Abstract
Mitochondrial dysfunction is a recent emerging research scope that proved to be involved in many cardiovascular diseases culminating in chronic heart failure (CHF), which remains one of the primary causes of morbidity and mortality. This study investigated the added cardio-protective effects of exogenous melatonin administration to conventional captopril therapy in isoproterenol (ISO) exposed rats with CHF. Five groups of Wistar rats were recruited; (I): Control group, (II): (ISO group), (III): (ISO + captopril group), (IV): (ISO + melatonin group) and (V): (ISO + melatonin/captopril group). Cardiac function parameters and some oxidant, inflammatory and fibrotic markers were investigated. Moreover; mRNA expression of mitochondrial mitophagy [parkin & PTEN induced kinase 1 (PINK1)], biogenesis [Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α)], fusion [mitofusin 2 (Mfn2)] and fission [dynamin-related protein 1 (DRP-1)] parameters in rat’s myocardium were evaluated. Rats’ myocardium was histo-pathologically and immunohistochemically evaluated for Beclin1 and Sirt3 expression. The present study revealed that captopril and melatonin ameliorated cardiac injury, oxidative stress biomarkers, and pro-inflammatory cytokines in ISO-exposed rats. These protective effects could be attributed to mitochondrial dynamic proteins control (i.e. enhanced the mRNA expression of parkin, PINK1, PGC-1α and Mfn2, while reduced DRP-1 mRNA expression). Also, Beclin1 and Sirt3 cardiac immunoreactivity were improved. Combined captopril and melatonin therapy showed a better response than either agent alone. Melatonin enhanced myocardial mitochondrial dynamics and Sirt3 expression in CHF rats and may represent a promising upcoming therapy added to conventional heart failure treatment.
Similar content being viewed by others
Introduction
Despite significant advancements in diagnosis and treatment, heart failure remains one of the world's major causes of morbidity and mortality1. Increased sympathetic excitability, aberrant humoral factor production, overexpression of inflammatory cytokines, imbalance of the immune system and cardiac interstitial fibrosis, are all important aspects of chronic heart failure (CHF) complex pathophysiology2,3.
Compared to other tissues, heart tissue contains an abundance of mitochondria, which comprise roughly 45% of the myocardial volume4. This abundance results from the cardiomyocytes' high energy requirements, which use more than 90% of the ATP produced by mitochondria5.
Lately, numerous dynamic mechanisms controlling mitochondrial function in mammals have been discovered. Fission/fusion, selective mitochondrial autophagy (mitophagy), and mitochondrial biogenesis are some of these mitochondrial dynamics (regulating mitochondrial number for cellular adaptation). Energy generation is regulated by mitochondrial dynamics, which is a balance between mitochondrial fusion and fission6.
Moreover, maintaining mitochondrial function and preventing cell death are both dependent on mitophagy, a selective form of autophagy for the targeted degradation of damaged mitochondria7. Defective mitochondria and dangerous reactive oxygen species (ROS), which can cross the plasma membrane, may accumulate as a result of damaged mitophagy8.
Several cardiac diseases are linked to mitochondrial dysfunction and excessive production of reactive oxygen species (ROS) which damage cellular lipids, proteins, enzymes, and DNA and are associated with apoptosis which accelerates cardiovascular damage6.
In addition, the upregulated renin–angiotensin–aldosterone system increases the production of reactive oxygen species (ROS), which causes an inflammatory state and myocyte degeneration in CHF9. Hence, angiotensin-converting enzyme inhibitors (ACE-I) are established therapy in patients with heart failure10. However, little literature investigated their effect on mitochondrial dynamic parameters.
Melatonin, an endogenous hormone released by the pineal gland has been handled in several studies as a cardioprotective agent in multiple heart diseases, such as atherosclerosis, hypertension, MI/R injury and heart failure as well as has shown to be critical in mitophagy and other mitochondrial activities11,12. Melatonin can traverse all cell membranes and collect in subcellular parts, especially mitochondria, due to its amphiphilic nature and tiny size13 where it counteracts lipid peroxidation and DNA injury14.
So, this study aims to evaluate the effects of both captopril and melatonin on cardiovascular remodelling and function in isoproterenol-exposed rats with CHF and evaluate their effects on mitochondrial dynamics and mitophagy.
Cytosolic Beclin 1 was evaluated as a marker of autophagy. Mitochondrial PTEN-induced kinase 1 (PINK1) and Parkin mRNA expression were assessed as markers of mitophagy. For assessment of mitochondrial biogenesis, the expression of cytosolic Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) was evaluated. Mitofusin2 (Mfn2) related mitochondrial fusion and [dynamin-related protein 1 (DRP-1) mediated mitochondrial fission was also investigated in response to added melatonin and captopril therapy.
Material and methods
Animals
50 healthy adult male Wistar rats (8–10 weeks old and 208–235 gm) obtained from the animal house of the Faculty of Veterinary Medicine—Zagazig University, were kept in hygienic conditions and received a commercial diet and ad libitum water, kept at a temperature of 24 ± 6 °C and on a 12 h light/dark cycle. The study protocol was approved by the Ethics of the guiding standard for the use of research animals by the ZU-IACUC committee, with the number ZU-IACUC/3/F/77/2022. Our study followed the ARRIVE and international ethical guidelines for the care and use of laboratory animals.
Drugs
Melatonin (5 mg tablet, Natrol Company, USA) and captopril (25 mg tablet, Smithkline Beecham, Giza, Egypt) were purchased. Isoproterenol hydrochloride powder was obtained from Sigma-Aldrich, Cairo, Egypt. Thiopental Sodium (farcopental 0.5 gm/vial, Powder) was obtained from Sigmatec Pharmaceutical Industries, Egypt.
Experimental design
Rats were randomly assigned to five equal groups: Group I (Control); rats were subcutaneously (S.C) injected with 5 ml/kg physiological saline (0.9%) once daily for 10 successive days, group II (ISO); isoproterenol hydrochloride, dissolved in physiological saline, was S.C injected (5 mg/kg body weight once daily for 10 days) to establish cardiac remodelling15, group III (ISO + captopril): Rats were treated with isoproterenol hydrochloride as group II with concurrent captopril administration at a dose of 13.5 mg/kg/day by gavage for 14 days starting from 1st day of ISO administration16, group IV (ISO + melatonin)): isoproterenol hydrochloride as group II was administered concurrently with melatonin at a dose of 10 mg/kg/day by gavage for 14 days starting from 1st day of ISO administration17, Group V (ISO + melatonin/captopril): isoproterenol was administered concurrently with both melatonin and captopril at the same doses and durations mentioned before.
Measurement of systolic blood pressure (SBP) and heart weight index (HWI)
SBP was measured on the 1st, 7th, and 14th days of the experiment in millimetres mercury (mm Hg) by a Non-Invasive Blood Pressure Monitor (NIBP 250, Serial No.: 21202-108, BIOPAC System, Inc.; USA)18. HWI was measured by dividing the heart weight by body weight.
Echocardiographic assessment
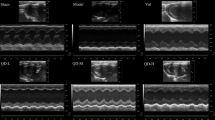
Echocardiographic examination was performed on day 14 to confirm cardiac remodeling and failure, using a VIVID 3 Ultrasound and 12 MHZ linear transducer and 5–8 MHZ sector transduced. Rats were anaesthetized with 100 mg/kg/i.p ketamine and the ultrasound probe was placed in the left sternal border after shaving their chest hair. Measurements of left ventricular diastolic dimension (LVDD), left ventricular systolic dimension (LVSD) and Ejection fraction were calculated from the M-mode at two-dimensional images obtained in the left parasternal long and short axes at the level of the papillary muscles after observation of at least 6 cardiac cycles.
Blood sample collection and preparation of tissue homogenate
Rats were anaesthetized with intraperitoneal thiopental (15 mg/kg) after overnight fasting and blood samples were collected from retro-orbital venous plexus, and serum was separated by centrifugation at 3000 rpm for 20 min and kept deep frozen (− 20 °C) until used to measure the serum levels of myocardial injury markers; lactate dehydrogenase (LDH), creatine phosphokinase muscle/brain (CPK-MB), cardiac troponin I (cTnI) by commercially available kits (Bayer Diagnostics Ltd., Baroda, India for LDH, BioMérieux Diagnostics, Milan, Italy for CK-MB and Sangon Biotech Co., Ltd., Shanghai, China for cTnI) according to the manufacturer’s instructions. Then animals were sacrificed, and hearts were excised immediately, washed with saline, then weighed and divided into two portions. A portion of the cardiac tissue was preserved immediately in liquid nitrogen, then homogenized with phosphate buffered solution (PBS, 7.4 pH), and then centrifuged at 12,000×g for 10 min at 4 °C, the supernatant portion was used for measuring cardiac biochemical parameters19. The other portion was rinsed in 10% formaldehyde solution and preserved for histopathological and immunohistochemical examination.
Measurement of lipid peroxidation and antioxidant status
Malondialdehyde (MDA) and superoxide dismutase (SOD) assay kits were used (Bio diagnostic, Egypt kits) according to their manufacturers’ protocols.
Determination of cardiac inflammatory/anti-inflammatory, apoptotic, and fibrotic markers (TNF-α, IL-6, IL-10, iNOS, caspase-3, TGF-β1, and collagen-I)
Rat ELISA kits for TNF-α, IL-6 and IL-10 (Bio diagnostic, Egypt kits), caspase-3 (CUSABIO, Baltimore, Maryland, USA) and TGF-β1, collagen-I and iNOS (Sigma-Aldrich Co., Egypt) were used to measure these parameters according to their manufacturers’ protocols.
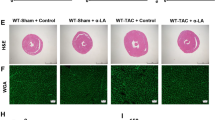
Histopathological analysis and immunohistochemical (IHC) study for Beclin-1 and Sirt3 expression
The remaining cardiac tissue was fixed in 10% buffered formalin and embedded in paraffin. Thick sections of 4–5 um were cut from the tissue block, mounted on glass slides then deparaffinized in xylene, and stained with haematoxylin and eosin (H & E) and Masson’s Trichome (MT) stains to observe collagen fibres deposits.
Serial sections (4–5 μ) cut from the paraffin blocks were boiled in 10 mM citrate buffer (AP9003) at pH 6 for 10 min to retrieve antigen, then incubated for 1 h with the primary antibodies of an anti-Beclin-1 antibody (ab217179) (rabbit anti-human polyclonal antibody, dilution 1:150, Abcam) and polyclonal Sirt3 (cat. no. sc-15404; Santa Cruz Biotechnology). After the automatic immunostainer (DAKO autostainer), IHC analysis was performed using the polymer Envision detection system; the Dako EnVision ™ kit (Dako, Copenhagen, Denmark). Finally, the sections were contrasted with Mayer’s haematoxylin at 0.1%. Analysis was performed by a blinded pathologist.
Morphometric study
Image J analysis software was used (Fiji image j; 1.51 n, NIH, USA) within 10 non-overlapping fields for each section. The area percent of collagen fibres and Beclin-1 & Sirt3 Immunoreaction were measured after light microscope magnification at 10× and 40× respectively.
Quantitative RT-PCR
Total RNA was extracted from rats’ homogenized myocardium using Trizol (Thermo Fisher Scientific, Waltham, MA, USA) and reversely transcribed to cDNA using a Revert Aid First Strand cDNA Synthesis kit (Fermentas, Vilnius, Lithuania). The cDNA was subjected to quantitative RT-PCR implemented in a StepOnePlus Real-Time PCR system (Applied Biosystems, USA) using iQ SYBR Green supermix (BioRad Laboratories, Hercules, CA, USA). The primer sequences (Thermo Fischer Scientific, USA) were as follows:
Forward | Reverse | |
|---|---|---|
Parkin | 5′-CTGGCAGTCATTCTGGACAC-3′ | 5′-CTCTCCACTCATCCGGTTTG)-3′ |
Pink1 | 5′-CATGGCTTTGGATGGAGAGT-3′ | 5′-TGGGAGTTTGCTCTTCAAGG-3′ |
PGC-1α | 5′-CGCAACATGCTCAAGCCAAA-3′ | 5′-GCGGTCTCTCAGTTCTGTCC-3′ |
Mfn2 | 5′-ATGCATCCCCACTTAAGCAC-3′ | 5′-CCAGAGGGCAGAACTTTGTC-3 |
DRP1 | 5′-CACCCGGAGACCTCTCATTC-3′ | 5′-CCCCATTCTTCTGCTTCCAC-3′ |
GAPDH | 5′-GGCACAGTCAAGGCTGAGAATG-3′ | 5′-ATGGTGGTGAAGACGCCAGTA-3′ |
The level of each gene expression was normalized to the level of the housekeeping gene, Gene—Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH). The results were detected as fold change by the 2–ΔΔCT method.
Statistical analysis
The SPSS program (version 20) was used to statistically evaluate the data, which were reported as mean ± SD (SPSS Inc. Chicago, IL, USA). One-way analysis of variance (ANOVA) was performed to assess group differences, and the student's least significant differences (LSD) test was applied for post hoc comparisons. Statistics were deemed significant at p < 0.05.
Ethics approval
The ZU-IACUC committee's Ethics of the guiding standard for the use of research animals accepted the study protocol, with number ZU-IACUC/3/F/77/2022.
Results
Effect of captopril and/ or melatonin on systolic blood pressure (SBP)
Compared to the normal control group, SBP was significantly reduced in the ISO group (p < 0.001). It was improved and increased in the treated captopril and melatonin groups when compared to the ISO untreated group (p < 0.001). Moreover, their combination therapy in the 5th group showed more significant SBP improvement than either drug alone (p < 0.01)] (Fig. 1).
Effect of captopril and/or melatonin on heart weight, heart weight index (HWI) and cardiac function and morphological alterations
Echocardiography revealed an increase in left ventricular dimensions, (LVESD and LVEDD), while, LVFS and LVEF were significantly decreased in the ISO group in comparison to the normal control group (p < 0.001). Also, the heart weight and heart weight index were increased in the ISO group. However, all these previous changes were significantly improved (LVESD, LVEDD, heart weight and heart weight index showed a significant decrease and LVFS and LVEF showed a significant increase) in treated groups and the most significant improvement was recorded in the combined therapy (group V) (Table 1, Fig. 2).
Echocardiographic views for control (a), (ISO) (b), ISO + captopril (c) ISO + melatonin (d) and ISO + melatonin/captopril (e). Statistical analysis showing the percentage of left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) in all groups (f). *p < 0.001 versus control, #p < 0.001 versus (ISO) group, €p < 0.001 versus ISO + captopril group and ₳p < 0.001 versus ISO + melatonin group.
Effect of captopril and/or melatonin on serum cardiac injury markers
The levels of serum LDH, CPK-MB and cTnI showed a significant increase in the ISO group compared to the control group (p < 0.001) which were significantly improved by melatonin and/ or captopril administration. Notably, the combined therapy in group V showed the best improvement than either drug alone (p < 0.001) (Fig. 3).
Effect of captopril and/or melatonin on cardiac oxidant/antioxidant markers
Cardiac MDA was significantly increased; however cardiac SOD activity was significantly decreased in the ISO group compared to the control group (p < 0.001). These effects of ISO were significantly mitigated with, melatonin and/ or captopril. Of note, the combination therapy showed a more significant improvement compared with that of monotherapy (p < 0.01) (Fig. 4).
Effect of captopril and/or melatonin on cardiac inflammatory/anti-inflammatory cytokines
Cardiac TNF-α, IL-6 as well and iNOS were significantly increased, however cardiac IL-10 level was significantly decreased in the ISO group, compared to the control group (p < 0.001). Melatonin and/ or captopril significantly ameliorated these changes with better improvement observed at their combination (Fig. 5).
Effects of captopril, melatonin, and their combination on cardiac inflammatory/ anti-inflammatory cytokines; (TNF-α) (a), (IL-6) (b), (iNOS) (c) and (IL-10) (d). *p < 0.001 versus control, #p < 0.001 versus (ISO) group, €p < 0.001 versus ISO + captopril group and ₳p < 0.001 versus ISO + melatonin group.
Effect of captopril and/or melatonin on cardiac apoptotic marker caspase 3 and fibrotic markers
Cardiac caspase-3 was significantly increased in the ISO group compared to the control (p < 0.001). On the other hand, significantly decreased with melatonin and/ or captopril compared to the ISO-untreated group (p < 0.001). Concurrent administration of captopril and melatonin showed more significant effects (p < 0.001) (Fig. 6). In addition, the ISO untreated group showed a significant increase in TGF-β1 and collagen-I cardiac tissue levels when compared to the control group (p < 0.001). However, melatonin and/ or captopril significantly (p < 0.001) suppressed this increase which was the best with their combination (Fig. 6).
Effect of captopril and/or melatonin on mRNA expression of mitochondrial dynamics and mitophagy parameters
Our study also revealed significantly impaired mitochondrial dynamics in untreated CHF model; reduced mRNA expression of mitochondrial mitophagy (parkin & PINK1), biogenesis (PGC-1α) and fusion (Mfn2) proteins compared to the control group (p < 0.001). Meanwhile, the mRNA expression of the mitochondrial fission Parameter (DRP-1) was significantly increased (p < 0.001). Both captopril and/or melatonin alleviated this impairment and significantly restored the mRNA expression of mitochondrial mitophagy (parkin/PINK1), biogenesis (PGC-1α) and fusion (Mfn2) parameters (p < 0.001). A significant reduction in the mRNA expression of the mitochondrial fission parameter (DRP-1) was also observed (p < 0.001) (Fig. 7). Better improvement was obvious at combination therapy, although sometimes the difference was insignificant.
Effects of captopril, melatonin, and their combination on mitochondrial dynamics in the rat model of CHF parkin (a) & (PINK1) (b), (PGC-1α) (c), (Mfn2) (d) and (DRP-1) (e) parameters in rat’s myocardium. *p < 0.001 versus control, #p < 0.001 versus (ISO) group, €p < 0.001 versus ISO + captopril group and ₳p < 0.001 versus ISO + melatonin group.
Effect of captopril and/or melatonin on myocardial morphology and fibrosis
Histopathological examination revealed distorted cardiac muscle fibres with patches of fibrosis, haemorrhage, inflammatory cells and dilated congested blood vessels with perivascular cuffing in ISO group. These manifestations were improved by melatonin and/or captopril administration with a more pronounced effect displayed with their combination (Figs. 8, 9).
H&E-stained heart tissue sections showing regularly arranged myofibrils (M) with narrow spaces in between in the control group (a,b). Distorted myofibrils with patches of fibrosis (F), haemorrhage (h) inflammatory cells (If), dilated congested blood vessels with perivascular cuffing (curved arrow) in ISO group (c–f). Improvement of myofibrils arrangement with wide spaces, dilated and perivascular cuffing in ISO/captopril group (g,h). Small areas of fibrosis (F), few cellular infiltration (If), perivascular cuffing and wide spaces in the ISO/melatonin group (i,j). Restoration of the normal arrangement of the myofibrils but areas of wide spaces and perivascular cuffing are still observed in the ISO/captopril/melatonin group (k,l).
Masson’s trichrome stained heart tissue sections showing collagen fibers (green color). In control group (a), wide areas of collagen deposition in the ISO group (b), few fibres of collagen around blood vessels in ISO + captopril (c) and ISO + melatonin group (d) but scanty patches of collagen fibers in noticed in ISO/captopril/melatonin (e). Morphometrical analysis shows an increased area percentage of collagen fibres in the ISO group (f). Scale bars 3A–3E = 200 µm.
Effect of captopril and/or melatonin on Beclin 1 and Sirt3 expression in myocardial tissue
Our immune-histochemical results also revealed decreased expression of Sirt3 and the mitophagy-related molecule, Beclin1 in ISO group that was abrogated by captopril and/or melatonin administration and similarly, the combined administration gave more significant results (Figs. 10, 11).
Expression of Beclin 1 in studied groups. The immunohistochemical results showed high positive cytoplasmic immunoreactivity (brown colour) is detected in most of myofibers of the control (a) group. The immunoreactivity is seen in a few myofibers of the ISO group (b). Positive immunoreactivity in some myofibers in both ISO/captopril (c) and ISO/melatonin (d) groups. Positive immunoreactivity in many myofibrils in ISO/captopril/melatonin (e) group. (f) Morphometrical analysis showing a decreased percentage of Beclin-1 immunoreactivity in the ISO group. Scale bars 4A–4E = 50 µm.
Expression of Sirt3 in studied groups. Sirt3 immuno-stained heart tissue sections showed decreased immunoreactivity (brown colour) of Sirt3 marker in ISO group (B) compared to other groups. (F) Morphometrical analysis showing a decreased percentage of Sirt3 immunoreactivity in the ISO group. Scale bars 5A–5E = 50 µm.
Discussion
The main factor in the development of heart failure and a crucial mechanism in ventricular remodelling is myocardial fibrosis (MF)16. When creating cardiovascular therapeutics, new modalities should target the major signalling pathways implicated in cardiac fibrosis20.
Many cardiovascular disorders have been linked to disturbed mitochondrial dynamics. Excessive mitochondrial fission is involved in ischemia/reperfusion injury, diabetic cardiomyopathy, and heart failure21,22. Mitochondrial fusion insufficiency was also found to be involved in myocarditis, heart failure, and cardiac hypertrophy23,24.
This study aimed to investigate the potential benefits of melatonin as a cardioprotective treatment for isoproterenol (ISO) induced chronic myocardial damage in rats, both alone and in combination with captopril and determine the possible underlying mechanisms concerning mitochondrial dynamics.
According to the dose and duration of its application, ISO treatment in rats has been shown to produce cardiac necrosis, hypertrophy, and fibrosis, allowing for the in vivo assessment of anti-fibrotic medicines25,26.
The current study demonstrated that isoproterenol administration causes myocardial hypertrophy (as indicated by heart weight and HWI increase) and cardiac dysfunction (as shown by the reduced SBP, LVEF and FS and the increase in cardiac dimensions, LVEDD and LVESD).
Moreover, myocardial histopathological examination showed distorted cardiac muscle fibres associated with exaggerated fibrosis with inflammatory cell infiltration and fibroblastic hyperplasia in the myocardium of the ISO group. Additionally, increased serum concentrations of myocardial enzymes; LDH, CPK-MB and cTnI as clinical markers for myocardial injury were observed. Myocardial enzymes increased in serum proportional to the number of myocardial necrotic cells or injury which alter the plasma membrane integrity and/or permeability.
ISO is a beta-adrenergic receptor (β) agonist that could stimulate both β1 and β2. However, excessive production or exogenous administration induces myocardial ischemia, necrosis, and fibrosis with decreased myocardial compliance, which causes diastolic and systolic dysfunction and closely resembles the alterations found in human heart failure27.
We observed in our study that administration of either captopril or melatonin significantly improved SBP, cardiac function and reduced cardiac injury-related protein levels, lessened the effects of ISO on myocardial cellular inflammatory infiltration, myofibrils disarray, extracellular matrix collagen deposition, and hypertrophy. Moreover, the coadministration of both compounds was more efficient in suppressing this damage than either agent alone, confirming their additive cardioprotective role in cardiac remodelling and fibrosis.
Abareshi et al.28 also showed that treatment with Captopril, a known ACE inhibitor, has a significant effect on preventing cardiac hypertrophy and left ventricular wall dilation, improved cardiac fibrosis, and lowered collagen content in heart tissue. Captopril has been widely used to alleviate left ventricular dysfunction after myocardial infarction29.
Additionally, melatonin has been reported to attenuate ISO-induced cardiac fibrosis and improve the SBP after long-term high-fat diet administration by blocking myocardial ROS production and cell apoptosis30,31.
In this study, The ISO-induced oxidative stress (indicated by increased MDA cardiac level as a marker for lipid peroxidation and by reduced SOD activity) was suppressed by captopril or/and melatonin. Several studies showed the protective effect of different anti‐oxidative stress therapies in CHF rat models32. Also, they inhibited ISO-induced inflammatory cytokines IL-6, TNF-α and iNOS levels, while IL-10, a cytokine that inhibits inflammation, was markedly elevated. This supports their antioxidants and anti-inflammatory properties reported in previous concordant studies12,17. Notably, the combined therapy of captopril and melatonin showed more improvement than either drug alone.
Melatonin has numerous antioxidant effects and due to its lipophilic property, it can easily reach mitochondria and maintain its adequate function by reducing oxidative stress, apoptosis and cell death. Via melatonin receptor, MT3, it acts as a quinone reductase, inhibits electron transfer from quinones and reduces oxidative stress33. Melatonin also acts directly as a free radical scavenger, donates electrons, and decreases reactive oxygen and nitrogen species, such as nitric oxide (NO), superoxide anion radical (O2−), and hydroxyl radical (OH)34. Melatonin via melatonin receptors MT1 and MT2 is also responsible for increasing the levels of antioxidant enzymes including superoxide dismutase, glutathione peroxidase and glutathione reductase, thus decreasing molecular damage35.
Similarly, captopril as a thiol-containing molecule confers properties other than ACE inhibition. The SH moiety easily undergoes oxidation and disulfide exchange reactions and is converted into disulfides through the interaction with free radicals. Through this process, it can serve as a free radical scavenger and improve cellular dysfunction. Captopril reduced ROS in several diseased models36,37.
Furthermore, the decrease of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-B) activation is another way that melatonin inhibits lipopolysaccharide-stimulated interleukin-1 beta (IL-1), IL-6, TNF-α and iNOS production12,38.
Moreover, we observed both captopril and melatonin therapy inhibited ISO-induced cardiomyocyte apoptosis by reducing the expression of caspase-3 and similarly, more improvement was observed in the combined therapy group. Melatonin's ability to inhibit apoptosis and modulate mitochondrial oxidative stress may result from its activation of p-Akt and p-STAT3, which in turn encourages antioxidant gene expression, prevents Bax translocation, increases the expression of the anti-apoptotic protein Bcl2, and inhibits the opening of the mitochondrial permeability transition pore (mPTP), which triggers caspase 339.
Indeed, our results showed that the increased cardiac level of the fibrogenic proteins, TGF-β1, as well as collagen-1 deposits in ISO-exposed myocardium, was significantly reduced by captopril and/or melatonin therapy, thus improving fibrosis and this is concordant to previous studies which reported the melatonin suppressing effect on fibrosis40. Melatonin could alleviate myocardial fibrosis via inhibiting TGF-β1/Smads signaling which increases collagen expression and deposition of fibrous tissue41,42. Moreover, this fibrosis-reducing effect of melatonin was confirmed in a continuous light-induced hypertension model and while only captopril prevented left ventricular hypertrophy development, only melatonin significantly reduced fibrosis. This antifibrotic action of melatonin may be protective in hypertensive heart disease43.
In our ISO-induced CHF model, we observed downregulation of mitochondrial mitophagy, biogenesis and fusion but, simultaneously upregulated mitochondrial fission. Similarly, Mukherjee et al.44 and Zhen et al.45 revealed that an imbalance between mitochondrial fusion and fission caused by ISO led to an accumulation of non-functional mitochondria and an overabundance of ROS, which in turn caused cell death.
Additionally, as damaged and dysfunctional mitochondria are significant producers of ROS, mitophagy, a particular type of autophagy, is one of the most efficient methods to remove damaged mitochondria, and its impairment is frequently noticed in the development of CHF46.
On the other hand, captopril therapy improved the disturbed mitochondrial dynamics in ISO-group by upregulating mitochondrial mitophagy, biogenesis and fusion while downregulating mitochondrial fission. This may be attributed to its reduction of oxidative stress so, protecting mitochondrial membrane potential (MMP) and improving mitochondrial energy production47,48.
Similarly, melatonin regained normal mitochondrial dynamics in our model, this is in line with Wang et al.49 who demonstrated the promoting effect of melatonin against impaired mitophagy activity by increasing Parkin translocation to the mitochondria in diabetic mice cardiomyopathy. Moreover, Pei et al.50 demonstrated that melatonin attenuates post-MI injury by increasing the expression of the mitochondrial fusion protein Mfn2, which is essential for balancing mitochondrial fusion and fission as well as autophagy. Mfn2 is involved in mitochondrial outer membrane fusion51. On the contrary, Wu et al.12 showed decreased mitophagy-related protein (Parkin and Beclin1) expression and suggested that the cardioprotective benefits of melatonin against anoxia/reoxygenation injury may be due to the suppression of excessive mitophagy. Melatonin modulates mitophagy by boosting or decreasing mitophagic activity, according to studies with various experimental designs52,53,54,55. These specific regulatory mechanisms need further investigation.
Furthermore, the immunohistochemical results highlighted the protective role of captopril and/or melatonin in their elevation of autophagy-mediator protein Beclin 1 in the heart tissue of treated groups. Autophagy is a conserved catabolic process that plays a critical role in cellular survival by sequestration of cytoplasmic organelles and long-lived proteins into the autophagosome to be degraded by lysosomal enzymes56. An important autophagy mediator is beclin-1, and influences both autophagy and apoptosis, thereby deeply affecting cardiomyocytes’ survival and death57. Myocardial hypertrophy models used in other studies similarly showed considerably reduced autophagy, which raises the possibility that autophagy might prevent hypertrophy of cardiac muscle58.
Moreover, the significant decrease in Sirt3 expression in the myocardium of the ISO group in our model has been reversed by captopril and/or melatonin therapy. Sirt3, a histone deacetylase found in the mitochondria, maintains the body's normal vital functions by balancing energy metabolism, controlling the cell cycle, and protecting against oxidative stress. This prevents the development of myocardial hypertrophy, myocardial fibrosis, and heart failure59.
Conclusion
In summary, combination therapy of melatonin and captopril can rescue chronic heart failure rats and provide an additional benefit to the protection with either agent alone. This is indicated by improved cardiac function, decreased cardiac tissue injury, and ameliorated interstitial fibrosis and hypertrophy. The pharmacologic actions are related to balancing mitochondrial dynamics and alleviating myocardial, inflammation, apoptosis, oxidative stress and fibrosis. Melatonin could be an added strategy for the treatment of heart failure and cardiac fibrosis with captopril.
Data availability
All data that support the results in the article are available as mean ± SE values, as well as statistical summaries as supporting information.
References
Savarese, G. & Lund, L. H. Global public health burden of heart failure. Card. Fail. Rev. 3(1), 7–11 (2017).
Ranganathan, P. et al. MicroRNA-150 deletion in mice protects kidney from myocardial infarction-induced acute kidney injury. Am. J. Physiol. Renal. Physiol. 309, F551–F558 (2015).
Wang, J., Shen, W., Zhang, J. Y., Jiaa, C. H. & Xie, M. L. Stevioside Attenuates isoproterenol-induced mouse myocardial fibrosis through inhibition of the myocardial NF-Κb/tgf-β1/Smad signaling pathway. Food Funct. 10, 1179–1190 (2019).
Gottlieb, R. A. & Gustafsson, A. B. Mitochondrial turnover in the heart. Biochim. Biophys. Acta. 1813, 1295–1301 (2011).
Tokarska-Schlattner, M., Zaugg, M., Zuppinger, C., Wallimann, T. & Schlattner, U. New insights into doxorubicin-induced cardiotoxicity: The critical role of cellular energetics. J. Mol. Cell Cardiol. 41, 389–405 (2006).
Siasos, G. et al. Mitochondria and cardiovascular diseases-from pathophysiology to treatment. Ann. Transl. Med. 6(12), 256 (2018).
Williams, J. A. & Ding, W. X. Mechanisms, pathophysiological roles and methods for analyzing mitophagy-recent insights. Biol. Chem. 399(2), 147–178 (2018).
Bienert, G. P. et al. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 282(2), 1183–1192 (2007).
Jafari-Vayghan, H., Saleh-Ghadimi, S., Maleki, V., Moludi, J. & Alizadeh, M. The effects of melatonin on neurohormonal regulation in cardiac cachexia: A mechanistic review. J. Cell Biochem. 120, 16340–16351 (2019).
McDonagh, T. A. et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 42(36), 3599–3726 (2021).
Pandi-Perumal, S. R. et al. Melatonin and human cardiovascular disease. J. Cardiovasc. Pharmacol. Ther. 22(2), 122–132 (2017).
Wu, G. C. et al. Melatonin receptor agonist protects against acute lung injury induced by ventilator through up-regulation of IL-10 production. Respir. Res. 21, 65 (2020).
Boga, J. A. et al. Therapeutic potential of melatonin related to its role as an autophagy regulator: A review. J. Pineal Res. 66(1), e12534 (2019).
Hosseinzadeh, A. et al. Apoptosis signaling pathways in osteoarthritis and possible protective role of melatonin. J.Pineal Res. 61(4), 411–425 (2016).
Liu, M., Feng, J., Du, Q., Ai, J. & Lv, Z. Paeoniflorin attenuates myocardial fibrosis in isoprenaline-induced chronic heart failure rats via inhibiting P38 MAPK pathway. Curr. Med. Sci. 40, 307–312 (2020).
Pan, J. et al. Huangqi Shengmai Yin ameliorates myocardial fibrosis by activating Sirtuin3 and inhibiting TGF-β/Smad pathway. Front. Pharmacol. 12, 722530 (2021).
Abdulwahab, D. A., El-Missiry, M. A., Shabana, S., Othman, A. I. & Amer, M. E. Melatonin protects the heart and pancreas by improving glucose homeostasis, oxidative stress, inflammation and apoptosis in T2DM-induced rats. Heliyon 7(3), e06474 (2021).
Abubakar, M., Ukwuani, A. & Mande, U. Antihypertensive activity of Hibiscus sabdariffa aqueous calyx extract in Albino rats. Sky J. Biochem. Res. 4(3), 16–20 (2015).
Lu, H. et al. Cardioprotective efficiency of tangeretin against heart failure induced by isoproterenol in rats. Int. J. Pharmacol. 14(8), 1145–1152 (2018).
Li, L., Fang, H., Yu, Y. H., Liu, S. X. & Yang, Z. Q. Liquiritigenin attenuates isoprenaline-induced myocardial fibrosis in mice through the TGF-β1/Smad2 and AKT/ERK signaling pathways. Mol. Med. Rep. 24(4), 686 (2021).
Givvimani, S., Pushpakumar, S., Veeranki, S. & Tyagi, S. C. Dysregulation of Mfn2 and Drp-1 proteins in heart failure1. Can. J. Physiol. Pharmacol. 92(7), 583–591 (2014).
Galloway, C. A. & Yoon, Y. Mitochondrial dynamics in diabetic cardiomyopathy. Antioxid. Redox Signal. 22(17), 1545–1562 (2015).
Wai, T. et al. Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science 350(6265), aad0116 (2015).
Hall, A. R. et al. Hearts deficient in both Mfn1 and Mfn2 are protected against acute myocardial infarction. Cell Death Dis. 7(5), e2238 (2016).
Wang, J. J. et al. Genetic dissection of cardiac remodeling in an isoproterenol-induced heart failure mouse model. PLoS Genet. 12, e1006038 (2016).
Wei, Y., Meng, T. & Sun, C. Protective effect of diltiazem on myocardial ischemic rats induced by isoproterenol. Mol. Med. Rep. 17, 495–501 (2018).
Li, H. et al. Trimetazidine ameliorates myocardial metabolic remodeling in isoproterenol-induced rats through regulating ketone body metabolism via activating AMPK and PPAR α. Front. Pharmacol. 14(11), 1255 (2020).
Abareshi, A. et al. Effect of angiotensin-converting enzyme inhibitor on cardiac fibrosis and oxidative stress status in lipopolysaccharide-induced inflammation model in rats. Int. J. Prev. Med. 8, 69 (2017).
Szeiffova, B. et al. Antiarrhythmic effects of melatonin and omega-3 Are linked with protection of myocardial Cx43 topology and suppression of fibrosis in catecholamine stressed normotensive and hypertensive rats. Antioxidants 9(6), 546 (2020).
Simko, F. et al. Melatonin reduces cardiac remodeling and improves survival in rats with isoproterenol-induced heart failure. J. Pineal Res. 57(2), 177–184 (2014).
Liu, Y. et al. Melatonin improves cardiac function in a mouse model of heart failure with preserved ejection fraction. Redox Biol. 18, 211–221 (2018).
Costa, C. et al. Progression of heart failure is attenuated by antioxidant therapy with N-acetylcysteine in myocardial infarcted female rats. Mol. Biol. Rep. 47, 8645–8656 (2020).
Maral, F. & Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 62, 472–479 (2018).
Reiter, R., Paredes, S., Manchester, L. & Tan, D. Reducing oxidative/nitrosative stress: A newly-discovered genre for melatonin. Crit. Rev. Biochem. Mol. Biol. 44, 175–200 (2009).
Tan, D., Manchester, L. C., Terron, M., Flores-Alvarado, L. & Reiter, R. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species?. J. Pineal Res. 42, 28–42 (2006).
Emekli-Alturfan, E., Ozdemir, E., Sağlam, E., Ozdamar, E. & Ozer, G. Angiotensin-converting enzyme inhibitor captopril ameliorates renal damage in a rat model of thermal injury. Asian Biomed. 7(3), 381–389 (2017).
Karimani, A., Mamashkhani, Y., Moghadam Jafari, A., Akbarabadi, M. & Heidarpour, M. Captopril attenuates diazinon-induced oxidative stress: A subchronic study in rats. Iran. J. Med. Sci. 43(5), 514–522 (2018).
Yu, G. M., Kubota, H., Okita, M. & Maeda, T. The anti-inflamFmatory and antioxidant effects of melatonin on LPS-stimulated bovine mammary epithelial cells. PLoS ONE 12(5), e0178525 (2017).
Huang, X. et al. Melatonin ameliorates myocardial injury by reducing apoptosis and autophagy of cardiomyocytes in a rat cardiopulmonary bypass model. PeerJ 15(9), e11264 (2021).
Xiao, H. et al. IL-18 cleavage triggers cardiac inflammation and fibrosis upon β-adrenergic insult. Eur. Heart J. 39(1), 60–69 (2018).
Gao, L. et al. TNAP inhibition attenuates cardiac fibrosis induced by myocardial infarction through deactivating TGF-β1/Smads and activating P53 signaling pathways. Cell Death Dis. 11, 44 (2020).
Che, H. et al. Melatonin alleviates cardiac fibrosis via inhibiting lncRNA MALAT1/miR-141-mediated NLRP3 inflammasome and TGF-β1/Smads signaling in diabetic cardiomyopathy. FASEB J. 34(4), 5282–5298 (2020).
Simko, F. et al. Hypertension and cardiovascular remodelling in rats exposed to continuous light: Protection by ACE-inhibition and melatonin. Mediators Inflamm. 2014, 703175 (2014).
Mukherjee, D. A. K. et al. Bandyopadhyay Mechanisms of isoproterenol-induced cardiac mitochondrial damage: Protective actions of melatonin. J. Pineal Res. 58(3), 275–290 (2015).
Zhen, D. et al. Cardioprotective effect of ethanol extracts of Sugemule-3 decoction on isoproterenol-induced heart failure in Wistar rats through regulation of mitochondrial dynamics. J. Ethnopharmacol. 292, 114669 (2022).
Korge, P., John, S. A., Calmettes, G. & Weiss, J. N. Reactive oxygen species production induced by pore opening in cardiac mitochondria: The role of complex II. J. Biol. Chem. 292, 9896–9905 (2017).
Taskin, E., Ozdogan, K., Kunduz Kindap, E. & Dursun, N. The restoration of kidney mitochondria function by inhibition of angiotensin-II production in rats with acute adriamycin-induced nephrotoxicity. Renal Fail. 36(4), 606–612 (2014).
Shokrzadeh, M., Zamani, E., Mollahasani, J. & Shaki, F. Captopril inhibited metamphetamine—Induced cardiac mitochondrial damage in hyperthermic condition via modulation of biochemical markers. Koomesh 18(1), 128–137 (2016).
Wang, S. et al. Melatonin activates Parkin translocation and rescues the impaired mitophagy activity of diabetic cardiomyopathy through Mst1 inhibition. J. Cell. Mol. Med. 22(10), 5132–5144 (2018).
Pei, H. et al. Melatonin prevents adverse myocardial infarction remodeling via Notch1/Mfn2 pathway. Free Radic. Biol. Med. 97, 408–417 (2016).
Xu, X., Su, Y. L., Shi, J. Y., Lu, Q. & Chen, C. MicroRNA-17-5p promotes cardiac hypertrophy by targeting Mfn2 to inhibit autophagy. Cardiovasc. Toxicol. 21(9), 759–771 (2021).
Onphachanh, X. et al. Enhancement of high glucose-induced PINK1 expression by melatonin stimulates neuronal cell survival: Involvement of MT2/Akt/NF-kappaB pathway. J. Pineal Res. 63, 2 (2017).
Zhou, H. et al. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J. Pineal Res. 63, 1 (2017).
Lee, W. J. et al. Melatonin promotes neuroblastoma cell differentiation by activating hyaluronan synthase 3-induced mitophagy. Cancer Med. 8(10), 4821–4835 (2019).
Chen, Y. et al. Melatonin ameliorates intervertebral disc degeneration via the potential mechanisms of mitophagy induction and apoptosis inhibition. J. Cell. Mol. Med. 23(3), 2136–2148 (2019).
Levine, B. & Yuan, J. Autophagy in cell death: An innocent convict?. J. Clin. Invest. 115, 2679–2688 (2005).
Maejima, Y., Isobe, M. & Sadoshima, J. Regulation of autophagy by Beclin 1 in the heart. J. Mol. Cell. Cardiol. 95, 19–25 (2016).
Wang, H. N. et al. Effects of Sirt3-autophagy and resveratrol activation on myocardial hypertrophy and energy metabolism. Mol. Med. Rep. 22(2), 1342–1350 (2020).
Jacobs, K. M. et al. SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression. Int. J. Biol. Sci. 4, 291–299 (2008).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
[S.F.E.], [A.M.A.] and [S.G.Z.] planned for the study. [A.M.A.] and [S.G.Z.] carried out the experiment. Data collection and analysis were performed by [S.M.M.M.] and [A.M.A.]. Histopathological studies were done by [D.I.E.]. The first draft of the manuscript was written by [S.F.E.], [A.M.A.] and [D.I.E.]. All authors revised and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Sayed, S.F., Abdelhamid, A.M., ZeinElabdeen, S.G. et al. Melatonin enhances captopril mediated cardioprotective effects and improves mitochondrial dynamics in male Wistar rats with chronic heart failure. Sci Rep 14, 575 (2024). https://doi.org/10.1038/s41598-023-50730-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50730-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.