Abstract
The treatment of fungal keratitis (FK) is challenging due to the subacute indolent course, and initial misdiagnosis. In this retrospective case series, we highlight both the diagnostic and therapeutic roles of corneal biopsy together with amniotic membrane transplantation (AMT) in patients with refractory clinically presumed FK. Debulking biopsy and tectonic AMT were performed during the initial presentation. Biopsy specimens were sent for KOH smears and cultures. After KOH smears confirmed the presence of fungal elements, topical voriconazole 1% was prescribed for the first 72 h then tailored according to the clinical response and the culture results. The outcome measures were complete resolution of infection and restoration of corneal integrity. Cases associated with culture proven bacterial keratitis were excluded. Twelve cases were included in the study. KOH smears confirmed the presence of fungal growth in all specimens. Cultures grew Aspergillus in 6/12 cases, sensitive to voriconazole (5/6) and amphotericin (3/6); Fusarium (4/12), sensitive to both voriconazole and amphotericin; and no growth in 2/12 cases. Amphotericin 0.15% eye drops were added to the 7 cases with proven sensitivity and to the remaining 2 culture negative cases. Gradual resolution of infection was seen in all cases after 35.6 ± 7.8 days. In FK, a debulking biopsy simultaneously with AMT help decrease the microbial load, suppress the inflammatory process, support the corneal integrity, confirm the presence of fungal pathogen.
Similar content being viewed by others
Introduction
Fungal infection of the cornea is one of the serious causes of microbial keratitis that can lead to sight-threatening complications and catastrophic sequelae if not correctly diagnosed and managed1,2. Fungal keratitis (FK) represents fewer than 10% of the infectious keratitis burden in developed countries versus 40% in developing countries with tropical climates3,4,5,6. In China, FK was found to account for up to 62% of severe microbial keratitis and was recognized as a frequent indication of keratoplasty7. Among the widely known species of fungi, molds, including Aspergillus and fusarium species, are the most encountered in patients with FK. Different modes of infection have been described, with the trauma of plant origin or organic matter being among the most familiar routes of acquiring the disease, mainly in developing countries6,8. A tropical climate, chronic use of topical steroids, chronic ocular surface disorders, and diabetes mellitus are other risk factors for FK. Also, fusarium species are ubiquitous in the surrounding environment, and the infection could be acquired via direct contact with fusarium-contaminated substances5,9, 10.
Diagnosis of FK is notoriously challenging and usually delayed due to the indolent course of the disease. Hence, patients could present with a descemetocele, corneal perforation, or intraocular spread of infection11,12 It is noteworthy that the patient may just complain of minimal ocular irritation in the presence of a severely infiltrated cornea13. Several invasive and non-invasive ways have been reported to aid the diagnosis of FK. Non-invasive methods include in vivo confocal microscopy (IVCM), which can visualize the pathogen in the corneal tissue. However, being expensive and needing expert interpretation have limited its use in routine clinical practice14. Invasive methods, which are more frequently used, including direct examination of corneal scrape or biopsy materials with 10% potassium hydroxide (KOH) smears under a light microscope for instantaneous detection of the fungal hyphae with an 80–99.3% sensitivity and an 83.8–99.1% specificity15. Also, polymerase chain reaction (PCR) has been reported as a fast diagnostic tool with small amounts of corneal tissue. However, the cost and requirement of a well-equipped diagnostic lab limited its routine clinical use16. Culture on different media, including Sabouraud dextrose agar (SDA), blood agar, and brain heart infusion broth, to detect the fungal spores. However, cultures take longer, which may delay the diagnosis, making the KOH smears a preferable primary approach for diagnosing patients with suspected FK17. False positive results, owing to contamination and non-pathogenic bystanders, have been reported. Thus caution must be given while interpreting culture results, especially if they are not concordant with the clinical findings14.
Treating FK can be a real challenge, especially in resistant cases. The first treatment line is topical antifungal agents, most of which are fungistatic agents like polyenes, including natamycin and amphotericin B, and azoles, including fluconazole and voriconazole. The ideal antifungal drug should have high efficacy with good penetration and be non-toxic to the corneal tissue. The fungistatic nature of topical antifungals and the poor penetration into deeper corneal tissue make them insufficient for eradicating the infection, especially in deeper corneal lesions. Usually, another route of administration and/ or an adjuvant treatment line is needed to protect the corneal integrity and improve visual outcomes18. Intrastromal and intracameral routes can be used to increase drug delivery19. Therapeutic penetrating keratoplasty (TPK) may be required, as a globe salvage procedure, in cases with corneal melting or perforation, albeit with a high risk of reinfection, rejection, and graft failure20.
Limited literature is currently available for using AM in cases of microbial keratitis, including those caused by fungal pathogens. Only a few reports have tested the efficacy and safety of AM Ref.21 as an adjuvant treatment option for cases with resistant FK (Table 1). In this retrospective case series, we report the outcomes of simultaneous debulking corneal biopsy and tectonic AMT in refractory clinically presumed FK.
Methodology
We conducted a hospital-based interventional 2-year retrospective case series that was approved by the Institutional Review Board of Benha University hospital and met the Declaration of Helsinki. Cases with refractory clinically presumed fungal keratitis were included. Clinical predictors of fungal keratitis were satellite lesions, deeper lesions, stromal melting, endothelial plaque, longer duration since onset and history of trauma22. Predictors of corneal perforation were presence of hypopyon, large infiltrate size and infiltrate involving the posterior third of the cornea regardless of the type of the fungal pathogen23. Polymicrobial and other microbial keratitis were excluded.
Prior to surgery, informed consent was obtained from all patients. Human AM was prepared and preserved as previously reported24. We did the procedure under peribulbar anesthesia. All discharge and loose epithelium were removed. Povidone iodine in the conjunctival sac was avoided not to compromise the culture results. Starting in the healthy cornea, 1–2 mm away from infiltrates, a deep anterior lamellar keratectomy was performed as a debulking biopsy. Briefly, a small crescent blade was used to cut into the deeper layers of the cornea, while holding the edge with a micro-notch forceps, till the deep layer of the infiltrate was reached. The biopsy specimen was subdivided and sent immediately for KOH smears, histopathological examination and cultures. At the same setting, adjuvant tectonic AMT was performed. AM, priorly soaked in voriconazole 1%, was cut into multiple pieces and fashioned slightly smaller to the post-debulking defect size, then layered epithelium-side-down to fill the facet. Then, a larger piece of AM was trimmed and sutured, as a patch graft, in an epithelial-side-up position with a running/interrrupted 10–0 nylon and not overlapping with the edge of the epithelial defect. On confirming the presence of fungal pathogen via the KOH smears, a compounded topical antifungal was prescribed hourly, and the corneal response was monitored daily till the culture results. Initial modification of treatment, before culture results, was planned when suboptimal response was observed by adding/switching the antifungal medication. The antifungal choices were either voriconazole 1% and/or amphotericin B 0.15%25.
The antifungal drug sensitivity tests were conducted using disc diffusion methods in adherence to the National Committee for Clinical Laboratory Standards (NCCLS) guidelines26. The control strain utilized was Candida albicans (ATCC 10231). The co-author (S.A) employed seven types of antifungal discs, including natamycin, voriconazole, amphotericin B, micronazole, fluconazole, ketoconazole, and itraconazole. Interpretation of clearance zone diameters was conducted in accordance with the guidelines proposed by the manufacturer.
Results
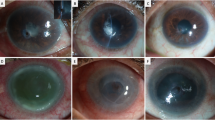
We report the clinical course of 12 eyes of 12 patients with refractory keratitis that met the clinical criteria of FK and underwent corneal debulking biopsy and a tectonic AMT. Table 2 elaborates the clinical criteria of study cases. The mean age of the patients was 50.5 ± 9.3 (median, 49; range, 36–67) years. Time between initial symptoms and referral to our hospital was 25.8 ± 10.2 (median, 22.5; range, 14–42) days. All patients were prescribed topical antibiotics, and only 5 patients were also given commercially available antifungal natamycin drops. However, cases #2, #11 and #12 were initially misdiagnosed as viral necrotizing stromal keratits and treated with topical prednisolone acetate 1%, preservative-free lubricants and systemic valacyclovir. The presenting vision, corneal signs, and prior eye drops are summarized in Table 2. KOH smears revealed fungal hyphae in all specimens. No empirical intrastromal or periocular or intracameral injections were done throughout the course of treatment. Surgeries were done uneventfully in all cases. Case #12 had iatrogenic microperforations during keratectomy that were sealed successfully with AMT (Fig. 1). The Sabouraud’s agar grew Aspergillus in 6/12 cases, sensitive to voriconazole (5/6) and amphotericin (3/6); and Fusarium (4/12), sensitive to both voriconazole and amphotericin. No growth was detected in 2/12 cases. Compounded amphotericin 0.15% eye drops were added to the 7 cases with proven sensitivity and to the remaining 2 culture negative cases. Complete resolution of infection, re-epithelialization of corneal surface, and restoration of corneal thickness were seen within 35.6 ± 7.8 (median, 36; range, 23–45) days in all cases. Topical treatment was gradually tapered after full resolution of infection. Elective optical PK was performed uneventfully in 10 eyes 4.5 ± 0.9 (median, 5; range, 3–6) months later.
(A) Case #12 presented with clinically presumed fungal keratitis with central descemetocele, and (B) had a corneal biopsy with tectonic amniotic membrane transplantation (B). (C) Slit-lamp photo showing complete corneal healing with a central leucoma non-adherent after 45 days from the surgery and topical voriconazole 1%.
Discussion
The treatment of FK is intriguing as symptoms are subtle, and keratitis is minimal early in the clinical course. Initial misdiagnosis and partial response to empirical topical antibiotics and steroids, along with the typically slow progression, have made the clinical diagnosis challenging as well as the referrals when complicated. Having clinical data regarding the onset and the stage of keratitis is crucial to interpret the results of microbiological workup. It is also known that fungi can proliferate and spread through the corneal channels after gaining access through the corneal epithelium. Without releasing chemotactic factors, fungi can colonize corneal tissue while delaying the host immune response. Further, being immune privileged with limited defense mechanisms, the cornea can be easily colonized by fungi yielding symbiotic germination that is required to promote fungal pathogenicity and drug resistance27. Debulking corneal biopsy has both diagnostic and therapeutic roles in refractory infectious keratitis, particularly those with progressive stromal infiltrates inaccessible to corneal scrapings28,29. In our series, we had our biopsy specimens examined histopathological and cultures. Pathological examination was positive in all cases, and cultures were positive in 83%. When compared to corneal scraping, biopsy possesses the benefit of extracting samples from the deeper layers of the cornea and/or reducing the infection’s size, resulting in an overall diagnostic success rate up to 82%28,30. Moreover, according to Younger et al., histopathologic examination of corneal biopsies demonstrated a higher rate of microorganism identification compared to culture alone (40% vs. 19%)31. Being inexpensive, fast, sensitive, and specific, we relied mainly on the KOH smear32 to start the antifungal eye drops immediately after the surgery. The common practice in identifying fungi in infectious keratitis cases involves using 10% KOH smears, which have an overall sensitivity ranging from 81 to 99%33. Similarly, KOH demonstrates a high sensitivity in detecting Acanthamoeba (84–91%)33 and microsporidia (97%)34. Other quick tools, such as PCR, were unavailable in our department. It is also essential to recognize false positive and false negative culture results if not consistent with the clinical presentation. Even though the culture results are of prime importance to identify the fungus species and to judge the fungus sensitivity to various antifungal drugs, direct examination of the biopsy specimens, using KOH, is considered superior in the diagnosis of FK29.
Although corneal biopsy is a valuable diagnostic procedure, it is crucial to acknowledge that it carries certain risks. There have been reported cases of inadvertent corneal perforation associated with biopsy, particularly when there is pre-existing corneal thinning, melting, and necrosis. These conditions can create a misleading appearance of a thick and swollen cornea. Thus, tectonic AMT was performed simultaneously in our case series to support the thinned residual corneal stroma, and seal inadvertent perforations. Table 1 summarizes seven reports on using AM in FK. AMT is well-known to have triple antimicrobial, anti-inflammatory, and tectonic properties. First, the antimicrobial activity is attributed to the existence of lysozyme, immunoglobulin, and transferrin in the amniotic fluid35. Second, regulation of T-cell function and secretion of an anti-inflammatory agent such as IL-1ra, sTNF, and VEGF-R constitute anti-inflammatory activities of the AM that also exhibit a long-term beneficial effect on the stability of corneal surface and reduce the severity and density of neovascularization36,37. Third, AM has shown to be aggressively repopulated by cornea stroma-derived cells, ultimately restoring corneal stromal integrity and helping obviate the necessity of emergent therapeutic keratoplasty in cases with impending or actual corneal perforation38,39. In the context of infectious keratitis, corneal transplantation has a high risk of rejection, reinfection, and ultimately failure. The AM is considered an inexpensive and effective alternative to tectonic therapeutic keratoplasty in cases of corneal melting and impending perforation40. Adequate debridement of necrotic corneal tissue and radical debridement of the corneal infiltrates help decrease the microbial load; nevertheless, it makes the cornea vulnerable to perforation. Hence, the AM provides invaluable tectonic support and helps maintaining corneal integrity during the initial postoperative period while waiting for the culture results. Together with corneal biopsy, we did AMT during the initial presentation to help start the antifungal drops as soon as possible. It is well established that early AMT helps accelerating corneal healing and improve visual outcomes in fungal and bacterial keratitis. Additionally, soaking the AM in antibiotics before transplantation sustains drug delivery during the early postoperative period41. Meanwhile, AM helps reducing the potential epithelial toxicity of compounded topical medications such as amphotericin B. In our cases, a reduction of the clinical severity of keratitis with subjective improvement was noted in the early postoperative period. This could be explained by the radical debridement therapeutic effect and AM's aforementioned inhibitory effect on endogenous and fungal proteinase activities. Successful corneal surface reconstruction and resolution of infection were achieved in our cases without complications.
Our study is not without limitations. First, we did not include other forms of microbial keratitis. It is noteworthy to mention that filamentous fungal keratitis is the predominant microbial keratitis in the Egyptian Delta region where the study was conducted42. Nevertheless, further studies are required to evaluate the efficacy of the aforementioned intervention in polymicrobial and non-fungal keratitis. Second, our results should be interpreted with caution given the small sample size, lack of control group and the retrospective design. Further prospective clinical trials with larger sample sizes are required to replicate our findings.
In conclusion, we recommend performing a debulking biopsy with tectonic AMT in refractory clinically presumed FK to decrease the microbial load, confirm the presence of fungal pathogen, suppress the inflammatory process, restore the stromal integrity, and promote epithelial healing.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- AMT:
-
Amniotic membrane transplantation
- BCVA:
-
Best corrected visual acuity
- FK:
-
Fungal keratitis
- IVCM:
-
In vivo confocal microscopy
- KOH:
-
Potassium hydroxide
- MMP:
-
Matrix metalloproteinases
- PCR:
-
Polymerase chain reaction
- SDA:
-
Sabouraud dextrose agar
- TKP:
-
Therapeutic keratoplasty
- NCCLS:
-
National Committee for Clinical Laboratory Standards
References
Srinivasan, M. et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, south India. Br. J. Ophthalmol. 81(11), 965–971 (1997).
Ung, L., Bispo, P. J., Shanbhag, S. S., Gilmore, M. S. & Chodosh, J. The persistent dilemma of microbial keratitis: Global burden, diagnosis, and antimicrobial resistance. Surv. Ophthalmol. 64(3), 255–271 (2019).
Green, M., Apel, A. & Stapleton, F. Risk factors and causative organisms in microbial keratitis. Cornea 27(1), 22–27 (2008).
Ritterband, D. C., Seedor, J. A., Shah, M. K., Koplin, R. S. & McCormick, S. A. Fungal keratitis at the New York eye and ear infirmary. Cornea 25(3), 264–267 (2006).
Sirikul, T., Prabriputaloong, T., Smathivat, A., Chuck, R. S. & Vongthongsri, A. Predisposing factors and etiologic diagnosis of ulcerative keratitis. Cornea 27(3), 283–287 (2008).
Acharya, M., Farooqui, J. H., Gaba, T., Gandhi, A. & Mathur, U. Delhi infectious keratitis study: Update on clinico-microbiological profile and outcomes of infectious keratitis. J. Curr. Ophthalmol. 32(3), 249 (2020).
Xie, L., Zhong, W., Shi, W. & Sun, S. Spectrum of fungal keratitis in north China. Ophthalmology 113(11), 1943–1948 (2006).
Iyer, S. A., Tuli, S. S. & Wagoner, R. C. Fungal keratitis: Emerging trends and treatment outcomes. Eye Contact Lens 32(6), 267–271 (2006).
Gopinathan, U. et al. The epidemiological features and laboratory results of fungal keratitis: A 10-year review at a referral eye care center in South India. Cornea 21(6), 555–559 (2002).
Thomas, P. Fungal infections of the cornea. Eye 17(8), 852–862 (2003).
Mahmoudi, S. et al. Fungal keratitis: An overview of clinical and laboratory aspects. Mycoses 61(12), 916–930 (2018).
Haseeb, A. A., Elhusseiny, A. M., Siddiqui, M. Z., Ahmad, K. T. & Sallam, A. B. Fungal Endophthalmitis: A Comprehensive Review. J. Fungi. 7(11), 996. https://doi.org/10.3390/jof7110996 (2021).
Ansari, Z., Miller, D. & Galor, A. Current thoughts in fungal keratitis: Diagnosis and treatment. Curr. Fungal Infect. Rep. 7(3), 209–218 (2013).
Kam, K. W., Rao, S. K. & Young, A. L. The Diagnostic Dilemma of Fungal Keratitis 1–2 (Nature Publishing Group, 2022).
Bharathi, M. J. et al. Microbiological diagnosis of infective keratitis: Comparative evaluation of direct microscopy and culture results. Br. J. Ophthalmol. 90(10), 1271–1276 (2006).
Kim, E. et al. Prospective comparison of microbial culture and polymerase chain reaction in the diagnosis of corneal ulcer. Am. J. Ophthalmol. 146(5), 714-723.e1 (2008).
Srinivasan, M. Fungal keratitis. Curr. Opin. Ophthalmol. 15(4), 321–327 (2004).
Kaur, I. P., Rana, C. & Singh, H. Development of effective ocular preparations of antifungal agents. J. Ocul. Pharmacol. Ther. 24(5), 481–494 (2008).
Saluja, G. et al. Comparison of safety and efficacy of intrastromal injections of voriconazole, amphotericin B and natamycin in cases of recalcitrant fungal keratitis: A randomized controlled trial. Clin. Ophthalmol. (Auckland, NZ) 15, 2437 (2021).
Xie, L., Dong, X. & Shi, W. Treatment of fungal keratitis by penetrating keratoplasty. Br. J. Ophthalmol. 85(9), 1070–1074 (2001).
Jamerson, E. C., Elhusseiny, A. M., ElSheikh, R. H., Eleiwa, T. K. & El Sayed, Y. M. Role of matrix metalloproteinase 9 in ocular surface disorders. Eye Contact Lens 46, S57–S63 (2020).
Jongkhajornpong, P., Nimworaphan, J., Lekhanont, K., Chuckpaiwong, V. & Rattanasiri, S. Predicting factors and prediction model for discriminating between fungal infection and bacterial infection in severe microbial keratitis. PLoS One 14(3), e0214076. https://doi.org/10.1371/journal.pone.0214076 (2019).
Prajna, N. V. et al. Predictors of corneal perforation or need for therapeutic keratoplasty in severe fungal keratitis: A secondary analysis of the mycotic ulcer treatment trial II. JAMA Ophthalmol. 135(9), 987–991. https://doi.org/10.1001/jamaophthalmol.2017.2914 (2017).
Lee, S.-H. & Tseng, S. C. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am. J. Ophthalmol. 123(3), 303–312 (1997).
Manikandan, P. et al. Fungal keratitis: Epidemiology, rapid detection, and antifungal susceptibilities of Fusarium and Aspergillus isolates from corneal scrapings. BioMed Res. Int. 2019, 1–9 (2019).
FR C. Performance standards for antimicrobial susceptibility testing. Approved Standard M100-S20. 2010;
Castano G EA, Mada PK. Fungal Keratitis. [Updated 2022 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan. https://www.ncbi.nlm.nih.gov/books/NBK493192/.
Alexandrakis, G., Haimovici, R., Miller, D. & Alfonso, E. C. Corneal biopsy in the management of progressive microbial keratitis. Am. J. Ophthalmol. 129(5), 571–576. https://doi.org/10.1016/S0002-9394(99)00449-3 (2000).
Ishibashi, Y. & Kaufman, H. E. Corneal biopsy in the diagnosis of keratomycosis. Am. J. Ophthalmol. 101(3), 288–293. https://doi.org/10.1016/0002-9394(86)90821-4 (1986).
Robaei, D. et al. Corneal biopsy for diagnosis of recalcitrant microbial keratitis. Graefes Arch. Clin. Exp. Ophthalmol. 256(8), 1527–1533. https://doi.org/10.1007/s00417-018-3981-1 (2018).
Younger, J. R. et al. Microbiologic and histopathologic assessment of corneal biopsies in the evaluation of microbial keratitis. American journal of ophthalmology. 154(3), 512-519.e2 (2012).
Sabapathypillai, S. L., James, H. R., Lyerla, R. R. & Hassman, L. The next generation of ocular pathogen detection. Asia-Pacific J. Ophthalmol. 10(1), 109–113 (2021).
Bharathi, M. J. et al. Microbiological diagnosis of infective keratitis: Comparative evaluation of direct microscopy and culture results. Br. J. Ophthalmol. 90(10), 1271. https://doi.org/10.1136/bjo.2006.096230 (2006).
Zhang, W., Yang, H., Jiang, L., Han, L. & Wang, L. Use of potassium hydroxide, giemsa and calcofluor white staining techniques in the microscopic evaluation of corneal scrapings for diagnosis of fungal keratitis. J. Int. Med. Res. 38(6), 1961–1967. https://doi.org/10.1177/147323001003800609 (2010).
Galask, R. P. & Snyder, I. S. Antimicrobial factors in amniotic fluid. Am. J. Obstetr. Gynecol. 106(1), 59–65 (1970).
Laranjeira, P. et al. Amniotic membrane extract differentially regulates human peripheral blood T cell subsets, monocyte subpopulations and myeloid dendritic cells. Cell Tissue Res. 373(2), 459–476 (2018).
Nicholas, M. P. & Mysore, N. Corneal neovascularization. Exp. Eye Res. 202, 108363 (2021).
Nubile, M. et al. In vivo analysis of stromal integration of multilayer amniotic membrane transplantation in corneal ulcers. Am. J. Ophthalmol. 151(5), 809-8221.e1 (2011).
Eleiwa, T. et al. Case series of perforated keratomycosis after laser-assisted in situ keratomileusis. Case Rep. Ophthalmol. Med. 2020, 1–8 (2020).
Qing L, Guiqiu Z, Jing L, Qian W, Yanli G, Ying L. Clinical efficacy of the combination of lesion debridement with amniotic membrane cover and drugs for fungal keratitis. Chin. J. Exper. Ophthalmol. 2014:824–828.
Kim, J.-S., Kim, J.-C., Hahn, T.-W. & Park, W.-C. Amniotic membrane transplantation in infectious corneal ulcer. Cornea 20(7), 720–726 (2001).
Sadik, N., Elzeiny, S. M., Ali, Y. E. & Sobeih, D. Fungal keratitis in the egyptian delta: Epidemiology, risk factors, and microbiological diagnosis. Ophthal. Epidemiol. 29(2), 198–205. https://doi.org/10.1080/09286586.2021.1914667 (2022).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
T.E. was a major contributor in writing the manuscript. T.E., G.Y. and A.K. implemented the segmentation algorithm. T.E., G.Y., I.A.E., S.A., A.K., acquired the data. T.E., I.A.E., S.A., A.K. analyzed and interpreted the data. All authors were involved in the concept, study design, and the writing and approval of the final manuscript. All co-authors have reviewed and agreed with the following responses and revisions made to the manuscript. All authors attest that they meet the current ICMJE criteria for Authorship.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eleiwa, T.K., Youssef, G.H., Elsaadani, I.A. et al. Debulking corneal biopsy with tectonic amniotic membrane transplantation in refractory clinically presumed fungal keratitis. Sci Rep 14, 521 (2024). https://doi.org/10.1038/s41598-023-50987-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50987-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.