Abstract
In order to evaluate the genetic effect caused by hybrid sterile loci, NILs with O. glaberrima fragment at six hybrid sterile loci under O. sativa genetic background (single-locus-NILs) were developed; two lines harboring two hybrid sterile loci, one line harboring three hybrid sterile loci were further developed. A total of nine NILs were used to test cross with O. sativa recurrent parent, and O. glaberrima accessions respectively. The results showed that the sterility of pollen grains in F1 hybrids deepened with the increase of the number of hybrid sterile loci, when the nine lines test crossed with O. sativa recurrent parent. The F1 hybrids were almost completely sterile when three hybrid sterile loci were heterozygeous. On the other hand, the single-locus-NILs had limited bridge effect on improving pollen grain fertility of interspecific hybrids. Compared single-locus-NILs, the multiple-loci-NILs showed increasing effect on pollen fertility when test crossing with O. glaberrima accessions. Further backcrossing can improve the fertility of pollen grain and spikelet of interspecific hybrids. The optimal solution to improve the fertility of interspecific hybrid can be utilization of pyramiding bridge parent plus backcrossing. This report has potential for understanding the nature of interspecific hybrid sterility, and overcoming the interspecific hybrid F1 pollen grain sterility between O. sativa and O. glaberrima.
Similar content being viewed by others
Introduction
Asian cultivated rice (Oryza sativa L.) is a prime food crop world-wide. However, advance in genetic improvement of rice has encountered problems owing to the narrow genetic diversity and the bottleneck of further yield increase1. African cultivated rice (O. glaberrima Steud.) is deemed to be a potential source of useful genes for improving Asian cultivated rice by hybridization as both cultivated species have the same AA genome and similar sequence arrangement2,3. However, there are strong reproductive barriers in the interspecific hybrids between the two cultivated species4. The F1 hybrids barely produce fertile pollen grain and as a result that the valuable genes are very difficult to be introgressed because of the strong hybrid sterility (HS). Therefore, HS is one of the main hindrances against the utilization of useful genes from the African cultivated rice for Asian cultivated rice improvement.
To date, at least 11 HS loci were reported as gamete eliminators or pollen killers between O. sativa and O. glaberrima5, and one of them were cloned6,7,8. The cumulative effects of these HS loci led to the complete male sterility in F1 hybrids of interspecific hybrid. O. glaberrima and O. sativa varieties possessed the genotype S-g and S-s, respectively, at the HS locus. Homozygotes of S-s/S-s and S-g/S-g show normal fertility, while S-s or S-g gametes are aborted when the sporophytic plants have the heterozygous genotype S-s/S-g in an O. sativa background. At the most HS loci reported, S-s gametes are aborted in the heterozygous genotype as the result the gametes of O. glaberrima are preferentially transmitted to the next generation and these HS loci were called African rice selfish loci. Recently, Feng et al.9 reported an Asian rice selfish locus S58, the S58-g gametes was aborted in the heterozygous genotype and caused a transmission advantage for the Asian rice allele of S58 in the hybrid offspring.
Based on the known genetic information, if the HS loci in a given O. sativa background could be substituted by the neutral alleles or the corresponding alleles from O. glaberrima, it would be possible to overcome the interspecific HS between O. sativa and O. glaberrima10,11. It was reported that the O. sativa lines carrying the S1-g allele from O. glaberrima can be used as bridge parents to improve the fertility of hybrids between O. glaberrima and O. sativa12,13. However, the bridge effect of others HS loci and their pyramided lines remain unknown.
In our previous study, six HS loci, S1, S19, S20, S37(t), S38(t), S39(t) were identified from the crosses between O. glaberrima and O. sativa14,15,16. The S1 and S37(t) loci functioned as the “gamete eliminator”: both male and female gametes carrying the allele of O. sativa were aborted in the heterozygotes. The S19, S20, S38(t), S39(t) locus functioned as the “pollen killer”: only male gametes carrying the allele of O. sativa were aborted in the heterozygotes.
In order to improve the bridge effect to overcome interspecific reproductive barrier, the near isogenic lines (NILs) carrying single and multiple O. glaberrima fragments at S1, S19, S20, S37(t), S38(t) and S39(t) were developed. The genetic effect of the HS loci were investigated in this study.
Results
Development of NILs harboring single and multiple O. glaberrima fragments at HS loci
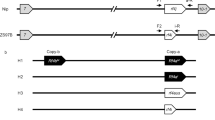
A genomics-based introgression of target single HS loci from the donors into DJY1 has been implemented using SSR markers. The high quality genotyping data for the candidate plants were provided on 6777 SNP markers. Six plants that carrying the target chromosomal fragment from the O. glaberrima accessions at HS loci of S1, S19, S20, S37(t), S38(t) and S39(t) respectively and the genetic background were similar to the recurrent parent DJY1 were selected and denoted as single-loci-NILs, and named as NILS1, NILS19, NILS20, NILS37(t), NILS38(t), NILS39(t) respectively (Supplementary Table 1). Actually, the NILS39(t) harbored another two fragments from the donor parent and the genomic regions containing S1, S37(t), S39(t) had large linkage fragments from their donor parents, which may have potential adverse genetic effects (Fig. 1).
Genetic background screen of six NILs with single hybrid sterile allele from O. glaberrima using C6AIR. Twelve chromosomes of rice are labelled from 1 to 12 and the triangles indicated the positions of the centromere. The reference genome is O. sativa DJY1. The circle indicate the positions of the target locus. The black lines indicated the positions of the SNP with homozygous genotypes where genomic fragments of the donor parent were introgressed, red lines indicated the positions of the SNP with heterozygous genotypes, and the genotypes of the rest genomic regions were the same as the recurrent parent DJY1.
All the F2 self-pollinated plants showing the homozygous to O. glaberrima alleles on S1 and S37(t) loci in the cross of NILS1/NILS37(t) because the S1 and S37(t) loci functioned as the “gamete eliminator” and as the result only the gametes carrying the allele of O. glaberrima survived (Table 1). The pyramiding line combining two HS loci of O. glaberrima allele was denoted as two-loci-NIL, or multiple-loci-NIL, and named this line as NILS1S37(t).
With the assistance of SSR markers linked to S1 and S20, the F2 self-pollinated plants from the cross of NILS1/NILS20 showing homozygous to O. glaberrima allele at the S1 and S20 loci were selected, then NILS1S20 was obtained. The selected plants with homozygous allele of O. glaberrima at the S1 and S20 loci also showed the normal pollen and spikelet fertility (Table 1).
To pyramid the three loci, S1, S20 and S37(t) of O. glaberrima alleles in O. sativa genetic background, the SSR markers linked to them were used to genotype the F2 plants in the cross of NILS1/NILS37(t)//NILS20. The plants homozygous to O. glaberrima allele at all three loci showing normal pollen and spikelet fertility were obtained (Table 1). The pyramiding line was designated as NILS1S20S37(t).
Genetic effect caused by HS loci between O. sativa and O. glaberrima
For the single-locus-NILs, the pollen grain and spikelet fertility of F1 plants were semi-sterile in the crosses between DJY1 and NILS1, NILS37(t); the pollen grains were semi-sterile and the spikelet fertility was normal in the crosses between DJY1 and NILS19, NILS20, NILS38(t), and NILS39(t) (Table 1).
When the multiple-loci-NILs were crossed with DJY1, the fertility of pollen grain was significantly lower than those of the single-locus-NILs. The NILS1S20 and NILS1S37(t) containing two HS loci caused about two-thirds of pollen abortion in hybrids and the F1 pollen grain fertility were 33.81% and 31.46%, respectively. In particular, the pollen grain of F1 plants was 3.35% in the cross between DJY1 and the NILS1S20S37(t) (Table 1), which was the lowest among the test hybrids. These results showed that the cumulative effect of these HS loci in F1 hybrids was remarkable. When NILS1 and NILS37(t) crossed with DJY1, the pollen and spikelet fertility of F1 plant were semi-fertility (Table 1) which followed the gamete eliminate model. However, the spikelet fertility of F1 plants was still semi-fertility (50.19%) when the NILS1S37(t) was crossed with DJY1 (Table 1). It is unclear in regard to the interaction between these two loci.
Bridge effect of single-locus-NILs and multiple-loci-NILs
There was no significant difference on pollen fertility between reciprocal F1s of all combinations (P = 0.94), indicated that no cytoplasmic effect was involved in hybrid sterility between the two O. glaberrima accessions and the O. sativa variety DJY1 (data not shown). The difference of F1 pollen grain fertility between the two interspecific crosses, DJY1 × IRGC102263 and DJY1 × IRGC103469 was not significant (P = 0.08), indicated that the compatibility between DJY1 and the two O. glaberrima accessions was consistent. The F1 pollen fertility data of reciprocal crosses between each NIL (or recurrent parent) and two African rice accessions was merged to obtain larger sample size.
The F1 progenies between the six single-locus-NILs and the O. glaberrima accessions showed poor pollen grain fertility although there were significant difference among them (Table 2), indicated that single-locus-NILs had limited bridge effect on improving pollen grain fertility of interspecific hybrids. Student's t test indicated that when NILS19, NILS20, and NILS37(t) were crossed with the test O. glaberrima accessions, the average pollen fertility of F1 was 0.49%, 1.72%, 0.85%, respectively, which was notably higher than that of the control (the crosses between DJY1 and O. glaberrima accessions) (0.15%). Among them, S20 locus had the largest effect on improving pollen grain fertility of interspecific F1 hybrids, followed by S37(t), and S19 was only significant at 0.05 level. While the progenies derived from NILS1, NILS38(t) and NILS39(t), were similar to the control.
The pollen grain fertility of F1 hybrids between the multiple-loci-NILs and O. glaberrima test accessions were notably higher than that of control (the crosses between DJY1 and O. glaberrima accessions). The F1 pollen fertility was 5.96% in the hybrids between NILS1S37(t) and O. glaberrima accessions, and 4.73% in the hybrids between NILS1S20 and O. glaberrima accessions. The tri-loci line NILS1S20S37(t) produced notably higher pollen fertility (3.90%) of F1 hybrids than that of single-locus-NILs. However, the F1 pollen fertility between the tri-loci-NIL and the test O. glaberrima accessions showed no significant difference from those of the F1 progenies between two-loci-NILs and test O. galberrima accessions (Table 3). Totally, multiple-loci-NILs showed higher bridge effect than the single-locus-NILs on overcoming interspecific hybrid sterility barriers between O. sativa and O. glaberrima. All of the F1 progenies from the crosses for bridge effect evaluation were complete spikelet sterility.
Backcrossing can be used to improve pollen fertility of interspecific hybrids after the hybridization
Compared with the F1 generation, the average pollen grain fertility of BC1F1 generation increased significantly. The average pollen grain fertility of the BC1F1 individuals involving the six single-locus-NILs ranged from 8.14% to 13.77% while that of the control’s was 5.06%. The highest pollen grain fertility reached 85.81% in the BC1F1 individuals from the cross between NILS1 and O. glaberrima, which was more than three times of the control (Table 3). However, the average pollen grain fertility in the BC1F1 segregation population derived from the six single-locus-NILs did not notably differ from the control because of the large standard deviation value. When O. glaberrima accessions were test crossed with the two-loci-NILs, NILS1S20 and NILS1S37(t), their average pollen grain fertility in the BC1F1 population was 44.04% and 49.50%, ranged from 3.11% to 97.00% and 19.57% to 81.71%, respectively (Table 4). The result indicated that the pyramiding lines with multiple hybrid sterility loci still had the more prominent bridge effect in the BC1F1 generation.
The average pollen fertility was notably higher in the BC2F1 generation than those of the BC1F1 generation. In the crosses between NILS1 and the O. glaberrima accessions, the average pollen fertility (51.01%) was notable higher than that of the control. The pyramiding lines with two hybrid sterility loci still showed better performance in the BC2F1 generation. The hybrids between NILS1S20 and O. glaberrima accessions had the highest average pollen grain fertility (78.92%). It is worth mentioning that the maximum pollen fertility reached normal range (> 95%) in all the hybridization crosses in BC2F1 generation (Table 4).
Discussion
Near isogenic lines should be one of the most effective methods on evaluating the HS loci genetic effect and bridge effect. As each NIL had the same DJY1 background and the difference was only on the specific chromosome fragments harboring the specific HS loci, the effect of genetic background could be readily eliminated and the difference between DJY1 and the NILs was originated from the HS locus. Our study showed that the presence of a single HS locus can cause about 50% of gametes sterility. The pollen grain and spikelet fertility of F1 plants were semi-sterile when involved S1 or S37(t); however, the pollen grains were semi-sterile and the spikelet fertility was normal when involved S19, S20, S38(t) or S39(t). These results indicated that all of the six HS loci followed the “one-locus sporo-gametophytic interaction model”17. S1, S37(t) acted as “gamete eliminator”, while S19, S20, S38(t), and S39(t) acted as the “pollen killer”, which is correspond with previous report. Theoretically, the presence of two HS loci can cause about two-thirds of pollen grain abortion and the pollen grain of F1 plants would be less than 5% if three HS loci are involved. However, the actual spikelet fertility (50.19%) of F1 plants was significantly higher than that of the theoretical value (25%) in the cross of DJY1×NILS1S37(t). The above results indicate that HS loci also involve in inter-loci interaction when they comply with the intra-locus allelic interaction model. Therefore, interspecific hybrid sterility has always been the focus and the difficulty in the rice genetic improvement. The interaction effects between HS loci should be considered in the interspecific hybrid breeding project.
In our case, it was confirmed that some of the “bridge parents” carrying a single HS loci produced interspecific F1 progenies with improved pollen fertility. Furthermore, present study showed that the pyramiding lines of multiple HS loci could significantly improve the F1 pollen grain fertility than those with single HS locus. In the presence of two HS loci, the pollen grain fertility of hybrid F1 increased 5–10 times compared with that of single HS locus when these NILs crossed with O. glaberrima. Because the interspecific hybridization involves more HS genes than that of the inter-subspecific hybridization10, the “bridge effect” of a single hybrid sterile allele is very limited, even the utilization of pyramiding lines with two or three hybrid sterile alleles cannot obtain ideal "bridge effect". In fact, in the interspecific hybrid breeding project, increasing pollen fertility from 0 to 5% has no remarkable breeding value.
As we known that interspecific hybrid sterility was shown quantitative trait controlled by multiple genes. Although the bridge lines with the O. glaberrima alleles can give homozygous genotype at the target loci, other HS loci were still heterozygous in the hybrids, which thus reduced fertility in the F1 populations. Backcrossing is an effective method to improve homogeneity of genetic background, and minimize the genetic variation of genetic background. Some of the HS loci become homozygous in the backcross population and as the result the fertility of BC1F1 plants has significantly improved compared to the F1 hybrids. By comparing the bridge effects of HS NILs among F1, BC1F1, and BC2F1 generations, the BC1F1 generation showed the most significant bridge effect. Furthermore, the BC1F1 individuals from the multiple-loci-NILs were notably more fertile than that of the single-locus-NILs. Therefore, the optimal solution to improve the fertility of interspecific hybrid can be utilization of pyramiding bridge parent plus backcrossing.
The hybrid sterility also occurs frequently in the inter-subspecific hybridization crosses in rice and the hybrid sterility is mainly affected by five loci involving four for F1 male sterility and one for F1 female sterility18. The indica-compatible japonica lines (ICJLs) were developed by pyramiding four indica allele and one neutral allele in japonica genetic background through marker-assisted selection. When the indica-compatible japonica lines were test-crossed with a set of typical indica and japonica varieties, the results indicated that the ICJLs were compatible with indica while incompatible with japonica rice. In the test crosses of the indica-compatible japonica lines with indica, the result showed that the F1 pollen and spikelet fertility reversed close to complete fertility when the indica-compatible japonica lines pyramided with four loci for “pollen killer” and one for “embryo sac killer”19. The study showed a great promise of overcoming the intersubspecific hybrid sterility by developing pyramiding lines at HS loci18. It can be deduced that the pyramiding lines with O. glaberrima alleles on five loci is still not enough on reversing the pollen and spikelet fertility to normal. More efforts are needed to elucidate the effect of various combinations of multiple hybrid sterile bridge loci, and to dissect their interaction or epistatic effect among HS loci.
Materials and methods
Developing single-locus-NILs
Five accessions of O. glaberrima (Supplementary Table 2) as the donor parents were backcrossed to the Dianjingyou 1 (DJY1), one O. sativa ssp. japonica variety from Yunnan province, P. R. China. As the result, a series of semi-sterile families of BC6F1 were obtained in the DJY1 background. In the previous work, six HS locus, S1, S19, S20, S37(t), S38(t), S39(t), for hybrid sterility were identified on chromosome 6, 3, 7, 1, 4, 12, respectively using these BC6F1 families14,15,16. Based on marker-assisted selection, the plants for the homozygous alleles of O. glaberrima on HS loci were obtained from corresponding mapping populations. A whole-genome SNP array (6 k) of rice designed by Cornell University was used to survey the genetic background of the plants with the target HS loci for single-locus-NILs developing19. The plants that the genetic backgrounds were similar to the recurrent parent DJY1 were selected as the NILs and designated as NILS1, NILS19, NILS20, NILS37(t), NILS38(t), NILS39(t).
Three NILs, NILS1, NILS20 and NILS37(t) were used to develop the pyramiding HS loci lines with molecular marker-assistant method and phenotype selection. The F2 self-pollinated plants from the crosses of NILS1/NILS37(t), NILS1/NILS20 and NILS1/NILS37(t)//NILS20 showing normal pollen and spikelet fertility and homozygous to O. glaberrima allele at target loci were selected as the pyramiding lines.
Evaluation genetic effect and bridge effect of HS loci
Six single-locus-NILs and three multiple-loci-NILs were used as test lines to cross with their recurrent parent DJY1 to evaluate genetic effect of HS loci.
In addition, DJY1 and its nine NILs were used as female and male parents to make reciprocal crosses with two O. glaberrima accessions to evaluate the bridge effect of the HS loci. The crosses of O. glaberrima with DJY1 were used as the control. Two O. glaberrima accessions, IRGC102263 and IRGC103469, from the International Rice Research Institute (IRRI), and O.sativa variety DJY1 were used as the tested lines in this study. The F1 plants were backcrossed as females to their corresponding NILs untill the BC2F1 generation was achieved.
All materials were planted at the Winter Breeding Station, YAAS, Sanya, Hainan Province, P. R. China. The first cropping season was from November to April of the following year, and the second cropping season was from July to October.
Phenotypic evaluation
The pollen grain and spikelet fertility for all parental lines, NILs, F1, BC1F1 and BC2F1 plants were evaluated. Pollen grain fertility was investigated following the instructions of Zhu20. Pollen grain fertility was measured using anthers collected from spikelets at 1 to 2 days before anthesis and stored in 70% ethanol21. Three to four anthers per floret per plant were mixed and stained with 1% I-KI solution, and more than 300 pollen grains were observed under a light microscope. Sterile types were further classified as typical, spherical or stained abortion types22. Three independent microscopic fields were scored for estimation of the percentage of the four types of pollen grains in each plant. Spikelet fertility was scored as the fertilized spikelet rate of three to five panicles on each plant.
Molecular marker and assay
The SSR molecular markers linked with S1, S19, S20, S37(t), S38(t), and S39(t) were selected on rice microsatellite maps23. The SSR markers linked with the target HS loci were used for developing the single-locus-NIL, confirming the true hybrid and optimizing the HS loci pyramiding process (Supplementary Table 3). Genomic DNA was extracted from the young leaves of each rice plant following simple DNA extraction method24. At least two SSR markers on each HS locus that have polymorphism between DJY1 and NILs were selected. Polymerase chain reaction (PCR) was performed according to McCouch et al.20 with minor modifications.
Total 22 plants with the introduced target fragment of O. glaberrima were examined using the Cornell_6K_Array_Infinium_Rice (C6AIR) SNP array19. Young leaves from each plant and recurrent parent DJY1 were used to isolate genomic DNA using the CTAB mothed. The quality of DNA was checked on 0.8% agarose gels, and the quantity was checked using a Nano-Drop spectrophotometer. The concentration of each DNA sample was adjusted to 50 ng/μl. DNA were used for genotying through the SNP array as described in Thomson et al.19. The genotypes of the called SNP were assigned as “A” (DJY1 genotype), “B” (donor parent genotype) and “H” (heterozygous genotype). An unambiguous graphic genotype for each NIL were achieved by R software.
Statistical analysis
Statistical analysis of the data was performed using one-way ANOVA, and the Student's test was used for further pairwise comparisons if ANOVA differences were significant. Pollen grain and spikelet fertility data as a percentage was transformed by function arcsine square root before the analysis but are listed as percentages.
Ethics declarations
The plant collection and use was in accordance with all the relevant guidelines.
Permissions statement
The rice cultivars involved in this paper have permission.
Data availability
All relevant data are within the paper.
References
Tanksley, S. D. & McCouch, S. R. Seed banks and molecular maps: Unlocking genetic potential from the wild. Science 277, 1063–1066 (1997).
Chang, T. T. The origin, evolution, cultivation, dissemination, and diversification of Asian and African rices. Euphytica 25, 425–441 (1976).
Ohmido, N. & Fukui, K. Cytolofical studies of African cultivated rice, Oryza glaberrima. Theor. Appl. Genet. 91, 212–217 (1995).
Sano, Y. The genic nature of gamete eliminator in rice. Genetics 125, 183–191 (1990).
Li, J. et al. New Insights into the nature of interspecifific hybrid Sterility in rice. Front. Plant Sci. 11, 555572. https://doi.org/10.3389/fpls.2020.555572 (2020).
Koide, Y. et al. Lineage-specifific gene acquisition or loss is involved in interspecifific hybrid sterility in rice. Proc. Natl. Acad. Sci. U. S. A. 115, E1955–E1962. https://doi.org/10.1073/pnas.1711656115 (2018).
Xie, Y. et al. Interspecifific hybrid sterility in rice is mediated by OgTPR1 at the S1 locus encoding a peptidase-like protein. Mol. Plant. 10, 1137–1140. https://doi.org/10.1016/j.molp.2017.05.005 (2017).
Xie, Y. et al. An asymmetric allelic interaction drives allele transmission bias in interspecifific rice hybrids. Nat. Commun. 10, 1–10. https://doi.org/10.1038/S41467-019-10488-3 (2019).
Feng, et al. Characterization and fine-mapping of a new Asian rice selfsh genetic locus S58 in Asian-African rice hybrids. TAG. 136, 87 (2023).
Heuer, S. & Miezan, K. M. Assessing hybrid sterility in Oryza glaberrima × Oryza sativa hybrid progenies by PCR marker analysis and crossing with wide compatibility varieties. Theor. Appl. Genet. 107, 902–909 (2003).
Tao, D. et al. Studies on hybrid sterility inheritance and mapping of sterile genes among near-isogenic lines derived from interspecific hybrid between cultivated rice species Oryza sativa L. and O. glaberrima Steud. Chin. J. Rice Sci. 17, 11–15 (2003).
Deng, X. et al. The role of S1-g allele from Oryza glaberrima in improving interspecific hybrid sterility between O. sativa and O. glaberrima. Breed. Sci. 60, 342–346 (2010).
Sano, Y. Sterility barriers between Oryza sativa and O. glaberrima. In Rice Genetics (ed. Institute-IRRI, I. R. R.) 109–118 (IRRI, Manila, 1986).
Li, J. et al. Identification of four genes for stable hybrid sterility and an epistatic QTL from a cross between Oryza sativa and Oryza glaberrima. Euphytica 164, 699–708 (2008).
Li, J. et al. Molecular mapping of sterility QTLs qSS-3, qSS-6a and qSS-7 as single Mendelian factors via NIL strategy. Rice Sci. 18, 110–115 (2011).
Xu, P. et al. Mapping three new interspecific hybrid sterile loci between Oryza sativa and O. glaberrima. Breed. Sci. 63, 476-482.22 (2014).
Oka, H. Analysis of genes controlling F1 sterility in rice by the use of isogenic lines. Genetics 77, 521–534 (1974).
Guo, J., Xu, X., Li, W., Zhu, W. & Zhang, G. Overcoming inter-subspecific hybrid sterility in rice by developing indica-compatible japonica lines. Sci. Rep. 6, 26878 (2016).
Thomson, M. J. et al. Large-scale deployment of a rice 6 K SNP array for genetics and breeding applications. Rice 10, 40. https://doi.org/10.1186/s12284-017-0181-2 (2017).
Zhu, Y. A. preliminary discussion about the classification of male sterile lines of rice in China. Acta Agron. Sin. 6, 17–26 (1979).
Doi, K., Yoshimura, A. & Iwata, N. RFLP mapping and QTL analysis of heading date and pollen sterility using backcross population between Oryza sativa L. and Oryza glaberrima Steud. Breed. Sci. 48, 395–399 (1998).
Li, Z. B. A preliminary discussion about the classification of male sterile lines of rice in China. Acta Agron. Sin. 6, 17–26 (1980).
McCouch, S. R. Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Res. 9, 199–207 (2002).
Edwards, K. et al. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 19, 1349 (1991).
Acknowledgements
We would like to thank Dr. Huihui Yu for his help in the process of making chromosome maps based on R Language. This research was funded by National Natural Science Foundation of China, grant number C130501, 31860372, 32160489, and 31000704; Basic Research Foundation of Yunnan Provincial Science and Technology Department, China, grant number 202201AS070072, 202101AS070286; Technology Talent and Platform program of Yunnan Provincial Science and Technology Department, China, grant number 202205AC160057; Applied Basic Research Foundation of Yunnan Academy of Agricultural Sciences JZ201801; and the Yunnan Seed Laboratory Program.
Author information
Authors and Affiliations
Contributions
Conceptualization, D.T.and J. L.; experiment implementation, J.L. and J.Z.; investigation, J.L., P.X., X.D., W.D., Y.Y, Y.Z., Y.L., Q.P.; writing—original draft preparation, J.L.; writing—revise and editing, D.T. and J.Z.. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Zhou, J., Xu, P. et al. Improving bridge effect to overcome interspecific hybrid sterility by pyramiding hybrid sterile loci from Oryza glaberrima. Sci Rep 13, 23057 (2023). https://doi.org/10.1038/s41598-023-49914-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-49914-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.