Abstract
Rosa canina L. (Rosaceae), commonly known as the rose hip, is originated from Europe, Africa, and Asia with a long history in medicinal applications. This study aimed to analyze the morphological traits, fatty acids profile, and content of phenolic compounds, anthocyanins, vitamin C, total carotenoid, total phenol, total flavonoid, and antioxidant activity of the fruits of eleven Iranian R. canina ecotypes (RCEs). The highest coefficient of variation was obtained in 1000 seed weight (46.57%). The seed oil varied from 8.08 ± 0.17% to 16.91 ± 0.35%. Linoleic (35.41 ± 0.78% to 49.59 ± 0.96%) and eicosanoic (17.67 ± 0.06% to 25.36 ± 0.54%) acids were the predominant fatty acids in the studied samples. The anthocyanin content in the fruits was ranged from 0.98 ± 0.03 to 4.41 ± 0.04 mg cyanidin 3-glucoside/100 g of dry weight (mg C3G/100 g DW). The high content of vitamin C (103.51 ± 1.24–419.70 ± 3.12 mg/100 g DW), total carotenoid (111.22 ± 0.78–206.98 ± 1.25 mg β-carotene equivalents per g of dry weight (mg β-CARE/g DW)), total phenol (52.87 ± 0.82–104.52 ± 0.23 mg GAE/g DW), and total flavonoid (14.20 ± 0.12–25.18 ± 0.47 mg RE/g DW) were observed in the studied samples. Catechin (20.42 ± 0.47–19.22 ± 0.13 µg/g DW) was the major phenolic compound. The high antioxidant activity in the fruits of the plant was recorded in the studied RCEs (IC50 = 12.54 ± 0.18–26.33 ± 0.13 μg/ml). A significant correlation between some phytochemical compounds (dependent variable) and morphological features (independent variable) was found. Based on our findings, the fruit of the studied ecotypes can be used for future breeding programs and drug development.
Similar content being viewed by others
Introduction
Nowadays, plants have gained special importance in the process of discovering and developing medications due to their specialized metabolites1,2. The development of spectroscopy in the nineteenth century rendered it possible to detect specialized metabolites in plants, which sped up their application in medicine production3. The separation, identification, and quantification of biologically active compounds in plants play a fundamental role in their use in the pharmaceutical industries4.
The genus Rosa belongs to the Rosaceae family and consists of 100–250 species5. The rose hip (Rosa canina L.) is a permanent and deciduous species whose height is between 2 and 3 m. It has imparipinnate compound leaves with 5–7 toothed leaflets and light pink flowers6. Rose hip is resistant to diverse environmental conditions (poor and rocky soils and water scarcity). So, it grows in wide regions of Europe, Northwestern Europe, and Western Asia. Rosa canica fruits (RCFs) are rich in polyphenols, e.g., flavonoids, anthocyanins, catechin, procyanidin, phenolic acids, including gallic and ellagic acids, kaempferol, apigenin, and resveratrol7,8. It is also an invaluable source of various vitamins, especially vitamin C6. Its fruits contain high levels of carotenoids, tocopherols, minerals (Ca, K, P, Na, Fe, Mn, and Zn), tannins, organic acids, amino acids, and pectin9. Saturated fatty acids (SFA) including palmitic and stearic acid and unsaturated fatty acids (USFA) such as linolenic and linoleic acids were found in the seeds of the rose hip10. Lycopene and β-carotene are the most important carotenoids in its fruits11.
The RCFs are traditionally used to cure arthritis, rheumatism, gout, sciatica, the cold, and infections, such as influenza, prevent gastritis and stomach ulcer, and treat skin diseases and lesions12,13. The most valuable part of the fruit is the pericarp which can be used in various products, such as medicinal products, herbal tea, jam, marmalade, syrup, jelly, and soft drinks, and has recently been employed as an ingredient of probiotic beverages, yogurt, and soup14,15,16. Its seed oil is mainly consumed in the cosmetic and pharmaceutical industries10,17.
The morphological traits of the RHFs, such as fruit weight and length, flesh percentage, and thickness are important traits, and their measurement and selection can help develop new cultivars18. The morphological and phytochemical diversity that is found in different types of plants is due to the interaction between environmental and genetic conditions. Phytochemical diversity is an important part of yield diversity. Therefore, adequate knowledge of the diversity of yield and economic traits is necessary for assessing genotypes and designing efficient breeding processes to achieve the breeding goals19. Research has revealed high phytochemical diversity in different Rosa species11,15,20,21,22. The breeding programs of the Rosa species have recently focused on the quality and quantity of bioactive compounds, e.g. vitamin C and phenol compounds of fruits23,24. Recently, the use of RCFs and their products is increasing25. It is important to include wild species that have valuable compounds in breeding programs. The present study analyzed the phenolic compounds, vitamin C content, total carotenoid content (TCC), seed oil yield, and fatty acids in different RCEs in Iran and introduced the best ecotypes for the initiation of breeding programs, cultivation, domestication, and application in pharmaceutical and food industries.
Materials and methods
Chemicals
The chemicals used in this study were in analytical grade. Standards and trifluoroacetic acid were supplied from Merck (Darmstadt, Germany). Butylated hydroxytoluene, Folin-Ciocalteu’s reagent, boron trifluoride, hydroxide potassium, sodium hydroxide, sodium nitrite, aluminum chloride, sodium carbonate, metaphosphoric acid, n-hexane, acetone, diethyl ether, methanol, and ethanol were purchased from Sigma-Aldrich company (USA).
Plant material
Fruits of the eleven RCEs were collected from eight Provinces of Iran. The geographical coordinates of the studied areas were shown in Table 1. The fruits were harvested at full ripening time. The distances of 2000 m were considered between the ecotypes in each collection region to avoid sampling clones of the chosen ecotypes. The samples were identified by Prof. Ali Sonboli, and voucher specimens were deposited at the Shahid Beheshti University herbarium (Table 1). The authors confirm that the necessary permissions to collect the samples have been obtained and also the present study complies with the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora.
Morphological analysis
Morphological traits were measured on eleven samples. The data were measured on 30 randomly selected fruit from each ecotype for six quantitative characteristics; fruit length (cm), fruit width (cm), fruit weight (g), pericarp weight (g), 1000 seed weight (g), and seed number per fruit (number). The weight for fruit, pericarp, and 1000 seed was recorded using an electronic balance (0.01 g precision).
Phytochemical analysis
Fatty acid analysis
Crude oil content was determined by the maceration method26. Initially, 500 mg dried powdered seed sample was added with 5 ml n-hexane and ultrasonicated for 60 min. The mixture was left at 23 ± 2 °C for 72 h and filtered with Whatman filter paper No. 1. n-Hexane was evaporated at room temperature. The crude oil was kept in airtight, colored bottles at − 18 °C until further analysis. The plant seed oils were esterified as methyl esters before analysis27 and then injected into a gas chromatography mass spectrometry (GC–MS) system (Agilent Technologies, 7890A, USA). The GC–MS system was installed with a universal column (HP5; 30 m, 0.325 mm, 0.25 μm; Agilent J&W GC column). Helium was used as carrier gas at a flow rate of 1.2 ml/min. The column temperature was increased from 150 to 240 °C at 3 °C/min and maintained for 20 min. The samples (1 ml) were injected in the split mode of 1:100. Determination and identification of fatty acids were used in the reference samples (NU-CHEK-PREP company, USA).
Extraction of phenolic compounds and HPLC analysis
Phenolic compound extraction was quantified as described by Demir et al.22. Concisely, 1 g powdered pericarp was ultrasonicated (Elma, S120H, Germany) with 100 ml methanol/water/ trifluoroacetic acid (90:10:0.02 v/v/v) for 30 min and centrifuged (Centrifuge Rotanta 460r, Hettich, Germany) at 1400g for 10 min at 4 °C. The extract was dried in a rotary evaporator (Heidolph Instruments GmbH, Schwabach Germany) at 35 °C. The extract was solved in 1 ml methanol and then filtered (0.22 µm). Phenolic compounds were determined using a high-performance liquid chromatography-photodiode array, with a Waters 2695 separations module equipped with a C18 column (250 × 4.6 mm) and a UV detector (Waters 2487). Mobile phases were methanol/water/trifluoroacetic acid (90:10:0.02 v/v/v) with 0.5 ml/min flow rate. Calibration curves were constructed by injecting standard mixture solutions at the seven concentrations of 2, 10, 50, 100, 250, 500, and 1000 ppm.
Total carotenoid content
Total carotenoid content (TCC) was measured according to the procedure detailed by Ghazghazi et al.11. Briefly, 1 g powdered pericarp was mixed with acetone/methanol/petroleum ether (3:2:1 v/v/v) and kept at ambient temperature for 5 h in the dark. The extract was filtered with Whatman filter paper. The extract was partitioned with 50 ml diethyl ether and dried in a rotary at 35 °C. The dry extract was solved in 10 ml ethanol and mixed with 60% potassium hydroxide and boiled for 10 min. The extract was partitioned with diethyl ether. The diethyl ether fraction was evaporated and the dry extract was dissolved in 10 ml ethanol. The absorbance was recorded at 470 nm, using a spectrophotometer (Shimadzu double beam UV–visible spectrophotometer-1800, Japan). The date was expressed as mg β-carotene equivalents per 100 g of dry weight (mg β-CARE/100 g DW).
Anthocyanins content
Evaluation of anthocyanin content was performed by the pH differential method9. Initially, 100 mg of dried powdered pericarp was added to 5 ml methanol/hydrochloric acid (1:1 v/v, pH = 2). Then 4 ml buffer solution (pH = 1) was mixed with 1 ml extract (pH = 4.5). The absorbance was calculated at wavelengths of 526 and 700 nm, using a spectrophotometer (Bio-Tek Instruments, Inc., USA). The anthocyanin content was calculated as follows equation:
MEC is the molar extinction coefficient (26,900 L/Mcm for cyanidin 3-glucoside), MW is the molecular weight (449.2 g/M for cyanidin 3-glucoside).
Data expressed as mg cyanidin 3-glucoside/100 g of dry weight (mg C3G/100 g DW).
Vitamin C assay
The AOAC28 method was used for vitamin C determination with the ascorbic acid standard. Initially, 1 g of powdered pericarp was mixed with 1 ml of metaphosphoric acid (3%) and centrifuged (Centrifuge Rotanta 460r, Hettich, Germany) at 1400 g for 10 min. The extract was titrated against 2,6-dichlorophenolindophenol dye solution (0.3%) to faint pink color. The amount of vitamin C was measured as follows formula:
\({\text{Vitamin C }}\left( {{\text{mg/}}100{\text{ g DW}}} \right) = \left( {{\text{A}}/{\text{B}}} \right) \times 100.\) A is the (Standard concentration (mg/ml) × Titre value of the sample (ml) × 10, B is the Titre value of standard (ml) × Sample volume (ml) × Sample weight (mg).
Total phenol and flavonoid content and antioxidant activity
Total phenol content (TPC) was determined as described previously by Singleton29. In summary, 25 μl pericarp methanolic extract (1000 ppm) and 125 μl Folin-Ciocalteu reagent, 100 μl sodium carbonate (7.5%) were taken in a test tube. The final volume was made up to 6 ml with distilled water. The solution was stored for 30 min in the dark. The absorbance was recorded at 765 nm using a spectrophotometer. The results are expressed as mg gallic acid equivalents (GAE)/per g of dry weight (mg GAE/g DW).
The total flavonoid content (TFC) was determined as described by Chang et al.30. Initially, 20 μl pericarp methanolic extract, 3.4 ml methanol (30%), 80 μl distilled water, 6 μl sodium nitrite (0.5 M), 6 μl aluminum chloride h (0.3 M) and 80 μl sodium hydroxide (1.0 M) was taken in a test tube and mixed well. The absorbance of the solution was determined against the reagent blank at 510 nm wavelength. The data were expressed as mg of rutin equivalents (RE) per g of dry weight (mg RE/g DW).
Antioxidant activity by the DPPH method was evaluated by Blois methods31. Briefly, 0.2 ml of methanolic extract and 4.0 ml DPPH solution was mixed into the test tube and incubated at room temperature for 20 min. The reduction of the DPPH radical was read using a spectrophotometer at 517 nm. Butylated hydroxytoluene was used as the control. The IC50 values were calculated as follows:
Abs0 is the absorbance of the control, Abs1 is the absorbance of the sample.
Statistical analysis
All the experiments in this study were performed in triplicate. The obtained results are expressed as the means ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to calculate significant differences between studied ecotypes in terms of the traits measured with SPSS 16.0 (SPSS Inc., Chicago, IL, USA). A post-hoc test was run using Duncan’s test at p < 0.05. Cluster analysis was drawn using Euclidean distance coefficient and Ward’s method. The correlation between two sets of data was performed by multiple regression analysis, using the “stepwise” method of “linear regression analysis”. The Origin software version 2021 was applied to draw the heat map, and correlation plot, and biplot. Canonical correspondence analysis (CCA) was estimated with PAST 4.13 software.
Results and discussion
Morphological features
The morphological traits of rose hips varied significantly among different RCEs (Table 2, Fig. 1). The coefficient of variation (CV%) was estimated at 21.13, 19.89, 33.92, 43.70, 31.92, and 46.57 for the traits of fruit length, fruit width, fruit weight, pericarp weight, number of seeds per fruit, and 1000 seed weight, respectively. A higher CV indicates a wider range of trait values, which offers more opportunities for selection32. The studied ecotypes differed in the morphological traits significantly (p < 0.05). The RC8 ecotype exhibited the maximum fruit length (2.00 ± 0.073 cm) and width (1.40 ± 0.026 cm), while the RC10 ecotype showed the minimum fruit length (0.97 ± 0.033 cm) and width (0.77 ± 0.005 cm). The lowest (0.46 ± 0.004) and highest (1.73 ± 0.023) fruit weight to g were recorded for the RC1 and RC8 ecotypes, respectively. The heaviest pericarps were 1.19 ± 0.044 g produced by the RC3 ecotype and the lightest were 0.21 ± 0.003 g produced by the RC1 ecotype. The RC4 ecotype was produced the highest number of seeds per fruit (on average, 43.67 seeds), and RC6 ecotype produced the lowest number (on average, 14.42 seeds). The minimum and maximum of 1000 seed weights were 97.92 ± 2.56 and 596.85 ± 4.55 g related to RC1 and RC3 ecotypes, respectively. The diversity of rose hip traits among RCEs has previously been reported by other researchers so far33,34. Guo et al.35 indicated that the wild edible fruits of the plant have more unique genetic diversity and genetic. Clearly, wild species can enhance the genetic diversity of crops36. The recurrent propagation of the wild fruit seeds in nature increases their genetic diversity37.
The pictures of shrub, fruit, and seed of Rosa canina ecotypes. For a detailed description of the plant ecotypes code, cf. Table 1.
Fatty acid profile
The knowledge about the seed oils and fatty acid profile of rose hip is extremely rare. The results of the GC analysis of some studied RCEs are presented in Table 3. The seed oil was ranged from 8.08 ± 0.17% to 16.91 ± 0.35%. The highest was observed in the RC5 ecotype. Javanmard et al.38 was reported 8.09 ± 0.15% to 11.43 ± 0.31% of the seed oil content in five RCEs.
Nine fatty acids were detected in the studied samples that represented 90.82 ± 0.44–99.90 ± 0.18% of the seed oil. The SFA and USFA ranged from 26.46 ± 0.025% to 36.44 ± 0.18% and from 55.22 ± 0.53% to 70.84 ± 1.11%, respectively. The RC5 and RC4 ecotypes had the lowest and highest SFA percentage, respectively. The maximum of USFA was obtained from the RC6 ecotype. Monounsaturated fatty acids (MUFA) varied from 15.06 ± 0.22% to 29.01 ± 0.15%, whereas polyunsaturated fatty acids (PUFA) varied from 37.44 ± 0.86% to 50.59 ± 0.81%. Eicosanoic acid (from 17.67 ± 0.06% to 25.36 ± 0.54%) was the main SFA in the studied ecotypes. It was the most abundant in the RC8 ecotype. Oleic acid (0.80 ± 0.01% to 19.25 ± 0.25%) was also primary PUFA that the highest level were found in RC3 ecotypes. Figure 2 displays the typical chromatogram of the fatty acids of several ecotypes.
Popovic-Djordjevic et al.26 reported linoleic acid (24.53 ± 0.96% to 46.68 ± 1.34%) and palmitic acid (9.77 ± 0.21% to 35.68 ± 1.22%) in two varieties and six RCEs from Serbia. Kulaitiene et al.39 stated that environmental conditions, genotype, and extraction method were effective on the oil content of R. canina seeds as well as fatty acids compounds. Due to the importance of USFA for human health, the high oil content, and USFA in the seeds of the studied ecotypes, the significance of the studied R. canina is evident.
Total phenol and flavonoid content and antioxidant activity
Figure 3 depicts the range of TPC and TFC for all studied RCEs. The ecotypes was differed in the TPC and TFC significantly (p < 0.05). The TPC was in the range of 52.87 ± 0.82–104.42 ± 0.23 mg GAE/g DW, and the TFC was in the range of 14.20 ± 0.12–25.18 ± 0.47 mg RE/g DW. The lowest TPC and TFC were obtained in RC6 and the highest was found in RC2. According to previous studies, the TPC of fresh fruits from Rosa species in different regions of the world was from 177 to 816 mg GAE/100 g FW40,41. Medveckiene et al.42 were obtained also the TPC of fresh fruits in the range of 150–299 mg GAE/100 g DW in various Rosa species. Jemaa et al.43 reported the TFC in the R. canina rose hip methanolic extract was 2.64 mg RE/g. Nadpal et al.’s44 study on R. canina and R. arvensis Huds. species revealed that the TFC was from 0.63 to 1.48 mg RE/g.
The studied RCEs was differed in TPC and TFC significantly. Polyphenolic compounds can capture free radicals due to their chemical structure and form complexes with metal ions. Therefore, these compounds showed good antioxidant activity. There are some methods to produce polyphenolic compounds in plants and various mechanisms for their distribution across different plant structures. Genetics, environmental conditions, climatic conditions, and the solvent used in extraction are some factors that influence the level of phenolic and flavonoid compounds derived from plants45.
The lower the half maximal inhibitory concentrate (IC50) is in the rose hip extract, the higher the antioxidant activity will be. The highest IC50 (μg/ml) was found in RC4 (26.33 ± 0.13) and RC6 (24.41 ± 0.24) ecotypes and the minimum was found in RC2 (12.54 ± 0.18), RC1 (13.70 ± 0.19), and RC10 (14.15 ± 0.08) ecotypes (Fig. 3).
The studied ecotypes exhibited significant diversity in antioxidant activity, which is consistent with Okatan et al.’s37 study on RCEs. Also, a significant diversity of antioxidant activity was detected among Romanian RCEs46. Shameh et al.47 were found a significant difference (p < 0.05) in the rose hip antioxidant activity between R. hemisphaerica Herrm. and R. canina ecotypes. They attributed this difference to genetics, geographical region, climatic conditions, and the type of sample used. Roby et al.48 have shown the extracts that are rich in phenolic compounds have much stronger antioxidant effects than extracts without these compounds. This study showed similarly that ecotypes containing more phenolic compounds had stronger antioxidant activity.
Total carotenoids, and anthocyanin contents, and vitamin C contents
A wide range of diversity in the TCC was detected among ecotypes (Fig. 4). The TCC was varied from the minimum value of 111.22 ± 0.78 mg β-CARE/g DW in RC4 to the maximum value of 206.98 ± 1.25 mg β-CARE/g DW in RC6.
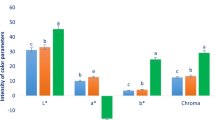
The red-to-blue color of the fruits is caused by their anthocyanins, which have strong anti-inflammatory and antioxidant activities. The studied ecotypes showed significant differences in the anthocyanin content (p < 0.05). The anthocyan in content varied from 0.98 ± 0.03 to 4.41 ± 0.04 mg C3G/100 g DW (Fig. 4). The RC2, RC1, and RC10 ecotypes had the highest anthocyanin contents of 4.41 ± 0.04, 3.98 ± 0.05, and 3.85 ± 0.05 mg C3G/100 g DW, respectively. However, the lowest content (0.98 ± 0.03 mg C3G/100 g DW) was obtained in RC4. The level of anthocyanin in R. canina has been reported to be 2.75, 2.82, and 2.94 mg CGE/100 g by Murathan et al.49, Yildiz and Alpaslan50, and Fascella et al.1, respectively. Szmagara et al.51 estimated the anthocyanin content of dried R. sweginzowii Koehne rose hips at 0.43–7.4 mg CGE/100 g.
The high antioxidant activity of rose hip is related to its high level of vitamin C52. The RCEs differed significantly in the compound (p < 0.05). The vitamin C content varied from 103.51 ± 1.24 to 419.70 ± 3.12 mg/100 g DW in different ecotypes. These values were observed in RC4 and RC2 ecotypes, respectively (Fig. 4). Kayahan et al.52 were reported vitamin C content of R. corymbifera Borkh., R. rugosa (Thunb.), R. alba L., and R. canina genotypes in the range of 180 to 965 mg/100 g. Our results are consistent with the reports of Erogul and Ogus53 and Bilgin et al.54. Kayahan et al.52 were mentioned that genotype is the key factor affecting the vitamin C content of Rosa genotypes.
Phenolic acids
The main phenolic compounds in the rose hips flesh included catechin, quercetin, gallic, chlorogenic, ferulic, p-coumaric, caffeic, 2,5-dihydroxy benzoic, and 4-hydroxy benzoic acid, kaempferol, salicylic acid, and apigenin (Fig. 5). In RC2 ecotype, the highest catechin, quercetin, gallic acid, chlorogenic acid, ferulic acid, p-coumaric acid, and kaempferol content were obtained 20.42 ± 0.47, 13.82 ± 0.04, 13.52 ± 0.21, 12.03 ± 0.13, 11.42 ± 0.12, 10.92 ± 0.45, and 7.32 ± 0.19 µg/g DW, respectively. The RC4 ecotype had the highest level (11.43 ± 0.14 µg/g DW) of caffeic acid whereas the lowest level (5.12 ± 0.03 µg/g DW) was observed in the RC10 ecotype. The 2,5-dihydroxy benzoic and 4-hydroxy benzoic acid content varied from 3.17 ± 0.019 (RC6) to 11.81 ± 0.02 µg/g DW (RC5) and from 3.98 ± 0.01 (RC5) to 11.86 ± 0.09 µg/g DW (RC9), reflecting the high diversity of the studied ecotypes. Salicylic acid content was in the range of 4.13 ± 0.05–5.98 ± 0.07 µg/g DW. The minimum and maximum levels of the salicylic acid were detected in RC8 and RC3 ecotypes, respectively.
Ozturk et al.55 reported that protocatechuic (1.4 mg/100 g), vanillic acid (6.9 mg/100 g), chlorogenic acid (8.5 mg/100 g), p–coumaric acid (24.9 mg/100 g), and ferulic acid (23.9 mg/100 g), and catechin (3.1 mg/100 g), were the most abundant phenolic compounds in R. canina. Shameh et al.47 reported chlorogenic and gallic acid as the most abundant phenolic compounds in rose hips from Iran. Chlorogenic, gallic, p–coumaric, and caffeic acid were the main phenolic compounds in five rose species grown in Turkey22. The catechin content was obtained in the range of 2.37 to 7.83 µg/g in rose hips species by Nadpal et al.15. The quantitative and qualitative differences in phenolic compounds among different RCEs may be related to different genetic and environmental factors (e.g. nutrition, light, and temperature), and maturity stages of rose hip56.
Correlation, cluster, and factor analysis
The relationships between phytochemical traits were calculated by Pearson’s correlation test and displayed by a heat map (Fig. 6). Vitamin C and anthocyanins had positive and significant correlations with linoleic acid, gallic acid, catechin, chlorogenic acid, 2,5–dihydroxy benzoic acid, p–coumaric acid, ferrulic acid, and quercetin had negative and significant correlations with myristic acid. The seed oil content and TCC, two important economic traits, had no significant correlations with other phytochemical traits. The awareness of the relationships between the traits is important for selection in breeding works.
Cluster analysis is a major method to group individuals, populations, and ecotypes in terms of various traits. The ecotypes were put into two main groups according to cluster based on phytochemical characters (Fig. 7). The first cluster (A) had two sub-clusters, the first (AI) including two ecotypes and the second (AII) including six ecotypes. The second cluster (B) contained three ecotypes. The studied ecotypes exhibited significant diversity in phytochemical traits.
According to the results of principal component analysis (PCA), the first to seventh factors accounted for 93.53 percent of the total variance (Table 4). Myristic, linoleic, vaccenic, eicosanoic, gallic, 4–hydroxy benzoic, chlorogenic, caffeic, 2,5–dihydroxy benzoic, p–coumaric, and ferulic acid, catechin, quercetin, apigenin, anthocyanins, vitamin C, TPC, TFC, and DPPH were the traits in the first component that had the highest impact factors and accounted for 40.85 percent of the total variance. The greatest impact factors in the second component were related to 1000 seed weight, lauroleic, palmitic, and salicylic acid, kaempferol, and TCC, which captured 14.72 percent of the total variance.
According to Fig. 8, RC5 ecotype was placed in the first zone of the biplot, which included positive values of both components. It was related to the traits of oil content, ferulic acid, linoleic acid, catechin, vitamin C, quercetin, TPC, chlorogenic acid, and anthocyanin. The second zone of the biplot, hosted RC3, RC6, and RC7 ecotypes, that included negative and positive values of the first and second components, respectively. The group was related to the traits of vaccenic acid, 1000 seed weight, TCC, salicylic acid, pericarp weight, fruit length, and weight, apigenin, and caffeic acid. The third zone of the biplot, in which the values of both components were negative, contained RC4 and RC9 ecotypes. These ecotypes were related to the traits of DPPH, arachidonic acid, myristic acid, 4–hydroxy benzoic acid, seed number per fruit, palmitic acid, and lauroleic acid, and stearic acid. Finally, the fourth zone contained RC10, RC2, RC1, RC8, and RC11 ecotypes, that were related to the traits of TFC, kaempferol, and gallic, p–coumaric, 2,4–dihydroxy benzoic, eicosanoic, and oleic acid. The results of comparing the cluster analysis and PCA revealed similarities between them. The ecotypes which were found by the cluster analysis to be superior in phytochemical traits were put in the same group by PCA.
Multiple regression analysis
The multiple regression analysis showed that the correlation between phenotypic data (as independent traits) and phytochemical traits (as dependent traits) was significant (p < 0.05; p < 0.01). The morphological trait of 1000 seed weight was related to palmitic acid (β = − 599), arachidonic acid (β = 679), and TCC (β = 449), whereas pericarp weight was correlated with vaccenic acid (β = 642), salicylic acid (β = 349), and TCC (β = − 620). In addition, the two variables of fruit length and fruit weight had significant relationships with salicylic acid (β = − 412) and apigenin (β = 722), respectively (Table 5). Therefore, morphological variables were involved in the synthesis and accumulation of these compounds. Khadivi-Khub et al.57, reported the relationship between morphological and phytochemical parameters. Research on the correlation of these traits can help plant breeders in selecting suitable ecotypes.
Canonical correspondence analysis
The CCA was performed to evaluate the correlation between the studied phytochemical compounds and three environmental factors such as mean annual precipitation (MAP), altitude, and mean annual temperature (MAT) (Fig. 9). The RCEs are distributed within the latitude of 30° 22ʹ N to 38° 16 ʹ N and longitude of 45° 34 ʹ E to 50° 55 ʹ E including different geographical regions. The mean rainfall of the RCEs is between 200 and 895 mm/year. The first CCA variable (CCA1) concerning environmental factors presented that MAP had a positive share, while MAT and altitude had a negative share on this CCA construction. The second CCA (CCA2) variable in connection to the phytochemical traits showed that the most of the compounds had a negative share in the formation of CCA variables. Vitamin C, anthocyanins content, and TPC had a positive share with MAP. Geographical conditions, genetic factors, and the different potency to synthesize are involved of specialized metabolite contents56.
Conclusion
Rose hip is used as a raw material in the pharmaceutical, cosmetic, and food industries. Production of modified varieties with desirable agromorphological and phytochemical traits based on the needs of these industries seems to be essential. In the present study, a considerable diversity between the Iranian RCEs was observed in terms of morphological, and phytochemical traits such as fatty acid compounds. This study offered novel information on the fatty acid composition in rose hip seeds and pertinent oils derived, as well as the content of vitamin C, anthocyanins, carotenoid, and phenolic compounds content of the fruit pericarp from Iranian wild RCEs. The initial evaluation of RCEs in terms of morphological, and phytochemical traits can help to introduce suitable genotypes for cultivation and use in the pharmaceutical, food, and cosmetic industries, and also the best parents can be selected for the improvement of this plant and used in breeding programs.
Data availability
The datasets used during the current study available from the corresponding author on reasonable request.
References
Fascella, G. et al. Bioactive compounds and antioxidant activity of four rose hip species from spontaneous Sicilian flora. Food Chem. 289, 56–64. https://doi.org/10.1016/j.foodchem.2019.02.127 (2019).
Mamat, S. F., Azizan, K. A., Baharum, S. N., Noor, N. M. & Aizat, W. M. GC-MS and LC-MS analyses reveal the distribution of primary and secondary metabolites in mangosteen (Garcinia mangostana Linn.) fruit during ripening. Sci. Hortic. 262, 109004. https://doi.org/10.1016/j.scienta.2019.109004 (2020).
Salem, M. et al. Metabolomics in the context of plant natural products research: From sample preparation to metabolite analysis. Metabolites. 10(1), 37. https://doi.org/10.3390/metabo10010037 (2020).
Dabrowska, M., Maciejczyk, E. & Kalemba, D. Rose hip seed oil: Methods of extraction and chemical composition. Eur. J. Lipid Sci. Technol. 121(8), 1800440. https://doi.org/10.1002/ejlt.201800440 (2019).
Lu, M., An, H. & Li, L. Genome survey sequencing for the characterization of the genetic background of Rosa roxburghii Tratt and leaf ascorbate metabolism genes. PLoS One. 11(2), e0147530. https://doi.org/10.1371/journal.pone.0147530 (2016).
Selahvarzian, A., Alizadeh, A., Baharvand, P. A., Eldahshan, O. A. & Rasoulian, B. Medicinal properties of Rosa canina L. Herb. Med. J. 3(2), 77–84. https://doi.org/10.22087/hmj.v3i2.703 (2018).
Dabic Zagorac, D.C., Fotiric Aksic, M.M., Glavnik, V., Gasic, U.M., Vovk, I., Tesic, Z.L. & Natic, M.M. Establishing the chromatographic fingerprints of flavan‐3‐ols and proanthocyanidins from rose hip (Rosa sp.) species. J. Sep. Sci. 43(8), 1431–1439. https://doi.org/10.1002/jssc.201901271. (2020).
Fetni, S., Bertella, N., Ouahab, A., Zapater, J. M. M. & Fernandez, S. D. P. T. Composition and biological activity of the Algerian plant Rosa canina L. by HPLC-UV-MS. Arab. J. Chem. 13(1), 1105–1119. https://doi.org/10.1016/j.arabjc.2017.09.013 (2020).
Tabaszewska, M. & Najgebauer-Lejko, D. The content of selected phytochemicals and in vitro antioxidant properties of rose hip (Rosa canina L.) tinctures. NFS J. 21, 50–56. https://doi.org/10.1016/j.nfs.2020.09.003 (2020).
Ilyasoglu, H. Characterization of rosehip (Rosa canina L.) seed and seed oil. Int. J. Food Prop. 17(7), 1591–1598. https://doi.org/10.1080/10942912.2013.777075 (2014).
Ghazghazi, H. et al. Phenols, essential oils and carotenoids of Rosa canina from Tunisia and their antioxidant activities. Afr. J. Biotechnol. 9(18), 2709–2716 (2010).
Gulbagca, F., Ozdemir, S., Gulcan, M. & Sen, F. Synthesis and characterization of Rosa canina-mediated biogenic silver nanoparticles for anti-oxidant, antibacterial, antifungal, and DNA cleavage activities. Heliyon. 5(12), e02980. https://doi.org/10.1016/j.heliyon.2019.e02980 (2019).
Maitra, S., Satya, P. & De, L. C. A positive outlook towards the lesser known: Wild rose brings hope. IJARB. 2(1), 25–30. https://doi.org/10.20431/2455-4316.0201004 (2016).
Ahmad, N. & Anwar, F. Rose hip (Rosa canina L.) oils. in Essential Oils in Food Preservation, Flavor and Safety, pp. 667–675. (Academic Press, 2016). https://doi.org/10.1016/B978-0-12-416641-7.00076-6.
Nadpal, J. D. et al. Comparative study of biological activities and phytochemical composition of two rose hips and their preserves: Rosa canina L. and Rosa arvensis Huds. Food Chem. 192, 907–914. https://doi.org/10.1016/j.foodchem.2015.07.089 (2016).
Teodorescu, A. A. et al. Customized technological designs to improve the traditional use of Rosa canina fruits in foods and ingredients. Plants. 12(4), 754. https://doi.org/10.3390/plants12040754 (2023).
Jovanovic, A. A. et al. Liposomal bilayer as a carrier of Rosa canina L. seed oil: Physicochemical characterization, stability, and biological potential. Molecules. 28(1), 276. https://doi.org/10.3390/molecules28010276 (2022).
Uggla, M., Gustavsson, K. E., Olsson, M. E. & Nybom, H. Changes in colour and sugar content in rose hips (Rosa dumalis L. and Rosa rubiginosa L.) during ripening. J. Hortic. Sci. Biotechnol. 80(2), 204–208. https://doi.org/10.1080/14620316.2005.11511918 (2005).
Sharma, D. C. et al. Phytochemical evaluation, antioxidant assay, antibacterial activity and determination of cell viability (J774 and THP1 alpha cell lines) of P. sylvestris leaf crude and methanol purified fractions. EXCLI J. 15, 85–94. https://doi.org/10.17179/excli2015-689 (2016).
Barros, L., Carvalho, A. M. & Ferreira, I. C. Exotic fruits as a source of important phytochemicals: Improving the traditional use of Rosa canina fruits in Portugal. Food Res. Int. 44(7), 2233–2236. https://doi.org/10.1016/j.foodres.2010.10.005 (2011).
Adamczak, A., Buchwald, W., Zielinski, J. & Mielcarek, S. Flavonoid and organic acid content in rose hips (Rosa L., sect. Caninae dc. Em. Christ.). Acta Biol. Cracov. Ser Bot. 54(1), 105–112. https://doi.org/10.2478/v10182-012-0012-0 (2012).
Demir, N., Yildiz, O., Alpaslan, M. & Hayaloglu, A. A. Evaluation of volatiles, phenolic compounds and antioxidant activities of rose hip (Rosa L.) fruits in Turkey. LWT Food Sci. Technol. 57(1), 126–133. https://doi.org/10.1016/j.lwt.2013.12.038 (2014).
Fan, C., Pacier, C. & Martirosyan, D. M. Rose hip (Rosa canina L.): A functional food perspective. Funct. Foods Health Dis. 4(12), 493–509. https://doi.org/10.31989/ffhd.v4i12.159 (2014).
Paunovic, D. et al. Assessment of chemical and antioxidant properties of fresh and dried rosehip (Rosa canina L.). Not. Bot. Horti Agrobot. 47(1), 108–113. https://doi.org/10.15835/nbha47111221 (2019).
Patel, S. Rose hip as an underutilized functional food: Evidence-based review. Trends Food Sci. Technol. 63, 29–38. https://doi.org/10.1016/j.tifs.2017.03.001 (2017).
Popovic-Djordjevic, J. et al. Fatty acids in seed oil of wild and cultivated rosehip (Rosa canina L.) from different locations in Serbia. Ind. Crops Prod. 191, 115797. https://doi.org/10.1016/j.indcrop.2022.115797 (2023).
Ozek, G., Chidibayeva, A., Ametov, A., Nurmahanova, A. & Ozek, T. Chemical composition of flower volatiles and seeds fatty acids of Rosa iliensis Chrshan, an endemic species from Kazakhstan. Rec. Nat. Prod. https://doi.org/10.25135/rnp.271.2105.2083 (2021).
AOAC. Official methods of analysis. Association of Official Analytical Chemists. Washington USA. Aromatic intermediates and derivatives. Paris, (22nd Edition). pp. A.IV.1–A.IV.17. (2014).
Singleton, V.L., Orthofer, R. & Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. in Methods in Enzymology (Vol. 299, pp. 152–178). (Academic Press, 1999). https://doi.org/10.1016/S0076-6879(99)99017-1.
Chang, C. C., Yang, M. H., Wen, H. M. & Chern, J. C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 10(3), 3 (2002).
Blois, M. S. Antioxidant determinations by the use of a stable free radical. Nature. 181(4617), 1199–1200. https://doi.org/10.1038/1811199a0 (1958).
Heydari, A. et al. Introduction of Thymus daenensis into cultivation: Analysis of agro-morphological, phytochemical and genetic diversity of cultivated clones. Ind. Crops Prod. 131, 14–24. https://doi.org/10.1016/j.indcrop.2019.01.033 (2019).
Celik, F., Kazankaya, A. & Ercisli, S. Fruit characteristics of some selected promising rose hip (Rosa spp.) genotypes from Van region of Turkey. Afr. J. Agric. Res. 4(3), 236–240 (2009).
Bozhuyuk, M. R. et al. Morphological and biochemical diversity in fruits of unsprayed Rosa canina and Rosa dumalis ecotypes found in different agroecological conditions. Sustainability. 13(14), 8060. https://doi.org/10.3390/su13148060 (2021).
Guo, M. et al. Genomic analyses of diverse wild and cultivated accessions provide insights into the evolutionary history of jujube. Plant Biotechnol. J. 19(3), 517–531. https://doi.org/10.1111/pbi.13480 (2021).
Ipek, P. & Balta, F. Fruit properties of rose hip (Rosa spp.) genotypes selected from Akkuş, Ordu Province. YYU J. Agr. Sci. 30(2), 338–344. https://doi.org/10.29133/yyutbd.680453 (2020).
Okatan, V., Colak, A. M., Guclu, S. F., Korkmaz, N. & Sekara, A. Local genotypes of dog rose from Interior Aegean region of Turkey as a unique source of pro-health compounds. Bragantia. 78, 397–408. https://doi.org/10.1590/1678-4499.20180386 (2019).
Javanmard, M., Asadi-Gharneh, H. A. & Nikneshan, P. Characterization of biochemical traits of dog rose (Rosa canina L.) ecotypes in the central part of Iran. Nat. Prod. Res. 32(14), 1738–1743. https://doi.org/10.1080/14786419.2017.1396591 (2018).
Kulaitiene, J. et al. Changes in fatty acids content in organic rosehip (Rosa spp.) seeds during ripening. Plants. 9(12), 1793. https://doi.org/10.3390/plants9121793 (2020).
Andersson, S. C., Rumpunen, K., Johansson, E. & Olsson, M. E. Carotenoid content and composition in rose hips (Rosa spp.) during ripening, determination of suitable maturity marker and implications for health promoting food products. Food Chem. 128(3), 689–696. https://doi.org/10.1016/j.foodchem.2011.03.088 (2011).
Fattahi, S., Jamei, R. & Hosseini, S. S. Antioxidant and antiradical activities of Rosa canina and Rosa pimpinellifolia fruits from West Azerbaijan. Iran. J. Plant Physiol. 2, 523–529. https://doi.org/10.30495/ijpp.2012.540789 (2012).
Medveckiene, B., Kulaitienė, J., Jariene, E., Vaitkeviciene, N. & Hallman, E. Carotenoids, polyphenols, and ascorbic acid in organic rosehips (Rosa spp.) cultivated in Lithuania. Appl. Sci. 10(15), 5337. https://doi.org/10.3390/app10155337 (2020).
Jemaa, H. B. et al. Antioxidant activity and α-amylase inhibitory potential of Rosa canina L. Afr. J. Tradit. 14(2), 1–8. https://doi.org/10.21010/ajtcam.v14i2.1 (2017).
Nadpal, J. D. et al. Phytochemical composition and in vitro functional properties of three wild rose hips and their traditional preserves. Food Chem. 241, 290–300. https://doi.org/10.1016/j.foodchem.2017.08.111 (2018).
Pourhosseini, S. H. et al. Diversity of phytochemical components and biological activities in Zataria multiflora Boiss. (Lamiaceae) populations. S. Afr. J. Bot. 135, 148–157. https://doi.org/10.1016/j.sajb.2020.08.024 (2020).
Soare, R., Babeanu, C., Bonea, D. & Panita, O. The content of total phenols, flavonoids and antioxidant activity in Rosehip from the spontaneous flora from south Romania. Sci. Papers Ser. A Agron. 58, 307–314 (2015).
Shameh, S., Alirezalu, A., Hosseini, B. & Maleki, R. Fruit phytochemical composition and color parameters of 21 accessions of five Rosa species grown in North West Iran. J. Sci. Food Agric. 99(13), 5740–5751. https://doi.org/10.1002/jsfa.9842 (2019).
Roby, M. H. H., Sarhan, M. A., Selim, K. A. H. & Khalel, K. I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 43, 827–831. https://doi.org/10.1016/j.indcrop.2012.08.029 (2013).
Murathan, Z., Zarifikhosroshahi, M., Kafkas, E. & Sevindik, E. Characterization of bioactive compounds in rosehip species from East Anatolia region of Turkey. Ital. J. Food Sci. 28, 314–325. https://doi.org/10.14674/1120-1770/ijfs.v198 (2016).
Yildiz, O. & Alpaslan, M. Properties of rose hip marmalades. Food Technol. Biotechnol. 50(1), 98–106 (2012).
Szmagara, A., Szmagara, M., Krzyszczak, A. & Sadok, I. Morphological and phytochemical characterization of Rosa sweginzowii fruit from Poland. LWT Food Sci. Technol. 173, 114349. https://doi.org/10.1016/j.lwt.2022.114349 (2023).
Kayahan, S., Ozdenir, Y. & Gulbag, F. Functional compounds and antioxidant activity of Rosa species grown in Turkey. Erwerbs-Obstbau. https://doi.org/10.1007/s10341-022-00688-5 (2022).
Erogul, D. & Oguz, H. I. Determining the physico-chemical characterstics of the rosehip genotypes grown naturally in Adiyaman Province. Erwerbs-Obstbau. 60(3), 195–201. https://doi.org/10.1007/s10341-017-0358-2 (2018).
Bilgin, N. A., Misirli, A., Sen, F., Turk, B. & Yagmur, B. Fruit pomological, phytochemical characteristic and mineral content of rosehip genotypes. Int. J. Food Eng. 6(1), 18–23. https://doi.org/10.18178/ijfe.6.1.18-23 (2020).
Ozturk, N., Tuncel, M. & Tuncel, N. B. Determination of phenolic acids by a modified HPLC: Its application to various plant materials. J. Liq. Chromatogr. Relat. Technol. 30(4), 587–596. https://doi.org/10.1080/10826070601093911 (2007).
Butkeviciute, A., Urbstaite, R., Liaudanskas, M., Obelevicius, K. & Janulis, V. Phenolic content and antioxidant activity in fruit of the genus Rosa L. Antioxidants. 11(5), 912. https://doi.org/10.3390/antiox11050912 (2022).
Khadivi-Khub, A., Karimi, E. & Hadian, J. Population genetic structure and trait associations in forest savory using molecular, morphological and phytochemical markers. Gene. 546(2), 297–308. https://doi.org/10.1016/j.gene.2014.05.062 (2014).
Acknowledgements
The authors gratefully acknowledge the Research Council of Shahid Beheshti University and Vesha Daro Pars herbal pharmaceutical Co. for their financial support. We also wish to thank Miss Haniyeh Dalvand, Dr. Hamid Ahadi, and Miss Marzieh Shakeri for their kind help in sample preparation, HPLC and GC analysis, respectively.
Author information
Authors and Affiliations
Contributions
Z.B.: Conceptualization, Methodology, Investigation, Formal analysis, Writing-original draft. G.E.: Supervision, Conceptualization, Methodology, Data curation, Writing-original draft. M.H.: Formal analysis, Validation, Writing-review and editing. M.H.M.: Conceptualization, Methodology, Data curation, Validation, Writing-review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bakhtiar, Z., Eghlima, G., Hatami, M. et al. Quantification of fatty acids in seed oil and important bioactive compounds in Iranian Rosa canina L. ecotypes for potential cosmetic and medicinal uses. Sci Rep 13, 22721 (2023). https://doi.org/10.1038/s41598-023-50135-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50135-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.