post-embryonic development of I. hookeri adults in the ticks lasted approximately 35 days, and adult I. hookeri stages emerged approximately 31 days after the nymphs had finished foraging and detached from the skin of the host.
The body mass increase in the parasitoid-infected nymphs compared to that in the non-infected specimens was greater in the I. ricinus nymphs than in the H. concinna specimens. Regardless of the tick species, the number of wasp specimens per 1 mg of body mass of an engorged nymph was similar. The present investigations confirm that the development of I. hookeri is not dependent on the host species.
The determinants of the number of eggs produced by one Ixodiphagus wasp female in natural conditions and the number of eggs laid in one tick have not been elucidated to date. Experimental studies showed that an I. hookeri female laid 1.5 ± 0.25 and 28.6 ± 5.5 eggs in an engorged and hungry A. variegatum nymph, respectively11. It has been estimated that one I. hookeri female can lay approximately 120 eggs during a lifetime77, while an I. texanus female can lay even 200 eggs21.
In the present study, the number of I. hookeri adults emerging from one H. concinna and I. ricinus nymph was similar to the number reported for I. ricinus nymphs from a German and French population13,44 and nymphs of the North American species I. scapularis15,23,78. In turn, as many as 73 wasp specimens (on average approx. 20) were found to develop in D. andersoni and D. variabilis ticks79, and from 8 to 40 wasp adults were reported to develop in A. variegatum75. Hoogstraal et al.80 described a Dermacentor auratus nymph that died upon parasitism of 36 specimens of Ixodiphagus mysorensis.
In the present study, we observed a variable sex ratio of I. hookeri emerging from the H. concinna and I. ricinus nymphs; nevertheless, there were slightly higher numbers of females of these endoparasitoids than males throughout the study. Similarly, females dominated in the group of adult wasps infesting I. ricinus in a German and French population of this tick species13,44. The case of emergence of only I. hookeri males from the H. concinna nymph may indicate a possibility of parthenogenetic development of eggs in this species. Confirmation or rejection of this hypothesis will require further research.
The course of reproduction in hymenopteran endoparasitoid wasps has not been fully elucidated. It has been found that symbiotic bacteria Wolbachia pipientis can stimulate thelytokous parthenogenesis (development of females from unfertilised eggs) in unfertilised Encarsia pergandiella females (Hymenoptera: Aphelinidae)81. A similar effect on the reproduction of Pnigalio soemius (Hymenoptera: Eulophidae) is exerted by Rickettsia sp.82. As suggested by Logan et al.71, thelytokous or arrhenotokous reproduction (development of males from unfertilised eggs) may also occur in Ixodiphagus wasps in certain conditions, but the association of bacteria with the reproduction type in representatives of this genus has not been studied to date.
The abundance of I. hookeri in some Central European habitats in such tick species as H. concinna and I. ricinus, which are competent vectors of tick-borne diseases, prompts the need to investigate their biological role and participation in the transmission of pathogens in this region.
Methods
Study area
The locality in the Slovak Karst National Park near the village of Hrhov (48°34.899 N, 20°46.743E) is located at an altitude of 200–220 m a.s.l. at the foothills on the southern steep slopes of the Horný vrch Plateau. The area has a warm and moderately wet climate due to its location at the border of two types of climate: oceanic and continental.
The environmental conditions in the Slovak Karst support the richness of the flora of xerothermic species, calciphytes, and mountain dealpine and prealpine species. The plains are covered by oak-hornbeam forests, whereas oak forests occupy the hills and spruce forests grow on the karst pits. In turn, the northern parts are covered by beech forests. The fauna species are characteristic of the steppe and forest-steppe zones. There numerous animals inhabiting the study area are potential hosts of various developmental stages of ticks.
Tick collection
Ticks were collected on the 19th of April (14 nymphs of H. concinna), the 29th of May (20 nymphs of H. concinna), and the 27th of June 2018 (17 nymphs of H. concinna and 33 nymphs of I. ricinus) in the early hours (between 9:00 and 11:00) for an hour each time. Unfed specimens were collected with the use of a 1-m2 white wool blanket swept over the vegetation along two 100-m-long transects. Ticks attached to the flag were transferred into transport containers. Concurrently, during each harvest, the temperature and humidity were measured in the habitat using electronic devices (Reed R6030 with an accuracy of 1 °C and 1% RH).
The species and developmental stage of the ticks were determined in laboratory conditions. All H. concinna and I. ricinus nymphs sampled in the field were used for investigation of thir development in laboratory conditions. Specimens that had ceased feeding were used in further analyses of their development.
Laboratory analyses
Prior to the beginning of the experiments, unfed nymphs of both species were kept at room temperature for approximately 7–10 days. Immediately before feeding, nymphs in pools of 10 specimens were weighed using the RadWag WPA 120/C analytical balance with an accuracy of 10–4 g 0.001 g. This was the basis for the determination of the average body mass of one hungry nymph of each tick species.
Next, the unfed nymphs were transferred into canvas bags attached to the shaved skin of Albinotic New Zealand rabbits (Oryctolagus cuniculus) kept at 20 °C ± 2 °C and 50% ± 5% humidity. Ten nymphs representing only one species were placed on one rabbit. The course of nymph feeding was checked at the same time every day. After detachment from the rabbit skin, engorged nymphs were removed from the host, weighed on the analytical balance, and transferred into rearing chambers with a temperature of 25 °C and 75% humidity. The study on the development of endoparasitoid wasps involved 12, 20, and 15 engorged H. concinna nymphs selected randomly from the first, second, and third collection round, respectively, and 18 engorged I. ricinus nymphs from the third collection.
One chamber contained one engorged nymph. At the same time every day, the nymphs were viewed using the Olympus SZX16 stereoscopic microscope until adult encyrtid wasps emerged. Chambers where the wasps emerged were checked on the subsequent days until the insects died. The dead adult wasps and tick nymphs were preserved in 75% ethyl alcohol. The species and sex of the endoparasitoid wasps were determined based on the morphological features, as described by Gahan38.
Photographs documenting the development of the wasps were taken using an Olympus SZX16 stereo microscope with an attached DP26 camera.
Based on the laboratory studies, the parameters of the development of the I. hookeri adults in the H. concinna and I. ricinus nymphs were determined. These included the length of the feeding period of wasp-infested nymphs (FP- from the beginning to the end of feeding), the length of development of adult wasps (from the beginning of feeding of infected nymphs to the emergence of the parasitoids), the length of the period between the end of nymph feeding and the emergence of the wasps, and the body mass of engorged nymphs infected with the parasitoid wasps (nymph engorgement mass NEM).
The following parameters characterising the I. hookeri-infected nymphs and the development of these parasitoids in both tick species were determined:
-
1.
Increased nymph body mass
INBM = body mass of an engorged nymph/body mass of a hungry nymph.
-
2.
Nymph feeding efficacy index in wasp-infected specimens
NFEI (g/d) = NEM/FP, where NEM—nymph engorgement mass, FP—length of the feeding period of a wasp-infested nymph.
-
3.
Number of endoparasitoid wasps per 1 mg of the body mass of an engorged nymph indicating the number of wasp specimens per 1 mg of nymph body mass after the end of feeding.
The same parameters and indicators were determined for nymphs that were not infected with I. hookeri wasps. They were used for comparison of the course of the parasitic and non-parasitic phases in the infected and uninfected nymphs.
Laboratory animals were provided with food and water ad libitum, and every effort was made to minimize their stress during the experiment. All experimental procedures described below were carried out in accordance with the standards of care and use of laboratory animals (Article 48 of the Act of January 15, 2015 on the protection of animals used for scientific or educational purposes, Polish Journal of Law Item 266 and Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes). The study was approved by the Local Ethical Committee for Animal Experiments at the University of Life Sciences in Lublin (approval no. 64/2018 from 16th April, 2018).
Statistical analysis
Measurable variables were described with the use of basic parameters: arithmetic mean, standard deviation (SD), and minimum and maximum values (min. and max.). The significance of the differences in the measurable variables between the two groups was determined with the following tests:
-
t test—in the case of the normal distribution of the measurable variable and homogeneity of variance.
-
the Cochran-Cox test—in the case of the normal distribution of the measurable variable but no homogeneity of variance.
-
the Mann–Whitney U test—in the case of non-normal distribution of the measurable variable.
A p value < 0.05 was considered statistically significant. Statistical calculations were performed using the STATISTICA 10 PL statistical package.
References
George, J. E., Pound, J. M. & Davey, R. B. Chemical control of ticks on cattle and the resistance of these parasites to acaricides. Parasitology 129(S1), 5353–5366 (2004).
Abbas, R. Z. et al. Acaricide resistance in cattle ticks and approaches to its management: The state of play. Vet. Parasitol. 203, 6–20 (2014).
Yessinou, R. E. et al. Resistance of tick Rhipicephalus microplus to acaricides and control strategies. J. Ent. Zool. Stud. 4, 408–414 (2016).
Bradberry, S. M. et al. Poisoning due to pyrethroids. Toxicol. Rev. 24, 93–106 (2005).
Klainbart, S. et al. Tremor salivation syndrome in canine following pyrethroid/permethrin intoxication. Pharm. Anal. Acta 5, 320 (2014).
Antwi, F. B. & Reddy, G. V. P. Toxicological effects of pyrethroids on non-target aquatic insects. Environ. Toxicol. Pharmacol. 40, 915–923 (2015).
Glorennec, P. et al. Determinants of children’s exposure to pyrethroid insecticides in western France. Environ. Int. 104, 76–82 (2017).
Alfeev, N. I. The utilization of Hunterellus hookeri How. for the control of the ticks, Ixodes ricinus L. and Ixodes persulcatus P. Sch. with reference to peculiarities of their metamorphosis under conditions of the Province of Lenningrad. Rev. Appl. Ent. B. 34, 108–109 (1946).
Hu, R., Hyland, K. E. & Oliver, J. H. A review on the use of Ixodiphagus wasps (Hymenoptera: Encyrtidae) as natural enemies for the control of ticks (Acari: Ixodidae). Syst. Appl. Acarol. 3, 19–28 (1988).
Mwangi, E. N. et al. The impact of Ixodiphagus hookeri, a tick parasitoid, on Amblyomma variegatum (Acari: Ixodidae) in a field trial in Kenya. Exp. Appl. Acarol. 21, 117–126 (1997).
Takasu, K. & Nakamura, S. Life history of the tick parasitoid Ixodiphagus hookeri (Hymenoptera: Encyrtidae) in Kenya. Biol. Control 46, 114–121 (2008).
Rehacek, J. & Kocianova, E. Attempt to infect Hunterellus hookeri Howard (Hymenoptera, Encyrtidae), an endoparasite of ticks, with Coxiella burnetti. Acta Virol. 36, 492 (1992).
Plantard, O. et al. Detection of Wolbachia in the tick Ixodes ricinus is due to the presence of the hymenoptera endoparasitoid Ixodiphagus hookeri. PLoS ONE 7, e30692 (2012).
Bohacsova, M. et al. Arsenophonus nasoniae and Rickettsiae infection of Ixodes ricinus due to parasitic wasp Ixodiphagus hookeri. PLoS ONE 11, e0149950 (2016).
Mather, T. N., Piesman, J. & Spielman, A. Absence of spirochete (Borrelia burgdorferi) and piroplasms (Babesia microti) in deer tick (Ixodes dammini) parasitized by Chalcid wasps (Hunterellus hookeri). Med. Vet. Entomol. 1, 3–8 (1987).
Noda, H., Munderloh, U. & Kurtti, T. Endosymbionts of ticks relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl. Environ. Microbiol. 63, 3926–3932 (1997).
Ahantarig, A. et al. Hard ticks and their bacterial endosymbionts (or would be pathogens). Folia Microbiol. 58, 419–428 (2013).
Duron, O. et al. Evolutionary changes in symbiont community structure in ticks. Mol. Ecol. 26, 2905–2921 (2017).
Vila, A. et al. Endosymbionts carried by ticks feeding on dogs in Spain. Ticks Tick Borne Dis. 10, 848–852 (2019).
Cooley, R. A. & Kohls, G. M. A summary of tick parasites. In Proceedings of the 5th Pacific Science Congress, Vol. 5, 3375–3381 (1934).
Bowman, J. L., Logan, T. M. & Hair, J. A. Host suitability of Ixodiphagus texanus Howard on five species of hard ticks. J. Agric. Entomol. 3, 1–9 (1986).
Mather, T. N., Piesman, J. & Spielman, A. Absence of spirochete (Borrelia burgdorferi ) and piroplasms (Babesia microti) in deer tick (Ixodes dammini) parasitized by Chalcid wasps (Hunterellus hookeri). Med. Vet. Entomol. 1, 3–8 (1987).
Hu, R., Hyland, K. E. & Mather, T. N. Occurrence and distribution in Rhode Island of Hunterellus hookeri (Hymenoptera: Encyrtidae), a wasp parasitoid of Ixodes dammini. J. Med. Entomol. 30, 277–280 (1993).
Stafford, K. C. 3rd., Denicola, A. J. & Kilpatrick, H. J. Reduced abundance of Ixodes scapularis (Acari: Ixodidae) and the tick parasitoid Ixodiphagus hookeri (Hymenoptera: Encyrtidae) with reduction of white-tailed deer. J. Med. Entomol. 40, 642–652 (2003).
Hu, R. & Hyland, K. E. Prevalence and seasonal activity of the wasp parasitoid, Ixodiphagus hookeri (Hymenoptera: Encyrtidae) in its tick host, Ixodes scapularis (Acari: Ixodidae). Syst. Appl. Acarol. 2, 95–100 (1997).
Lopes, A. J. O. et al. Parasitism by Ixodiphagus Wasps (Hymenoptera: Encyrtidae) in Rhipicephalus sanguineus and Amblyomma Ticks (Acari: Ixodidae) in Three Regions of Brazil. J. Econ. Entomol. 5, 1979–1981 (2012).
Fiedler, O. G. H. A new African tick parasite, Hunterellus theilerae sp. n. Onderstepoort. J. Vet. Res. 26, 61–63 (1953).
Hoogstraal, H. & Kaiser, M. N. Records of Hunterellus theileri Fielder (Encyrtidae: Chalcidoidea) parasitizing Hyalomma ticks on birds migrating through Egypt. Ann. Ent. Soc. Am. 54, 616–617 (1961).
Mwangi, E. N., Newson, R. M. & Kaaya, G. P. A hymenopteran parasitoid of the Bont tick Amblyomma variegatum Fabricius (Acarina: Ixodidae) in Kenya. Discov. Innov. 5, 331–335 (1993).
Shastri, U. V. Some observations on Hunterellus hookeri Howard, a parasitoid of Hyalomma-anatolicum anatolicum Koch, 1844 in Marathwada region Maharashtra State. Cheiron 13, 67–71 (1984).
Gaye, M. et al. Hymenopteran parasitoids of hard ticks in western Africa and the Russian Far East. Microorganisms 8, 1992 (2020).
Oliver, J. H. A wasp parasite of the possum tick, Ixodes tasmani, Australia. Pan-Pac. Entomol. 40, 227–230 (1964).
Doube, B. M. & Heath, A. C. G. Observations on the biology and seasonal abundance of an encyrtid wasp, a parasite of ticks in Queensland. J. Med. Entomol. 12, 433–447 (1975).
Heath, A. C. G. & Cane, R. P. A new species of Ixodiphagus (Hymenoptera: Chalcidoidea: Encyrtidae) parasitizing seabird ticks in New Zealand. N. Z. J. Zool. 37, 147–155 (2010).
Costa Lima, A. The chalcid Hunterellus hookeri Howard, a parasite of the tick Rhipicephalus sanguineus Latreille, observed in Rio de Janeiro. Rev. Vet. Zoot. 5, 201–203 (1915).
Philip, C. B. Occurrence of a colony of the tick parasite Hunterellus hookeri Howard in West Africa. US Public Health Serv. Rpts. 46, 2168–2172 (1931).
Bishopp, F. C. Record of hymenopterous parasites of ticks in the United States. Proc. Entomol. Soc. Wash. 36, 87–88 (1934).
Gahan, A. B. On the identities of chalcidoid tick parasites (Hymenoptera). Proc. Entomol. Soc. Wash. 36, 89–97 (1934).
Munaf, H. B. The first record of Hunterellus hookeri parasitizing Rhipicephalus sanguineus in Indonesia. South Asian J. Tropic. Med. Public Health 7, 492 (1976).
Cheong, W. H., Rajamanikam, C. & Mahadevan, S. A case of Hunterellus hookeri parasitization of ticks in Pentaling Jaya, Peninsula Malaysia. South Asian J. Tropic. Med. Publ. Health 9, 456–458 (1978).
Coronado, A. Ixodiphagus hookeri Howard, 1907 (Hymenoptera: Encyrtidae) in the brown dog tick Rhipicephalus sanguineus Latreille, 1806 (Acari: Ixodidae) in Venezuela. Entomotropica 21, 61–64 (2006).
Bezerra Santos, M. et al. Larvae of Ixodiphagus wasps (Hymenoptera: Encyrtidae) in Rhipicephalus sanguineus sensu lato ticks (Acari: Ixodidae) from Brazil. Ticks Tick Borne Dis. https://doi.org/10.1016/j.ttbdis.2017.03.004 (2017).
Řehaček, J. Uzitočný cudzopasnik. Enviromagazin 3, 19 (1998).
Collatz, J. et al. A hidden beneficial: Biology of the tick-wasp Ixodiphagus hookeri in Germany. J. Appl. Entomol. 135, 351–358 (2011).
Tijsse-Klasen, E. et al. Parasites of vectors—Ixodiphagus hookeri and its Wolbachia symbionts in ticks in the Netherlands. Parasit. Vectors 4, 228 (2011).
Ramos, R. A. et al. Occurrence of Ixodiphagus hookeri (Hymenoptera: Encyrtidae) in Ixodes ricinus (Acari: Ixodidae) in southern Italy. Ticks Tick Borne Dis. 6, 234–236 (2015).
Sormunen, J. J. et al. First evidence of Ixodiphagus hookeri (Hymenoptera: Encyrtidae) parasitization in Finnish castor bean ticks (Ixodes ricinus). Exp. Appl. Acarol. 79, 395–404 (2019).
Krawczyk, A. I. et al. Tripartite interactions among Ixodiphagus hookeri, Ixodes ricinus and deer: Differential interference with transmission cycles of tick-borne pathogens. Pathogens 9, 339 (2020).
Pervomaisky, G. S. On the infestation of Ixodes persulcatus by Hunterellus hookeri How. (Hymenoptera). Zool. Zh. 22, 211–213 (1943).
Alfeev, N. I. & Klimas, Y. V. Experience in cultivating ichneumon flies, Hunterellus hookeri, obtained from United States, which destroy ixodid ticks of Soviet fauna. Priroda 2, 98–101 (1938).
Brumpt, E. Utilisation des insectes auxiliares entomophages dans la lutte contre les insectes pathogenes. Presse Med. Paris 36, 359–361 (1913).
Klyushkina, E. A. A parasite of the ixodid ticks, Hunterellus hookeri How. in the Crimea. Zool. Zh. 37, 1561–1563 (1958).
Slovak, M. Finding of the endoparasitoid Ixodiphagus hookeri (Hymenoptera, Encyrtidae) in Haemaphysalis concinna ticks in Slovakia. Biologia 58, 890 (2003).
Brumpt, E. Parasitisme latent de l’Ixodiphagus caucurtei chez les larves gorgées et les nymphes á jeun de divers ixodines (Ixodes ricinus et Rhipicephalus sanguineus). Comptes Rendus de l’Académie des Sciences de Paris 191, 1085–1087 (1930).
Boucek, Z. & Černy, V. A parasite of ticks, the chalcid Hunterellus hookeri in Czechoslovakia. Zool. Listy 3, 109–111 (1954).
Heglasová, I. et al. Ticks, fleas and rodent-hosts analyzed for the presence of Borrelia miyamotoi in Slovakia: The first record of Borrelia miyamotoi in a Haemaphysalis inermis tick. Ticks Tick Borne Dis. 11, 101456 (2020).
Nosek, J. The ecology, bionomics and behavior of Haemaphysalis (Haemaphysalis) concinna tick. Z. Parasitenkd. 36, 233–241 (1971).
Nosek, J. The ecology and public health importance of Dermacentor marginatus and D. reticulatus ticks in central Europe. Folia Parasitol. 19, 93–102 (1972).
Széll, Z. et al. Temporal distribution of Ixodes ricinus, Dermacentor reticulatus and Haemaphysalis concinna in Hungary. Vet. Parasitol. 141, 377–379 (2006).
Harnok, S. & Farkas, R. Influence of biotope on the distribution and peak activity of questing ixodid ticks in Hungary. Med. Vet. Entomol. 23, 41–46 (2009).
Bartosik, K., Wiśniowski, L. & Buczek, A. Abundance and seasonal activity of adult Dermacentor reticulatus (Acari: Amblyommidae) in eastern Poland in relation to meteorological conditions and the photoperiod. Ann. Agric. Environ. Med. 18, 340–344 (2011).
Egyed, L. et al. Seasonal activity and tick-borne pathogen infection rates of Ixodes ricinus ticks in Hungary. Ticks Tick Borne Dis. 3, 90–94 (2012).
Hornok, S. et al. Ixodid ticks on ruminants, with on-host initiated moulting (apolysis) of Ixodes, Haemaphysalis and Dermacentor larvae. Vet. Parasitol. 187, 350–353 (2012).
Buczek, A. et al. Threat of attacks of Ixodes ricinus ticks (Ixodida: Ixodidae) and Lyme borreliosis within urban heat islands in south-western Poland. Parasit. Vectors 7, 562 (2014).
Chitimia-Dobler, L. Spatial distribution of Dermacentor reticulatus in Romania. Vet. Parasitol. 214, 219–223 (2015).
Pfäffle, M., Littwin, N. & Petney, T. Host preferences of immature Dermacentor reticulatus (Acari: Ixodidae) in a forest habitat in Germany. Ticks Tick Borne Dis. 6, 508–515 (2015).
Collatz, J. et al. Being a parasitoid of parasites: Host finding in the tick wasp Ixodiphagus hookeri by odours from mammals. Ent. Exp. Appl. 134, 131–137 (2010).
Takasu, K. et al. Host recognition by the tick parasitoid Ixodiphagus hookeri (Hymenoptera: Encyrtidae). Environ. Entomol. 32, 614–617 (2003).
Demas, F. A. et al. Cattle and Amblyomma variegatum odors used in host habitat and host finding by the tick parasitoid, Ixodiphagus hookeri. J. Chem. Ecol. 26, 1079–1093 (2000).
Alfeev, N. I. & Klimas, Y. V. On the possibility of developing ichneumon flies, Hunterellus hookeri in climatic conditions of the USSR. Sovet. Vet. 15, 55 (1938).
Logan, T. M., Bowman, J. L. & Hair, J. A. Parthenogenesis and overwintering behavior in Ixodiphagus texanus Howard. J. Agric. Entomol. 2, 272–276 (1985).
Wood, H. P. Notes on the life history of the tick parasite Hunterellus hookeri Howard. J. Econ. Entomol. 4, 425–431 (1911).
Cooley, R. A. & Kohls, G. M. Egg laying of Ixodiphagus caucurtei du Buysson in larval ticks. Science 67, 656 (1928).
Hu, R. Identification of the wasp parasitoid of the deer tick, Ixodes dammini, in Rhode Island and its implication in the control of Lyme disease. M.S. thesis, University of Rhode Island, USA (1990).
Mwangi, E. N. et al. Parasitism of Amblyomma variegatum by a hymenopteran parasitoid in the laboratory, and some aspects of its basic biology. Biol. Control 4, 101–104 (1994).
Hu, R. & Hyland, K. E. Effects of the feeding proces of Ixodes scapularis (Acari: Ixodidae) on embryonic development of its parasitoid, Ixodiphagus hookeri (Hymenoptera: Encyrtidae). J. Med. Entomol. 35, 1050–1053 (1998).
Knipling, E. F. & Steelman, C. D. Feasibility of controlling Ixodes scapularis ticks (Acari: Ixodidae), the vector of Lyme disease, by parasitoid augmentation. J. Med. Entomol. 37, 647–652 (2000).
Stafford, K. C. 3rd., Denicola, A. J. & Magnarelli, L. A. Presence of Ixodiphagus hookeri (Hymenoptera: Encyrtidae) in two Connecticut populations of Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol. 33, 183–188 (1996).
Cole, M. M. Biological control of ticks by the use of hymenopterous insects. A review. World Health Organization (WHO/EBL/43.66) 43, 1–12 (1965).
Hoogstraal, H., Santana, F. J. & van Peenen, P. F. D. Ticks (Ixodoidea) of Mt. Sontra, Danang, Republic of Vietnam. Ann. Ent. Soc. Am. 61, 722–729 (1968).
Zchori-Fein, E. et al. A newly discovered bacterium associated with parthenogenesis and a change in host selection behawior in parasitoid wasps. PNAS 98, 12555–12560 (2001).
Giorgini, M. et al. Rickettsia symbionts cause parthenogenic reproduction in the parasitoid wasp Pnigalio soemius (Hymenoptera: Eulophidae). Appl. Environ. 8, 2589–2599 (2010).
Author information
Authors and Affiliations
Contributions
Conceptualization: A.B.; Methodology: A.B., W.B., K.B., J.K., and M.S.; Formal analysis: A.B., W.B., K.B.; Investigation: A.B., W.B., K.B., J.K., and M.S.; Resources: A.B.; Supervision: A.B.; Visualization: A.B., W.B, and K.B.; Writing—original draft preparation: A.B.; Writing—review and editing: A.B., W.B., K.B., and M.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buczek, A., Buczek, W., Bartosik, K. et al. Ixodiphagus hookeri wasps (Hymenoptera: Encyrtidae) in two sympatric tick species Ixodes ricinus and Haemaphysalis concinna (Ixodida: Ixodidae) in the Slovak Karst (Slovakia): ecological and biological considerations. Sci Rep 11, 11310 (2021). https://doi.org/10.1038/s41598-021-90871-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-90871-7
This article is cited by
-
First detection of Ixodiphagus hookeri (Hymenoptera: Encyrtidae) in Ixodes ricinus ticks (Acari: Ixodidae) from multiple locations in Hungary
Scientific Reports (2023)
-
First record of the parasitoid wasp Ixodiphagus hookeri (Hymenoptera: Encyrtidae) infesting the tick Amblyomma nodosum (Acari: Ixodidae)
Parasitology Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.