Abstract
Effects of valproate (VPA) dose and treatment discontinuation during the first trimester of pregnancy on the risks of spontaneous abortions (SAB) and major birth defects were analyzed. Pregnancies with first trimester VPA exposure (n = 484) prospectively recorded by the German Embryotox center in 1997–2016 were compared with a randomly selected, non-exposed cohort (n = 1446). The SAB risk was not significantly increased in the VPA cohort [HRadj 1.31 (95% CI 0.85–2.02)] but major birth defects were significantly more frequent [8.7% vs. 3.4%; ORadj 2.61 (95% CI 1.51–4.50)]. Risk was even higher in pregnancies with no VPA discontinuation in first trimester [ORadj 3.66 (95% CI 2.04–6.54)]. Significant ORs were found for nervous system defects in general [ORadj 5.69 (95% CI 1.73–18.78)], severe microcephaly [ORadj 6.65 (95% CI 1.17–37.68)], hypospadias [ORadj 19.49 (95% CI 1.80–211)] and urinary system defects [ORadj 6.51 (95% CI 1.48–28.67)]. VPA dose had a stronger effect than antiepileptic poly- versus monotherapy; for VPA dose ≥ 1500 mg/day the ORadj was 5.41 (95% CI 2.32–12.66)]. A daily dose increase of 100 mg was calculated to raise the risk for major birth defects by 15% [OR 1.15 (95% CI 1.08–1.23)]. Overall, maternal first trimester treatment regimen had a relevant impact on birth defect risk.
Similar content being viewed by others
Introduction
The teratogenicity of valproate (VPA) is well-established, with evidence having accumulated since the 1980’s1,2,3,4,5,6,7,8,9,10. Specific birth defects like neural tube defects2,6,10,11, cardiac septal defects2,6,9,10, oro-facial clefts2,6,10, hypospadias2,6,9,10 and skeletal and limb malformations6,10 have been associated with VPA. Previous research revealed dose-dependency of VPA’s teratogenicity3,4,5,6,8,9,12. However, applied dose categories were heterogeneous and study results difficult to compare. Antiepileptic (AED) polytherapy including VPA was discussed to carry a higher risk than VPA monotherapy3,12,13,14,15. Moreover, neurodevelopmental disorders in childhood have been associated with VPA exposure in pregnancy16,17. Interestingly, data regarding the risk of spontaneous abortions (SAB) and VPA exposure during pregnancy are scarce and conflicting1,9,18,19,20,21,22.
The European Commission Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) issued new warnings and restrictions of VPA use in 201423 with further tightening in 201824, aiming at avoidance of VPA for girls and women of childbearing potential. Following these restrictions, VPA should only be considered in women of childbearing age when safer alternatives are ineffective such as in some types of generalized epilepsy25. Ethical as well as risk–benefit aspects of VPA treatment for mother and unborn child have been widely discussed26,27,28,29,30,31,32,33. Several studies showed increased risks of unsatisfactory seizure control after withdrawal of VPA or switch to another AED during pregnancy26,34, or in women of childbearing potential in general27,28,29,30. In contrast, there are no studies on the time-dependent effects of VPA discontinuation during the first trimester on the risks of birth defects and pregnancy loss. So far, there has been only one study by Vajda et al.26, which analyzed the risk of major birth defects after pre-conception withdrawal of VPA treatment.
It is important to address changes in organ-specific susceptibility to toxic agents when analyzing drug exposure effects on pregnancy outcomes. Exposure to harmful substances can lead to negative effects on the embryo or fetus throughout pregnancy. The period most sensitive to teratogens is the first trimester, where the defined development of anatomical structures and organs mainly takes place and toxic exposure may result in major birth defects or spontaneous abortion. We analyzed the German Embryotox database focusing on the impact of VPA dose, treatment discontinuation, and mono- versus polytherapy within the first trimester on the risk of major birth defects and SAB.
Methods
The Embryotox Center of Clinical Teratology and Drug Safety in Pregnancy (Embryotox) offers consultation on drug safety in pregnancy for health care professionals (HCP) and patients, with about 15,000 requests per year. Upon contact to Embryotox, details on all drug exposures (duration of treatment, dose, ATC-codes), treatment indications (MedDRA) and maternal medical history are recorded. Approximately eight weeks after the expected date of delivery, follow-up data are obtained by a standardized procedure using a questionnaire with a response rate of approximately 75%. Details on further drug exposures, course of pregnancy and delivery, and neonatal outcome including congenital anomalies are asked for. Data are generated iteratively from first contact in pregnancy to follow-up. In cases of incomplete or inconsistent response, particularly with respect to details of drug exposure and pregnancy outcome, the patient and/or her HCP are contacted for further information and medical records. Following this case by case plausibility check, data are archived (Embryotox database VigilanceOne, PharmApp Solutions GmbH). In addition, Embryotox serves as a national clearinghouse for suspected adverse drug reactions in pregnancy. A description of Embryotox procedures can be found in Dathe et al.35.
Study design
This observational cohort study is based on prospectively ascertained pregnancies exposed to VPA at least during first trimester (independent of treatment duration) and with complete follow-up. Our study follows the recommendation of the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement36,37. Pregnancies enrolled between January 1997 and December 2016 and exposed to VPA were compared to a randomly selected cohort unexposed to known teratogens or fetotoxicants at any stage of pregnancy, matched at a ratio 3:1 by year of enrollment. Definition of exclusion criteria, exposure, pregnancy outcomes and mono- and polytherapy are summarized in Table S1. Assignment to major birth defects was performed according to EUROCAT38 independently by two experienced clinicians blinded to exposure status.
Approval was obtained from the ethics committee of the Charité—Universitätsmedizin Berlin (EA2/130/18). The study was registered at the German Clinical Trial register (DRKS00015636, date of registration: 23.10.2018) and listed at the WHO International Clinical Trials Registry Platform. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Statistical analysis
Crude rates for SAB, elective termination of pregnancy (ETOP), stillbirth and live birth can be biased due to delayed study entry and competing risks39. Instead, cumulative incidences (CIF) were calculated using event history analysis for cause-specific sub-distributions of competing risks incorporating left truncation39,40. The effect of VPA exposure was estimated using a Cox proportional hazards regression model. The time-dynamic pattern of VPA exposure was accounted for using multistate models41. The multistate model by Bluhmki et al.42 was used, which classifies women for each point in time during pregnancy into the states “exposed” or “non-exposed (discontinued)” to VPA. Landmarking43 for probability estimation of pregnancy outcomes was then applied. The VPA cohort was stratified by discontinuation time into the groups “early discontinuation” (≤ GW 5 + 0), “moderate discontinuation” (between GW 5 + 1 and GW 7 + 0) and “late discontinuation” (at GW 7 + 1 or later)42,44. To avoid conditioning on the future45, the analysis was conditioned on observed and event free women at GW 7 + 1, which corresponds to the lower limit for late VPA discontinuers. For each discontinuation group, the probability for pregnancy outcomes was calculated. The effect of discontinuation on the hazard of SAB was estimated by applying two different Cox proportional hazards models42. First, the impact of time-dependent discontinuation on SAB risk was estimated by comparing the relative change in hazard of SAB between women having discontinued VPA in the first trimester and women remaining exposed to VPA throughout the first trimester. Second, time-specific discontinuation was estimated in the subgroup of all women having discontinued VPA in the first trimester and measures the relative change in the hazard of SAB for discontinuing VPA one week later.
Crude rates of major birth defects excluding genetic disorders were calculated by the number of live-born infants and fetuses affected with major birth defects divided by all live-born infants plus pregnancy losses with major birth defects. Effect estimation for major birth defects regarding VPA treatment, discontinuation groups (early discontinuation ≤ GW 5 + 0, moderate discontinuation GW 5 + 1 to 7 + 0, late discontinuation GW 7 + 1 to 12 + 6 and no discontinuation in first trimester ≥ GW 13 + 0), maximum dose in first trimester, mono- and polytherapy, and for preterm birth was based on odds ratios (OR) using logistic regression. ORs for affected EUROCAT organ systems and subgroups of congenital anomalies38 were calculated if there were more than three events per category. Extreme values of maximum doses due to suicide attempts (30,000 mg/day, 18,000 mg/day and 9000 mg/day) were excluded from descriptive statistics and logistic regression models. Dose category groups based on the maximum dose in first trimester were chosen according to Tomson et al.12 (< 700 mg/day, 700 to < 1500 mg/day, ≥ 1500 mg/day).
Birth weight and head circumference were evaluated using the percentile values derived from the German perinatal survey46,47. The effect of VPA was assessed using the standard deviation score (SDS) in a linear regression model. All regression models are presented with 95% confidence intervals (CI).
To reduce possible confounding, all regression models were adjusted for the same set of relevant baseline covariates (labeled in Table S2) by either incorporating the covariates directly in the regression models or by using a stratified analysis based on the quintiles of the propensity score (PS)48. Imputation of missing values and estimation of the PS was performed based on standard procedures by Embryotox44. R version 4.0.2 (R Core Team) was used.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Results
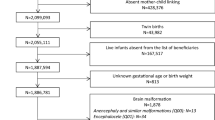
During the study period 1997–2016, n = 484 VPA exposed pregnancies met the study criteria. These were compared with n = 1446 prospectively ascertained pregnancies not exposed to VPA or other teratogens (Fig. S1).
Maternal characteristics
Maternal characteristics differed significantly between the VPA and comparison cohort (Table S2). Women in the VPA cohort were younger (29 years vs. 32 years), had a higher BMI (24.4 vs. 22.8), a lower level of advanced education (28.2% vs. 62.1%) and smoked more (26.3% vs. 12.1%). They had less frequently planned their pregnancy (78.4% vs. 90.7%), had a higher rate of previous ETOP (12.7% vs. 6.8%) and more often previous children with major birth defects (2.7% vs. 0.7%). In addition, they started folic acid intake less often preconception (24.6% vs. 42.1%) and the first contact to Embryotox was earlier (GW 7 + 6 vs. GW 8 + 6).
Treatment indications and exposure to VPA
Epilepsy was the most frequent treatment indication for VPA (69%), followed by bipolar disorder (11%), and other psychiatric disorders or migraine; further information on treatment indication is listed in Table S3. Discontinuation status in first trimester was known for the majority of women (89%, n = 430/484). Almost all women were already on VPA exposure at the beginning of pregnancy (96%, n = 467/484) and the majority of women took valproate on a regular basis (98%, n = 476/484). Nearly half of the women (42%, n = 179/430) discontinued VPA in the first trimester (median GW 5 + 6, IQR GW 4 + 6 − 7 + 0), and additional 22 women discontinued after the first trimester (Fig. S2).
VPA treatment during first trimester was monotherapy in 68% (n = 292/428) and polytherapy in 32% (n = 136/428). For the remaining n = 56 pregnancies, assignment to mono- or polytherapy was inconclusive. Lamotrigine was the most frequent substance used in a polytherapy regimen (47%, n = 64), followed by levetiracetam (16%, n = 22) and carbamazepine (13%, n = 18). Additional substances of polytherapy are summarized in Table S4. In contrast to other treatment indications, women with epilepsy were almost six times longer exposed during pregnancy (median duration 250 days), less often discontinued VPA in first trimester (23% vs. 60–94%) and used VPA more often in antiepileptic polytherapy regimen (41% vs. 10–14%) (Table S3).
Pregnancy outcome
Pregnancy outcomes are summarized in Table 1. The risk for SAB was slightly but not significantly increased after VPA exposure (Table 1, Fig. S3). Cumulative incidences for SAB were independent of the time of VPA discontinuation (early < GW 5 + 0; moderate GW 5 + 1 to 7 + 0; late ≥ GW 7 + 1) (Fig. 1). The results are in line with the Cox proportional hazards models including discontinuation as a time-varying covariate. The risk of SAB was not increased for continued exposure to VPA in first trimester in contrast to discontinuation [HR 0.71 (95% CI 0.35–1.46)], and was not increased by later discontinuation time in the subset of women with first trimester discontinuation [HR 1.07 (95% CI 0.79–1.45)].
Stacked cumulative incidences for the pregnancy outcomes live birth, spontaneous abortion (SAB), elective termination of pregnancy (ETOP) and stillbirth stratified by early (≤ gestational week (GW) 5 + 0), moderate (GW 5 + 1 ≤ GW 7 + 0) and late (still exposed at GW 7 + 1) treatment discontinuation with the number of pregnancies at risk for each gestational day (dotted line). For multiple pregnancies with identical outcomes, only one outcome was considered. Pregnancies with incomplete information of discontinuation (seven live births, seven SAB, 13 ETOP) and pregnancies ending before GW 7 + 1 (three SAB) were excluded.
The rate for ETOP was significantly higher after VPA exposure (Table 1, Fig. S3) and there was a slight increase for ETOP with longer duration of VPA treatment (Fig. 1).
Neonatal characteristics
Neonatal parameters are summarized in Table S5. The rate of preterm birth was higher in the VPA cohort but not statistically significant after adjustment. Birth weight and head circumference were lower in the VPA cohort but adjusted SDS differences were not statistically significant.
Birth defects
Major birth defects, affected organ systems and subgroups are shown in Table 2. Overall, the risk of major birth defects after VPA exposure was significantly increased and in particular for defects within the group nervous system in general, severe microcephaly, urinary system defects and genital defects (driven by a statistically significant increase in the risk of hypospadias in male infants). The risks for spina bifida, cardiac defects in general, atrial septal defects, oro-facial clefts, limb disorders overall, limb reduction defects, polydactyly and the group of other anomalies/syndromes were increased, but statistical significance was not reached. Details of VPA exposed pregnancies with major birth defects are summarized in Table S6.
The first trimester maximum daily dose was higher for women with affected children than for women with children without major birth defects (median 1200 mg/day vs. 900 mg/day). Table 3 demonstrates the rising birth defect risks with higher daily doses, with the highest risk associated with exposure ≥ 1500 mg/day [ORadj 5.41 (95% CI 2.32–12.66)]. By using a logistic regression model, an increase of the maximum dose by 100 mg/day raised the risk of major birth defects by 15% [OR 1.15 (95% CI 1.08–1.23)] (Fig. S4). Effects of VPA in antiepileptic polytherapy versus VPA monotherapy are summarized in Table 4 and Table S7. In comparison with the unexposed cohort, major birth defect risks were increased for both polytherapy [ORadj 3.87 (95% CI 1.89–7.92)] and monotherapy [ORadj 2.36 (95% CI 1.27–4.38)] (Table S7). However, for mono- and polytherapy (excluding teratogenic co-medication) with VPA dose < 1000 mg/day, the risk was not significantly increased (Table 4).
Treatment discontinuation
Effects of VPA discontinuation time during the first trimester on the risk of major birth defects are shown in Table 5 and Table S8. In contrast to the comparison cohort, the risk increase reached statistical significance when VPA was not discontinued during the first trimester of pregnancy [ORadj 3.66 (95% CI 2.04–6.54)] (Table 5). However, women who discontinued VPA in the first trimester had a lower maximum dose of VPA compared to women who continued treatment of VPA throughout the first trimester [900 mg/day (IQR 500–1012.5 mg/day) vs. 1000 mg/day (IQR 600–1200 mg/day)]. Moreover, women with VPA monotherapy more frequently discontinued VPA in the first trimester than women on VPA polytherapy [39% (n = 98/251) vs. 31% (n = 29/95)], and only 22% (n = 57/257) discontinued within the epilepsy cohort. To address these confounders, several sensitivity analyses within the valproate exposed cohort were performed (Table S8). The risk of major birth defects was increased for continued treatment throughout first trimester in contrast to discontinuation until GW 13 [continued treatment 26/227 (11.5%) vs. discontinuation 8/150 (5.3%); OR 2.30 (95% CI 1.01–5.22)], and remained within the same range after adjusting for maximum dose, treatment indication, and mono- versus polytherapy, but did not reach statistical significance (Table S8).
Discussion
This is the first cohort study analyzing the effect of VPA discontinuation at different gestational stages during first trimester on the risk of SAB and congenital birth defects.
The overall risk of major birth defects determined by other studies varied between 6 and 15%1,2,3,4,5,6,7,8,9,10, which is in line with the rate of 8.7% in our study. Similar to other studies2,6,9,10,11 we found significantly increased risks for birth defects of the nervous system overall, the urinary system and particularly hypospadias in male infants. In addition, we observed increased ORs for spina bifida, the group of congenital heart defects, oro-facial clefts, limb reduction defects and polydactyly, although they did not reach statistical significance possibly due to small numbers of events. A finding not yet reported by other working groups is the significantly increased rate of severe microcephaly [5/385 (1.3%) vs. 3/1299 (0.2%), ORadj 6.65 (95% CI 1.17–37.68)]. At Embryotox, the diagnosis of microcephaly is based on reliable head circumference measures and not limited to formal coding of microcephaly, which may have been missed in clinical practice. It is worth mentioning, however, that severe microcephaly did not occur isolated in all cases, i.e. additional organ systems were affected (P0309 and P0600, Table S6). In addition, in four out of five infants affected with severe microcephaly, VPA monotherapy during the first trimester was reported (Table S6). All infants affected with severe microcephaly were born at term, while three infants were additionally small for gestational age (Table S6). To the best of our knowledge, only Jentink et al.10 reported an elevated but not significant risk for severe microcephaly [ORadj 2.5 (95% CI 0.3–9.7)]. More recently, Blotíere et al.2 investigated the risk of severe microcephaly after monotherapy of ten different antiepileptic drugs, but could not find an increased risk for VPA. However, definition of severe microcephaly (both microcephaly < 3 SD without craniosynostosis (MedDRA Q02), but in addition with brain MRI by Blotiére et al.), time-point of diagnosis (at 24 months vs. at birth by Embryotox), and type of data (ICD-10 coding of health care data vs. measured head circumference at Embryotox) complicates a direct comparison.
It has been widely shown that the risk of birth defects is higher with increasing daily dose of VPA, although applied dose categories and associated risks vary from study to study3,4,5,6,8,9,12. Our study results are consistent with the risks and dose-categories applied by Tomson et al.4,12. However, we found slightly lower risks than their working group for VPA < 700 mg/day and ≥ 1500 mg/day4,12, which might be explained by differences in inclusion and exclusion criteria and length of follow-up. We omitted four cases with major birth defects from the analysis because of imprecise specification of the high dose (≥ 1000 mg/day). The effect of the maximum dose in first trimester was modelled linearly using a logistic regression. Based on this calculation, a risk increase of 15% was shown for each additional 100 mg/day VPA in the first trimester (Fig. S4). Risk calculation based on VPA plasma concentrations would have been advantageous but these data were not available.
AED polytherapy involving VPA has been associated with higher risks of birth defects than monotherapy3,12,13,14,15. We also found higher risk for VPA polytherapy than monotherapy (12.3% vs. 7.8%), which were both significantly increased compared to the non-exposed cohort. However, when assigning cases to either < 1000 mg/day or ≥ 1000 mg/day VPA, risks for mono- and polytherapy were comparable when teratogenic co-medication was excluded (Table 4). In contrast, birth defect risk was the highest when co-medication included teratogens, irrespective of the maximum VPA dose. The results of the present study support previous findings of Tomson et al.12 that VPA dose is of utmost importance in both mono- and polytherapy and that monotherapy in high doses might be more hazardous than polytherapy with lower VPA doses.
The most striking result of our study was the time-dependence of birth defect risk with a more than threefold risk increase compared to the non-exposed cohort when VPA treatment was not discontinued in the first trimester [11.5% vs. 3.4%, ORadj 3.66 (95% CI 2.04–6.54)]. If VPA treatment was discontinued up to GW 5 + 0 the risk of major birth defects was only slightly increased (Table 5). The effect of continued treatment throughout the first trimester in contrast to treatment discontinuation remained significantly increased when comparing women in the VPA exposed cohort (Table S8, unadjusted OR). Several sensitivity analyses were performed to adjust for differences in maximum dose, mono- and polytherapy and treatment indication. The effects of continued treatment throughout the first trimester remained within the same range, but did not reach statistical significance possibly due to small event counts (Table S8). In addition, we were only able to adjust for one confounder at a time. Therefore, larger studies are needed to further investigate the effect of treatment discontinuation in the first trimester in a more complex setting. Moreover, we cannot rule out that treatment discontinuation is linked to other unmeasured risk factor such as severity of maternal disease, which might have biased our results.
Lower birth defect risk with early discontinuation during the first trimester was also observed with other teratogens such as vitamin K-antagonists44. However, in contrast to vitamin K-antagonists, no recommendations have been defined for AED treatment changes during pregnancy. Instead, a careful risk–benefit assessment is required in each individual patient, taking into account relapse risk in cases, where VPA has been shown to be the most effective treatment26,27,28,29,30,31,32,33.
In contrast to birth defects, there are only limited and conflicting data available regarding the risk of SAB associated with VPA. While some studies found increased risks for SAB18,19,20,22, others did not1,9,21. Our study neither indicates an overall increased rate of SAB [HRadj 1.31 (95% CI 0.85–2.02)], nor dependence on treatment discontinuation. However, the high rate of ETOP as a competing event possibly results in an underestimation of SAB risk. A larger proportion of unwanted pregnancies, fear of VPA fetotoxicity, and severity of the underlying maternal disease may have contributed to the high ETOP rate among VPA exposed women. A limitation of our study is that information on treatment duration obtained through follow-up questionnaire was less complete in cases of pregnancy losses than in live births. Furthermore, estimation of gestational week in early pregnancy is mainly based on the first day of the last menstrual period (LMP), which might lead to slight inaccuracies in the estimation of pregnancy duration. A possible association between time of VPA discontinuation and SAB risk may therefore remain undetected.
Further strengths and limitations for pregnancy outcome studies using observational data were discussed in detail by Schaefer et al.36. Both cohorts of our study were collected following the Embryotox standardized data recording and processing protocol, making a substantial bias unlikely. Although the lost-to-follow-up rate is about 18%, Stegherr et al.49 showed that the difference in baseline covariates between response and non-response in the Embryotox cohort is unlikely to confound study results of drug toxicity. In addition, pregnant women registered at Embryotox may not be representative of pregnant women on VPA in Germany in general. For example, higher educational level of women who themselves or their treating physician seeking advice at the Embryotox center has already been demonstrated by Beck et al.50. On the other hand, in the study cohort analyzed here treatment indications for VPA are very similar for women of reproductive age in Germany as shown by claims data51.
Conclusion
Our data suggest that treatment discontinuation of VPA in the first trimester may lower the risk of major birth defects. If VPA treatment during pregnancy cannot be avoided, the lowest possible dose should be prescribed in either mono- or polytherapy to achieve seizure control in patients with epilepsy.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Seshachala, B. B. et al. Valproate usage in pregnancy: An audit from the Kerala Registry of epilepsy and pregnancy. Epilepsia 62(5), 1141–1147 (2021).
Blotiere, P. O. et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology 93(2), e167–e180 (2019).
Vajda, F. J. E. et al. Antiepileptic drugs and foetal malformation: Analysis of 20 years of data in a pregnancy register. Seizure 65, 6–11 (2019).
Tomson, T. et al. Comparative risk of major congenital malformations with eight different antiepileptic drugs: A prospective cohort study of the EURAP registry. Lancet Neurol. 17(6), 530–538 (2018).
Thomas, S. V., Jose, M., Divakaran, S. & Sankara, S. P. Malformation risk of antiepileptic drug exposure during pregnancy in women with epilepsy: Results from a pregnancy registry in South India. Epilepsia 58(2), 274–281 (2017).
Campbell, E. et al. Malformation risks of antiepileptic drug monotherapies in pregnancy: Updated results from the UK and Ireland epilepsy and pregnancy registers. J. Neurol. Neurosurg. Psychiatry 85(9), 1029–1034 (2014).
Vajda, F. J., O’Brien, T. J., Graham, J. E., Lander, C. M. & Eadie, M. J. Dose dependence of fetal malformations associated with valproate. Neurology 81(11), 999–1003 (2013).
Hernandez-Diaz, S. et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology 78(21), 1692–1699 (2012).
Diav-Citrin, O. et al. Pregnancy outcome after in utero exposure to valproate: Evidence of dose relationship in teratogenic effect. CNS Drugs 22, 325–334 (2008).
Jentink, J. et al. Valproic acid monotherapy in pregnancy and major congenital malformations. N. Engl. J. Med. 362(23), 2185–2193 (2010).
Lindhout, D. & Meinardi, H. Spina bifida and in-utero exposure to valproate. Lancet 2(8399), 396 (1984).
Tomson, T. et al. Dose-dependent teratogenicity of valproate in mono- and polytherapy: An observational study. Neurology 85(10), 866–872 (2015).
Vajda, F. J. E. et al. Antiepileptic drug polytherapy in pregnant women with epilepsy. Acta Neurol. Scand. 138, 115 (2018).
Keni, R. R. et al. Teratogenicity of antiepileptic dual therapy: Dose-dependent, drug-specific, or both? Neurology 90(9), e790–e796 (2018).
Holmes, L. B., Mittendorf, R., Shen, A., Smith, C. R. & Hernandez-Diaz, S. Fetal effects of anticonvulsant polytherapies: Different risks from different drug combinations. Arch. Neurol. 68(10), 1275–1281 (2011).
Meador, K. J. et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): A prospective observational study. Lancet Neurol. 12(3), 244–252 (2013).
Whelehan, A. & Delanty, N. Therapeutic strategies for treating epilepsy during pregnancy. Expert Opin. Pharmacother. 20(3), 323–332 (2019).
Trivedi, M., Jose, M., Philip, R. M., Sarma, P. S. & Thomas, S. V. Spontaneous fetal loss in women with epilepsy: Prospective data from pregnancy registry in India. Epilepsy Res. 146, 50–53 (2018).
Thomas, S. V., Sindhu, K., Ajaykumar, B., Sulekha Devi, P. B. & Sujamol, J. Maternal and obstetric outcome of women with epilepsy. Seizure 18(3), 163–166 (2009).
Pittschieler, S. et al. Spontaneous abortion and the prophylactic effect of folic acid supplementation in epileptic women undergoing antiepileptic therapy. J. Neurol. 255(12), 1926–1931 (2008).
Tomson, T. et al. Antiepileptic drugs and intrauterine death: A prospective observational study from EURAP. Neurology 85(7), 580–588 (2015).
Bech, B. H. et al. Use of antiepileptic drugs during pregnancy and risk of spontaneous abortion and stillbirth: Population based cohort study. BMJ 349, g5159 (2014).
European Medicines Agency. Assessment Report. Procedure Under Article 31 of Directive 2001/83/EC Resulting from Pharmacovigilance Data. Substances Related to Valproate. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Valproate_and_related_substances_31/Recommendation_provided_by_Pharmacovigilance_Risk_Assessment_Committee/WC500177352.pdf (2014).
European Medicines Agency. New Measures to Avoid Valproate Exposure in Pregnancy Endorsed. https://www.ema.europa.eu/en/news/prac-recommends-new-measures-avoid-valproate-exposure-pregnancy (2018).
Tomson, T., Battino, D. & Perucca, E. Valproic acid after five decades of use in epilepsy: Time to reconsider the indications of a time-honoured drug. Lancet Neurol. 15(2), 210–218 (2016).
Vajda, F. J. E. et al. Pregnancy after valproate withdrawal—Fetal malformations and seizure control. Epilepsia 61(5), 944–950 (2020).
Vajda, F. J. E. et al. The outcome of altering antiepileptic drug therapy before pregnancy. Epilepsy Behav. 111, 107263 (2020).
Cerulli Irelli, E. et al. Doing without valproate in women of childbearing potential with idiopathic generalized epilepsy: Implications on seizure outcome. Epilepsia 61(1), 107–114 (2020).
Vajda, F. J. E. et al. Valproate-associated foetal malformations—Rates of occurrence, risks in attempted avoidance. Acta Neurol. Scand. 139(1), 42–48 (2019).
Tomson, T. et al. Declining malformation rates with changed antiepileptic drug prescribing: An observational study. Neurology 93(9), e831–e840 (2019).
Tomson, T. et al. Valproate in the treatment of epilepsy in girls and women of childbearing potential. Epilepsia 56(7), 1006–1019 (2015).
Perucca, P., O’Brien, T. J., Eadie, M. & Vajda, F. J. Valproate still has a place in women with epilepsy. Epilepsia 56(7), 1175–1176 (2015).
Van McCrary, S. Commentary: Revised recommendation from CMDh on use of valproate in women is ethically incomplete and neglects the interests of women. Epilepsia 56(7), 1020–1022 (2015).
Tomson, T. et al. Withdrawal of valproic acid treatment during pregnancy and seizure outcome: Observations from EURAP. Epilepsia 57(8), e173–e177 (2016).
Dathe, K. & Schaefer, C. Drug safety in pregnancy: The German Embryotox institute. Eur. J. Clin. Pharmacol. 74(2), 171–179 (2018).
Schaefer, C., Ornoy, A., Clementi, M., Meister, R. & Weber-Schoendorfer, C. Using observational cohort data for studying drug effects on pregnancy outcome—Methodological considerations. Reprod. Toxicol. 26(1), 36–41 (2008).
von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 370(9596), 1453–1457 (2007).
European Surveillance of Congenital Anomalies (Version 28/12/2018). Complete EUROCAT Guide 1.4 and Reference Documents. https://eu-rd-platform.jrc.ec.europa.eu/sites/default/files/Full_Guide_1_4_version_28_DEC2018.pdf (2019).
Meister, R. & Schaefer, C. Statistical methods for estimating the probability of spontaneous abortion in observational studies—Analyzing pregnancies exposed to coumarin derivatives. Reprod. Toxicol. 26(1), 31–35 (2008).
Kalbfleisch, J. D. & Prentice, R. L. The Statistical Analysis of Failure Time Data 2nd edn. (Wiley, 2011).
Beyersmann, J., Allignol, A. & Schumacher, M. Competing Risks and Multistate Models with R (Springer, 2011).
Bluhmki, T. et al. Multistate methodology improves risk assessment under time-varying drug intake—A new view on pregnancy outcomes following coumarin exposure. Pharmacoepidemiol. Drug Saf. 28(5), 616–624 (2019).
van Houwelingen, H. & Putter, H. Dynamic Prediction in Clinical Survival Analysis 1st edn. (CRC Press, 2011).
Huttel, E., Padberg, S., Meister, R., Beck, E. & Schaefer, C. Pregnancy outcome of first trimester exposure to the vitamin K antagonist phenprocoumon depends on duration of treatment. Thromb. Haemost. 117(5), 870–879 (2017).
Matok, I., Azoulay, L., Yin, H. & Suissa, S. Immortal time bias in observational studies of drug effects in pregnancy. Birth Defects Res. 100(9), 658–662 (2014).
Voigt, M. et al. New percentile values for the anthropometric dimensions of singleton neonates: Analysis of perinatal survey data of 2007–2011 from all 16 states of Germany. Z. Geburtsh. Neonatol. 218(5), 210–217 (2014).
Voigt, M. et al. New percentile values for the anthropometric dimensions of twin neonates: Analysis of perinatal survey data of 2007–2011 from all 16 states of Germany. Z. Geburtsh. Neonatol. 218(6), 254–260 (2014).
D’Agostino, R. B. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 17(19), 2265–2281 (1998).
Stegherr, R., Beck, E., Hultzsch, S., Schaefer, C. & Dathe, K. Can non-responding mask or mimic drug effects on pregnancy outcome? Evaluation of case characteristics based on the national Embryotox cohort. Reprod. Toxicol. 111, 129–134 (2022).
Beck, E., Lechner, A. & Schaefer, C. Who seeks teratology information service’s advice? Assessing the risk of selection bias in observational cohort studies on drug risks in pregnancy. Reprod. Toxicol. 67, 79–84 (2017).
Wentzell, N. et al. Prescribing valproate to girls and women of childbearing age in Germany: Analysis of trends based on claims data. Bundesgesundh. Gesundh. Gesundh. 61(8), 1022–1029 (2018).
Acknowledgements
The authors would like to thank Angela Kayser for classifying the birth defects according to EUROCAT as the second expert and Katja Meixner for study data cleaning. They additionally thank our Embryotox team who answered the inquiries, and all patients and health care professionals for contacting Embryotox.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was performed with financial support from the German Federal Institute for Drugs and Medical Devices (BfArM).
Author information
Authors and Affiliations
Contributions
A.K.F., M.O., S.P. and K.D. were responsible for data collection and designed the study jointly with C.S. A.K.F. outlined and performed the analysis, and developed the first draft of the manuscript. All authors significantly contributed to the interpretation of the data and revised the manuscript for important intellectual content. Each author approved the final version for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fietz, AK., Onken, M., Padberg, S. et al. Impact of maternal first trimester treatment regimen on the outcome of valproate exposed pregnancies: an observational Embryotox cohort study. Sci Rep 14, 674 (2024). https://doi.org/10.1038/s41598-023-50669-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50669-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.