Abstract
Background
Respiratory care protocol including less invasive śsurfactant administration (LISA) in ≤29 weeks’ gestational age (GA) infants introduced in October 2018.
Methods
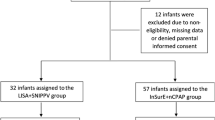
Retrospective study of infants admitted on continuous positive airway pressure (CPAP) October 2018 to December 2021. Maternal and neonatal variables were compared between infants managed on CPAP with and without LISA. Infants who received LISA and subsequently required mechanical ventilation (MV) within 72 h of life (HOL) [LISA failure (LF)] were compared with those who required no MV [LISA success (LS)].
Results
249 infants were admitted on CPAP, 5 were intubated prior to LISA, 143 required LISA and 101 remained on CPAP without surfactant. Of those receiving LISA, 108 were LS and 35 were LF. Compared to LS, LF infants were of lower GA and birth weight, required higher fractional inspired oxygen (FiO2), and CPAP level at birth, admission, one HOL, and an hour after LISA. Moreover, LF infants had higher mortality and morbidity. Together GA ≤ 25 weeks’ and FiO2 ≥ 0.3 an hour after LISA best predicted LF.
Conclusions
Over 80% of infants admitted on CPAP avoided MV within 72 HOL. Early predictors of LF provide targets for future interventions to decrease need for MV in preterm infants.
Impact
-
Less invasive surfactant administration (LISA) decreases the need for mechanical ventilation (MV) and improves outcomes. However, some infants require MV within 72 h of life (HOL) despite LISA (LISA failure).
-
Over 80% of ≤29 weeks’ gestational age (GA) infants can be successfully managed on CPAP with or without surfactant in the first 72 HOL.
-
A combination of factors including ≤25 weeks’ GA and fraction of inspired oxygen ≥0.3 an hour after LISA predict LISA failure.
-
Evaluation of a noninvasive respiratory support strategy including LISA provides targets for intervention to decrease need for MV in preterm infants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Suresh, G. K. & Soll, R. F. Overview of surfactant replacement trials. J. Perinatol. 25, S40–S44 (2005).
Schmolzer, G. M. et al. Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. BMJ 347, f5980 (2013).
Subramaniam, P., Ho, J. J. & Davis, P. G. Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database Syst. Rev. 6, CD001243 (2016).
Stoll, B. J., Hansen, N. I. & Bell, E. F. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 314, 1039–1051 (2015).
Bell, E. F. et al. Mortality, in-hospital morbidity, care practices, and 2-year outcomes for extremely preterm infants in the Us, 2013-2018. JAMA 327, 248–263 (2022).
Morley, C. J. et al. Nasal Cpap or intubation at birth for very preterm infants. N. Engl. J. Med. 358, 700–7008 (2008).
Finer, N. N. et al. Early Cpap versus surfactant in extremely preterm infants. N. Engl. J. Med. 362, 1970–1979 (2010).
Sweet, D. G., Carnielli, V. & Greisen, G. European consensus guidelines on the management of respiratory distress syndrome—2016 update. Neonatology 111, 107–125 (2017).
Kribs, A. et al. Nonintubated surfactant application vs conventional therapy in extremely preterm infants: a randomized clinical trial. JAMA Pediatr. 169, 723–730 (2015).
Göpel, W. et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (Amv): an open-label, randomised, controlled trial. Lancet 378, 1627–1634 (2011).
Kanmaz, H. G., Erdeve, O., Canpolat, F. E., Mutlu, B. & Dilmen, U. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics 131, e502–e509 (2013).
Dargaville, P. A. et al. Effect of minimally invasive surfactant therapy vs sham treatment on death or bronchopulmonary dysplasia in preterm infants with respiratory distress syndrome: the optimist-a randomized clinical trial. JAMA 326, 2478–2487 (2021).
Abdel-Latif, M. E., Davis, P. G., Wheeler, K. I., De Paoli, A. G. & Dargaville, P. A. Surfactant therapy via thin catheter in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database Syst. Rev. 5, CD 011672 (2021).
Kakkilaya, V. & Gautham, K. S. Should less invasive surfactant administration (Lisa) become routine practice in Us Neonatal Units? Pediatr. Res. 93, 1188–1198 (2023).
Herting, E., Härtel, C. & Göpel, W. Less invasive surfactant administration (LISA): chances and limitations. Arch. Dis. Child. Fetal Neonatal Ed. 104, F655–F659 (2019).
Härtel, C. et al. Association of administration of surfactant using less invasive methods with outcomes in extremely preterm infants less than 27 weeks of gestation. JAMA Netw. Open 5, e2225810 (2022).
Kakkilaya, V. B. et al. Decreasing continuous positive airway pressure failure in preterm infants. Pediatrics 148, e2020014191 (2021).
Weiner, G. M. Textbook of neonatal resuscitation. 8th ed. Elk Grove Village, IL: American Academy of Pediatrics and American Heart Association; 2021.
Kakkilaya, V. et al. Quality improvement project to decrease delivery room intubations in preterm infants. Pediatrics 143, e20180201 (2019).
Kakkilaya, V. et al. Early predictors of continuous positive airway pressure failure in preterm neonates. J. Perinatol. 39, 1081–1088 (2019).
Sahni, R. & Wung, J. T. Continuous positive airway pressure (Cpap). Indian J. Pediatr. 65, 265–271 (1998).
Walsh, M. C., Szefler, S. & Davis, J. Summary proceedings from the bronchopulmonary dysplasia group. Pediatrics 117, S52–S56 (2006).
Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500 G. J. Pediatr. 92, 529–534 (1978).
International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch. Ophthalmol. 123, 991–999 (2005).
Härtel, C. et al. Less invasive surfactant administration and complications of preterm birth. Sci. Rep. 8, 8333 (2018).
Janssen, L. C. et al. Minimally invasive surfactant therapy failure: risk factors and outcome. Arch. Dis. Child. Fetal Neonatal Ed. 104, F636–F642 (2019).
Balazs, G. et al. Incidence, predictors of success and outcome of lisa in very preterm infants. Pediatr. Pulmonol. 57, 1751–1759 (2022).
Dargaville, P. A., Aiyappan, A. & Paoli, A. G. Continuous positive airway pressure failure in preterm infants: incidence, predictors and consequences. Neonatology 104, 8–14 (2013).
Gulczyńska, E., Szczapa, T., Hożejowski, R., Borszewska-Kornacka, M. K. & Rutkowska, M. Fraction of inspired oxygen as a predictor of Cpap failure in preterm infants with respiratory distress syndrome: a prospective multicenter study. Neonatology 116, 171–178 (2019).
Lemyre, B., Davis, P. G., De Paoli, A. G. & Kirpalani, H. Nasal Intermittent Positive Pressure Ventilation (NIPPV) Versus Nasal Continuous Positive Airway Pressure (NCPAP) for preterm neonates after extubation. Cochrane Database Syst. Rev. 2, CD003212 (2017).
Buzzella, B., Claure, N., D’Ugard, C. & Bancalari, E. A randomized controlled trial of two nasal continuous positive airway pressure levels after extubation in preterm infants. J. Pediatr. 164, 46–51 (2014).
Kidman, A. M. et al. Protocol for a randomised controlled trial comparing two cpap levels to prevent extubation failure in extremely preterm infants. BMJ open 11, e045897 (2021).
Dobson, N. R. et al. Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants. J. Pediatr. 164, 992–998 (2014).
Lodha, A. et al. Association of early caffeine administration and neonatal outcomes in very preterm neonates. JAMA Pediatr. 169, 33–38 (2015).
Katheria, A. C. et al. A pilot randomized controlled trial of early versus routine caffeine in extremely premature infants. Am. J. Perinatol. 32, 879–886 (2015).
Ines, F. et al. Multicentre, randomised trial of preterm infants receiving caffeine and less invasive surfactant administration compared with caffeine and early continuous positive airway pressure (CaLI trial): study protocol. BMJ 11, e038343 (2021).
Speer, C. P. et al. Randomized European multicenter trial of surfactant replacement therapy for severe neonatal respiratory distress syndrome: single versus multiple doses of curosurf. Pediatrics 89, 13–20 (1992).
Mehler, K. et al. Outcome of extremely low gestational age newborns after introduction of a revised protocol to assist preterm infants in their transition to extrauterine life. Acta Paediatr. 101, 1232–1239 (2012).
Arattu Thodika, F. M. S. et al. Outcomes following less-invasive-surfactant-administration in the delivery-room. Early Hum. Dev. 167, 105562 (2022).
LISA in the Delivery Room for Extremely Preterm Infants, https://clinicaltrials.gov/study/NCT04715373.
Kattwinkel, J. et al. High-versus low-threshold surfactant retreatment for neonatal respiratory distress syndrome. Pediatrics 106, 282–288 (2000).
Acknowledgements
Dr. Kakkilaya acknowledges the support from Parkland Community Health Plan.
Author information
Authors and Affiliations
Contributions
C.S.C. provided substantial contributions to drafting and revising the article for important intellectual content. S.A. provided substantial contributions to the acquisition of data. M.C. provided substantial contributions to the acquisition of data. L.S.B. provided substantial contributions to the analysis and interpretation of data through statistical support. P.B. provided substantial contributions to the acquisition of data. V.S. provided substantial contributions to the acquisition of data. K.G. provided substantial contributions to the acquisition of data. K.M. provided substantial contributions to the acquisition of data. M.A.J. provided substantial contributions to conception and design. M.H.W. provided substantial contributions to conception and design. V.S.K. provided substantial contributions to conception and design. V.K. provided substantial contributions to conception, design, data analysis and interpretation, drafting and revising of the article. All authors gave final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
C.S.C. has no interests to declare. M.C. has no interests to declare. S.A. has no interests to declare. L.S.B. has no interests to declare. P.B. has no interests to declare. V.S. has no interests to declare. K.G. has no interests to declare. K.M. has no interests to declare. M.A.J. has no interests to declare. M.H.W. has no interests to declare. V.S.K. has no interests to declare. He acknowledges support by RO1HD104970-01 grant from the NIH. V.K. has received grant support from Chiesi Pharmaceuticals and SPARK Biomedical, Inc, USA. There is no conflict of interest relevant to this article with any of the entities.
Consent Statement
Patient consent was not required for this retrospective review.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chan, C.S., Chiu, M., Ariyapadi, S. et al. Evaluation of a respiratory care protocol including less invasive surfactant administration in preterm infants. Pediatr Res (2023). https://doi.org/10.1038/s41390-023-02963-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-023-02963-x
This article is cited by
-
To intubate or not to intubate, is that the question?
Pediatric Research (2024)