Abstract
The successful implementation of heterosis in rice has significantly enhanced rice productivity, but the genetic basis of heterosis in rice remains unclear. To understand the genetic basis of heterosis in rice, main-effect and epistatic quantitative trait loci (QTLs) associated with heterosis for grain yield-related traits in the four related rice mapping populations derived from Xiushui09 (XS09) (japonica) and IR2061 (indica), were dissected using single nucleotide polymorphism bin maps and replicated phenotyping experiments under two locations. Most mid-parent heterosis of testcross F1s (TCF1s) of XS09 background introgression lines (XSILs) with Peiai64S were significantly higher than those of TCF1s of recombinant inbred lines (RILs) with PA64S at two locations, suggesting that the effects of heterosis was influenced by the proportion of introgression of IR2061’s genome into XS09 background. A total of 81 main-effect QTLs (M-QTLs) and 41 epistatic QTLs were identified for the phenotypic variations of four traits of RILs and XSILs, TCF1s and absolute mid-parent heterosis in two locations. Furthermore, overdominance and underdominance were detected to play predominant effects on most traits in this study, suggesting overdominance and underdominance as well as epistasis are the main genetic bases of heterosis in rice. Some M-QTLs exhibiting positive overdominance effects such as qPN1.2, qPN1.5 and qPN4.3 for increased panicle number per plant, qGYP9 and qGYP12.1 for increased grain yield per plant, and qTGW3.4 and qTGW8.2 for enhanced 1000-grain weight would be highly valuable for breeding to enhance grain yield of hybrid rice by marker-assisted selection.
Similar content being viewed by others
Introduction
Food security is one of the major concerns of the rapidly growing population of the world, which can be solely resolved through the sustainable food production. In particular, rice is a staple food for more than half of the population globally1, which is usually classified into two main types, indica and japonica. These two subspecies considerably differ from each other on the basis of morphology and physiology, while inter-subspecies hybrid usually demonstrated a strong level of heterosis for numerous agronomic traits2.
Heterosis, also known as hybrid vigor, plays a key role in the enhancement of crop yield and thus, regarded as a far superior phenotypic performance of hybrids in comparison with their parents3. F1-hybrid plants possess appreciable features such as increased yield, biomass, vegetative growth rate, and tolerance of biotic and abiotic stresses4. The much enhanced yield of F1-hybrid has been utilized in numerous crops and vegetables5. Jones has firstly reported the heterosis in rice and suggested that few F1-hybrid plants had more culms and higher yield as compared with their parents6. Later, numerous researchers have reported heterosis for yield and yield-related traits7,8,9,10. Notably, China had successfully achieved the application of heterosis of rice into agronomic industries, and the planted land of hybrid rice ever rose to as high as 57% of the total rice area in China11. However, the traditional heterosis cannot satisfy the breeding demands of improved yield12.
Several researchers revealed that the indica–japonica hybrids have large sink and genetic source, capable of increasing the yield potential. As a result, many studies investigated the potential of indica–japonica hybrids13,14,15. However, hybrid sterility seriously restricts the development of high-yield hybrid rice15. Thus, the development of indica–japonica background lines is prerequisite for high yielding hybrid16. Numerous inter-subspecific lines have been developed for hybrid varieties with stronger heterosis and normal seed set17,18,19. Recently, China has made a great success in utilization of heterosis of indica–japonica, releasing many super hybrid combinations such as Yongyou 6, Yongyou 9, Yongyou 12, Chunyou658, Chunyou84, Chunyou 927 by introgressing japonica composition and the wide-compatibility gene (S5n) into indica restorer line background20,21. In spite of the extensive research, the genetic basis of heterosis of indica–japonica are not yet completely understood.
With the advancement of second-generation genome sequencing technologies, genome-wide association study (GWAS) has been acquired to identify quantitative trait loci (QTLs) for rice agronomic traits22,23. GWAS provided insights into the genetic architecture of the heterosis for yield traits in rice24,25, and permitted the rapid identification of single nucleotide polymorphisms (SNPs) associated with heterosis. Based on QTL analysis, dominance26, overdominance17,27,28 and epistasis29,30 could be the key determinants of heterosis in rice31. Testcrossing is one the most regular method to identify superior hybrid in plant breeding. Therefore, testcross populations have been extensively used to identify QTLs associated with yield heterosis in rice, suggesting that these QTL effects may sufficiently describe the genetic basis of heterosis32,33. While, the high perseverance of genetic and field data was required to detect significant QTLs in the rice genome associated with hybrid performance34,35,36. However, the reports demonstrated the SNPs analysis of heterosis in indica–japonica background lines is surprisingly rare. By using 1,654,030 SNPs of 1,495 hybrids and their parental lines of rice, Li et al. reported the genetic basis of heterosis and the involvement of superior allele to heterosis37. Meanwhile, Zhen et al. performed GWAS using 50 K SNP for F1-hybrid and identified many QTLs related to yield traits, and genes including Hd3a, qGL3 and LAX2 within these QTLs were detected25.
In this study, we performed genotyping using a customized rice 56 K SNP array to Xiushui09 (XS09) background introgression lines (XSILs) and recombinant inbred lines (RILs) derived from indica–japonica varieties and their testcross F1 (TCF1) populations separately, and identified 81 QTLs underlying heterosis for four yield traits with a number of already identified genes within these QTLs. We further investigated divergence and the gene actions of QTLs responsible for yield in the inbred line populations and heterosis in the testcross populations to understand the relative significance of additive and non-additive gene actions in rice improvement.
Results
Phenotypic performances
The performance for the four yield traits of the parents (XS09 and IR2061) of the RILs and XSILs, the tester line Peiai64S (PA64S) (or PA64), the relative F1 plants (XS09 × IR2061 and PA64S × XS09), and theirs mid-parent heterosis (HMP) in Lingshui (LS) and Wenzhou (WZ) were shown in Table 1. Significant differences were observed for filled grain number per panicle (FGNP) and 1000-grain weight (TGW) between XS09 and IR2061 in both locations. The F1 (XS09 × IR2061) plants had significantly higher panicle number per plant (PN) and FGNP, and similar TGW and grain yield per plant (GYP) compared with the two parents in the two locations. The HMP of the F1 (XS09 × IR2061) plants for PN, FGNP and GYP was 25.9%, 37.2%, and − 0.6% in LS, and 41.0%, 37.7%, and 3.6% in WZ, respectively, showing strong heterosis for PN and FGNP between the two parents and low heterosis for GYP with low fertility of F1 plants in the two locations, resulting from incompatibility between the two subspecies parents. As compared with XS09, the tester line PA64S had significantly higher PN, FGNP, and GYP, and significantly lower TGW in LS. In the WZ experiment, PA64 (the recurrent parent of PA64S) was used instead of PA64S due to its sterility, which showed significantly higher PN, lower FGNP and TGW than XS09, and similar GYP with XS09. The F1 (PA64S × XS09) plants showed significantly increased FGNP and GYP, and similar PN compared with the better parent PA64S/PA64 in both locations, and similar TGW in LS and significantly lower TGW in WZ compared with the better parent XS09. Significant HMP of the F1 (PA64S × XS09) plants were found with 27.0% for PN, 59.0% for FGNP and 60.7% for GYP in LS, and 27.7% for PN, 94.8% for FGNP and 53.3% for GYP in WZ.
The ranges of variations for each trait in the lines (RILs and XSILs), TCF1s (TCF1s of RILs with PA64S [RILTCF1s] and TCF1s of XSILs with PA64S [XSILTCF1s]) and their HMPs were shown in Fig. 1. Wider distributions of all traits were found in the RIL population than XSIL population in both locations. The RILTCF1s had significantly lower TGW and higher PN, FGNP and GYP than the RILs at the two locations, while similar results were observed for all traits except for TGW between XSILTCF1s and XSILs in WZ (Fig. 1, Table 1). The mean TGW and GYP in RILTCF1s and the mean PN, FGNP and GYP in XSILTCF1s were all higher than those of the check F1 (PA64S × XS09) plants in LS. There were 7 (3.3%), 18 (8.6%), 27 (12.9%), and 8 (3.8%) RILTCF1s and 16 (7.2%), 16 (7.2%), 0, and 3 (1.4%) XSILTCF1s having significantly higher PN, FGNP, TGW, and GYP than the F1 (PA64S × XS09) plants in LS, respectively. Similarly, in the WZ experiment, the mean PN in both RILTCF1s and XSILTCF1s and the mean GYP in XSILTCF1s were higher than the check F1 (PA64S × XS09) plants. There were 28 (13.4%), 2 (1.0%), 1 (0.5%), and 1 (0.5%) RILTCF1s and 39 (17.6%), 1 (0.5%), 0, and 4 (2.0%) XSILTCF1s having significantly higher PN, FGNP, TGW, and GYP than the F1 (PA64S × XS09) plants, respectively. On average, the RILTCF1s had higher TGW and GYP and lower PN and FGNP than XSILTCF1s in LS, while a similar trend was observed in RILTCF1s for PN, FGNP and TGW, but lower GYP compared with XSILTCF1s in WZ.
Phenotypic distribution for the four yield-related traits of two populations at the two locations. RILs recombinant inbred line derived from a cross between Xiushui09 and IR2061, XSILs introgression lines under Xiushui09 background with IR2061 as a donor, TCF1 testcross F1 between RIL and XSIL and one common maternal tester line PA64S, HMP mid-parent heterosis, LS Lingshui, WZ Wenzhou, GYP grain yield per plant, FGNP filled grain number per panicle, TGW 1000-grain weight, PN effective panicle number per plant.
The performance of the hybrid plants was determined by their mid-parental value and the heterosis level. Here, the most HMP of the TCF1s showed positive heterosis in all traits except for TGW in both locations (Fig. 1, Table 1). Moreover, the HMP of the TCF1s for FGNP and GYP were generally higher than those of PN and TGW. We found that 39.2%, 10.5%, 53.6%, and 54.1% of the RILTCF1s and 58.6%, 19.4%, 0, and 64.0% of the XSILTCF1s with higher HMP of PN, FGNP, TGW and GYP compared with the check F1 (PA64S × XS09) plants in LS, respectively. Similarly, 37.3%, 25.8%, 68.9%, and 77.5% of the RILTCF1s and 69.4%, 42.8%, 50.9%, and 88.3% of the XSILTCF1s with higher HMP of PN, FGNP, TGW and GYP compared with the check F1 (PA64S × XS09) plants in WZ, respectively. The mean HMP of the XSILTCF1s was higher for PN, FGNP and GYP but lower for TGW than those of the RILTCF1s at the two locations, suggesting that the effects of heterosis may be influenced by the proportion of introgression of IR2061’s genome into the XS09 background, when RILs or XSILs were tested with the common indica tested parent PA64S. Taking together, the observed heterosis for GYP in the TCF1s was mainly attributed to yield-component traits PN and FGNP.
Correlations of yield traits among lines, testcross F1s and mid-parent heterosis
Correlation analyses on each trait across the lines (RILs or XSILs), the TCF1s (RILTCF1s and XSILTCF1s), and their HMPs are presented in Table 2. The HMPs of TCF1s were determined by the trait values of the TCF1s and the corresponding lines (RILs or XSILs). In other words, the performance of individual TCF1 plants largely depends on the cumulative effect of the trait value of the relative line and the heterosis. There were significant and highly positive correlations between the lines and the relative TCF1s for all traits in the two locations except FGNP between XSILs and the XSILTCF1, and GYP between RILs and the RILTCF1 in WZ, and the correlation coefficients in LS were generally higher than those in WZ. The significant and highly positive correlations (r ≥ 0.53, P < 0.01) between TCF1s and their HMPs were also observed for PN, FGNP and GYP in both locations, while the correlation of TGW was slightly weak between RILTCF1s and their HMPs (r = 0.26) and between XSILTCF1s and their HMPs (r = 0.15) in LS. In contrast, most correlations between the HMP of TCF1s and the relative lines for FGNP, TGW and GYP were significantly negative and were much stronger than PN in both locations (Table 2). In addition, the correlations between the lines and their HMP for these yield traits differed according to different locations. The above results suggested that the role of heterotic loci for yield should be affected by the environment.
Effects of proportion of Japonica genome in male lines on heterosis of testcross
PA64S shared the same genotypes with XS09 at 9646 (38.1%) of all 25,296 SNPs. Within the RIL population, individual RILs varied considerably in their ratios of homozygous XS09 alleles ranging from 0 to 91% with a median of 42%, much smaller than the ratios of homozygous XS09 alleles in the XSILs ranging from 72 to 100% with a median of 92% due to two-time consecutive backcrossing (Fig. 2A). Furthermore, most XSILTCF1s had a higher ratio of homozygous XS09 alleles (a median of 36% with a range from 27 to 40%) and a higher ratio of heterozygous genotype (a median of 60% with a range from 50 to 68%) than RILTCF1s. In view of a continuous distribution of japonica genome (XS09 genome) in the two inbred line (XSILs and RILs) populations, the two populations could be also merged into one combined population for further analysis of the proportional effect of japonica genome on heterosis of the combined testcross populations. Thus, at all tested SNPs, the ratio of japonica genome (XS09-genome) could range from 0 to 100% in the combined inbred population consisted of all RILs and XSILs, and from 0 to 40% in a combined TCF1 population consisted of RILTCF1s and XSILTCF1s. Correlation analyses between the ratios of XS09 genome of individual inbred line and the trait value or HMP of the testcross for the four yield traits were shown in Fig. 2B and Table S1. The results indicated that there were significant negative correlations for TGW, significant weak positive correlations for PN and FGNP and no significant correlations for GYP between the trait values or HMP of the TCF1s or the combined TCF1s and the ratios of XS09 genome in the RILs, XSILs and the combined line population (RILs and XSILs). It should be noted that these significant correlations found in the combined line population were much stronger than those only in the RILs or XSILs. It was indicated that there was no inevitable connection between heterosis of the testcross and the proportion of japonica genome in paternal lines in our tested populations.
Relationship between the ratio of heterozygous and homozygous japonica genotype and the heterosis in rice. (A) Distribution of the ratio of heterozygote and homozygous XS09 genotypes in RIL and XSIL populations and testcross F1s. (B) Correlations between the ratio of the heterozygous and homozygous genotype of rice individuals and the value of relative TCF1s and mid-parent heterosis. ‘Homo’ and ‘H’ indicates homozygote and heterozygote, respectively.
Main-effect QTL mapping of yield-related traits and mid-parent heterosis
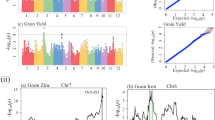
A total of 81 main-effect QTLs (M-QTLs) were identified for phenotypic variations of the four traits of the lines (RILs and XSILs), TCF1s and absolute mid-parent heterosis (AHMP) in LS and WZ (Table 3, Fig. 3). The detected M-QTLs were distributed on all the 12 chromosomes, including 54 additive, 10 dominance-type (D-type), 6 overdominance-type (OD-type), and 11 underdominance-type (UD-type) M-QTLs.
QTLs for four yield-related traits detected in Lines, TCF1s, and for their HMP in Lingshui and Wenzhou. Symbols above the QTLs represent known genes related to the trait as candidate genes. QTL actions of ‘A’, ‘D’, ‘OD’, and ‘UD’ represent additive, dominance, overdominance, underdominance, respectively. The sizes of the shapes represent the percentage of phenotypic variance explained by the QTL.
Nine M-QTLs were identified for PN in LS, explaining 22.3%, 20.3% and 16.4% of the total phenotypic variance in the RILs, RILTCF1s and corresponding AHMPs, and 22.7%, 8.2% and 5.6% of the total phenotypic variance in the XSILs, XSILTCF1s and corresponding AHMPs, respectively. Similarly, 12 M-QTLs were detected for PN in WZ, explaining 29.4% and 13.1% of the total phenotypic variance in the RILs and RILTCF1s, and 24.5%, 16.6% and 7.1% of the total phenotypic variance in the XSILs, XSILTCF1s and corresponding AHMPs, respectively. Among them, qPN1.2 and qPN1.5 appeared to be OD-type M-QTLs, which increased AHMPs values by 4.0 in WZ and 1.3 in LS, respectively. The other OD-type M-QTL qPN4.3 was found in the RILTCF1s and corresponding AHMPs. Three M-QTLs qPN1.3, qPN7.2 and qPN9.2 appeared to be UD-type QTLs, which decreased AHMPs values by 2.1 and 1.3 in LS and 8.5 in WZ, respectively. The remaining 15 M-QTLs were detected as additive types, including 8 M-QTLs (qPN1.1, qPN1.4, qPN3, qPN4.2, qPN4.4, qPN5, qPN6.2 and qPN6.3) detected only in the RILs or XSILs, 1 (qPN2) both in the RILs and XSILs, and 6 (qPN4.1, qPN6.1, qPN7.1, qPN9.1, qPN11 and qPN12) only in the RILTCF1s or XSILTCF1s. Notably, five genes Os08g0323700 (OsCCC1), Os09g0410500 (OsTb2), Os09g0470500 (Oshox4), Os09g0466400 (OsZHD1) and Os09g0457900 (OsEATB) reportedly contributing to PN were located nearby the UD-type M-QTL qPN9.2 in WZ, which had a largest negative effect − 8.5 on PN from heterozygote in the TCF1s. Moreover, qPN6.2, with a negatively additive effect of 1.4 from XS09 allele and the largest logarithm of the odds (LOD) score detected in the lines in WZ, contained two known genes Os06g0157700 (Hd3a) and Os06g0181300 (AID1) for PN.
Of 12 M-QTLs, each six were identified for FGNP in LS and WZ, including ten additive (qFGNP1, qFGNP4.1, qFGNP4.2, qFGNP4.3, qFGNP6.2, qFGNP6.3, qFGNP7.2, qFGNP10, qFGNP11 and qFGNP12) and two UD-type (qFGNP6.1 and qFGNP7.1) QTLs, which collectively explained 23.6% (20.4%), 3.6% (10.6%) and 0 (14.9%) of the total phenotypic variance in the RILs, RILTCF1s and corresponding AHMPs in LS (WZ), and 22.5% (10.1%) and 6.7% (9.9%) of the total phenotypic variance in the XSILs and XSILTCF1s in LS (WZ), respectively. Among them, the UD-type M-QTL qFGNP7.1 was only detected in the AHMPs of RILTCF1s, with the heterozygote reduced FGNP by 17.8 in WZ. The other UD-type M-QTL qFGNP6.1, simultaneously detected in the RILTCF1s and corresponding AHMPs, with a dominance effect of 16.6 for reduced FGNP in WZ.
A total of 33 M-QTLs were detected for TGW, including 20, 12, and 1 QTLs identified only in LS, WZ, and both locations, respectively. These 21 M-QTLs (8 additive, 9 D-type, 1 OD-type and 3 UD-type QTLs) detected in LS together accounting for 44.2% (47.6%), 25.4% (42.6%), and 23.4% (10.8%) of the total phenotypic variance in the RILs (XSILs), RILTCF1s (XSILTCF1s) and their AHMPs, respectively. Of them, three (qTGW1.1, qTGW2.2 and qTGW6.1) of nine D-type M-QTLs were adjacent to three known TGW-related genes GW5L, OsGS1 and OsKASI. Two (qTGW3.3 and qTGW3.6) of three UD-type M-QTLs had a negative overdominance effect causing decreased TGW by 0.82 g and 0.73 g, which co-located or were close to the known TGW-related genes, GS3, OsMADS34 and Pho1. In the WZ experiment, 13 TGW M-QTLs (10 additive, 1 OD-type and 2 UD type QTLs) were identified together accounting for 27.9% (21.4%), 33.0% (15.4%) and 13.7% of the total phenotypic variance in the RILs (XSILs), RILTCF1s (XSILTCF1s) and AHMPs of XSILTCF1s, respectively. Only OD-type M-QTL (qTGW8.2) was detectable in AHMPs of XSILTCF1s, with the heterozygote associated with increased TGW by 3.84 g.
Fifteen M-QTLs were detected for GYP, including 13 QTLs (10 additive, 2 OD-type and 1 UD-type) together explained 20.9% (20.6%), 24.6% (10.1%) and 19.7% (5.7%) of the total phenotypic variance in RILs (XSILs), RILTCF1s (XSILTCF1s) and their AHMPs in LS, respectively, and 2 additive QTLs explained 6.5% in XSILs and 3.5% (1.2%) in RILTCF1s (XSILTCF1s) in WZ. Of these, only UD-type QTL (qGYP10.1) was identified with a negative dominance effect of 5.4 g in LS, which contained a known gene OsPQT3 controlling rice grain yield in the field conditions. In addition, two OD-type M-QTLs qGYP9 and qGYP12.1 were only detected in AHMPs of RILTCF1s and both with positive dominance effects of 6.0 g and 7.4 g in WZ.
Not any co-located QTL was detected among 32 QTLs for the traits in the RIL and the AHMP populations at the two locations, and only one co-located QTL (qTGW1.1) was detected at LS among 34 QTLs for the traits in the XSIL and the AHMP populations (Table 3).
Epistatic QTL mapping of yield-related traits and mid-parent heterosis
In order to test whether these M-QTLs had epistatic effects on the relevant traits, we checked all epistatic QTL (E-QTL) pairs identified in the RILs or XSILs, their TCF1s and AHMPs. Eight, 21 and 12 E-QTL pairs were identified in line, TCF1 and AHMP populations, respectively, which involved one M-QTL and the other random loci (Table 4). For PN, 3 and 4 E-QTLs in the RILs and XSILs, 6 E-QTLs in XSILTCF1s, and 4 E-QTLs in AHMPs of XSILTCF1s were identified. The total phenotypic variation explained (PVE) of E-QTLs for PN was 6.84% for RILs in LS and 11.10% for XSILs in WZ, 6.32% for XSILTCF1s in WZ, and 6.40% and 1.12% for AHMPs of XSILTCF1s in LS and WZ, respectively. For GYP, only one E-QTL in the RILs, 15 E-QTLs in XSILTCF1s, and 8 E-QTLs in AHMPs of XSILTCF1s were identified. The total PVE of E-QTLs for GYP was 13.05% for RILs in LS, 3.52% for XSILTCF1s in WZ, and 1.86% for AHMPs in WZ. Among M-QTLs involved epistasis on GYP and PN, qGYP1 and qPN1.3 on chromosome 1 were the most important loci contributing to the TCF1 performances and heterosis of GYP and PN both in XSIL and the corresponding AHMP populations. No E-QTLs were identified for TGW and FGNP. No E-QTL between two M-QTLs was observed.
Discussion
QTLs play an important role to enrich our understanding of the genetic basis of heterosis in rice. This study permitted the direct measurement of heterosis for all the measured traits using two sets of lines (RILs and XSILs) together with their TCF1s and HMPs that exaggerated the capacity to more precisely resolve different types of gene actions for identified QTLs that were responsible for trait performance and heterosis.
In this study, the phenotypic performance of four yield traits was evaluated in two locations, LS and WZ. The performance of hybrid plants was mainly determined by their mid-parental value and the heterosis level. In both the locations, the XSILTCF1s had higher HMPS of PN, FGNP, and GYP but lower TGW than the RILTCF1s, which suggested that the portion of introgression of IR2061 (indica)’s genomic fragment into XS09 (japonica) background may influence the effect of heterosis. Recently, Lin et al. reported that the introduction of japonica germplasm played an important role in indica hybrid breeding36. In their study, only 3.31% of the genome in the parents of the indica hybrids were contributed by japonica germplasm, which affected about half of the grain yield heterotic loci36. To evaluate the effects of the proportion of indica and japonica genome on heterosis, we explored the correlations between the ratios of heterozygous or homozygous XS09 alleles and the heterosis for four yield-related traits (Fig. 2). We observed a weak trend for higher ratios of homozygous XS09 genotype in RILs/XSILs or heterozygous genotype in their TCF1s with higher heterosis for most yield-related traits except for TGW. This trend is even more pronounced in the combined line populations (RILs and XSILs). Indeed, moderate genomic differences between parents of indica–japonica cross do improve, at least to some extent, grain yield and its degree of heterosis in rice30,37,38. However, the genomic differences between parents of indica–japonica cross for attaining strongest heterosis varies according to the cross, and actually there is no a fixed proportion of introgression of indica genome into japonica background or japonica genome into indica background for the parents, as indicated by indica–japonica hybrid breeding practices in China37,39,40.
Our study provides a possibility to identify significant genetic factors for heterosis by comparing QTLs detected from different datasets. A total of 81 M-QTLs were identified for phenotypic variation of four traits of lines (RILs and XSILs) or TCF1s and AHMP values in both locations. The most striking finding was the presence of two predominant types of M-QTLs for yield-related traits except for TGW, the additive M-QTLs and OD/UD-type M-QTLs, without M-QTLs exhibiting complete and partial dominance (Fig. 3, Table 3). Previously, it has been reported that the genetic basis of heterosis is mainly determined by dominance and overdominance effects14,17,41. Furthermore, Wen et al. used F1 hybrids in the NCII design to dissect the genetic basis of heterosis by investigating the factors that mainly affect the heterosis were dominance, dominance-by-dominance, overdominance, and complete dominance QTL42. Here, 83.3%, 80.0%, 71.4% and 16.7%, 20.0%, 23.8% of the detected M-QTLs were attributed by additive QTL actions and OD/UD-type QTL actions in FGNP, GYP and PN, respectively. However, 9 of 10 D-type M-QTLs in this study were detected in TGW, which may result in a relatively low proportion (51.5%) of the detected 33 M-QTLs for TGW with additive QTL actions. On the other hand, it is worth noting that 13 of 81 M-QTLs were also detected as E-QTLs with another random locus without significant M-QTL. Our results confirmed the previous reports of epistasis and overdominance as the major genetic basis of heterosis in rice8,43, and these findings are also consistent with Li et al.44 and Melchinger et al.45. However, almost no genetic overlap was found between the QTLs affecting the traits in inbred line populations (RILs and XS09) and the ones underlying heterosis in their testcross populations (XSILTCF1s and RILTCF1s) under the two locations, suggesting that different genetic mechanisms involved in trait itself and its heterosis.
In the present study, GYP showed a strongest heterosis among the four yield-related traits studied, and TGW had a weakest heterosis in XSILTCF1s and RILTCF1s (Table 1). Heterosis for GYP in TCF1s was mainly attributed to yield-component traits FGNP and PN both in the two sets of testcrosses, consistent with the findings of previous studies performed on rice8,30,37,43. In TCF1s, the heterosis of GYP and yield component traits (PN and FGNP) was mainly produced by the overdominance of heterotic loci, indicating that non-additive gene actions are pivotal to grain yield. This finding is consistent with He et al. as they used RILs based NCII design for E-QTLs to estimate genomic position, digenic interactions of QTL, additive and dominance effects, and they noted that non-additive gene actions mainly contribute to heterosis46. Further, we explored the correlations among the lines, TCF1s and HMPs, and found highly positive correlations between TCF1s and HMPs and lower positive correlations between the lines and TCF1s for FGNP and GYP in both locations (Table 2). These results suggested that non-additive QTL was a contributor to TCF1s for FGNP and GYP traits. Contrarily, a high positive correlation was found between the lines and TCF1s for TGW compared to the low positive correlation between TCF1s and HMPs in LS, indicating that additive QTL mainly contribute to TCF1s for TGW only in LS. However, the correlations between the lines and TCF1s for TGW in WZ exhibited contradictory results as indicted in LS. The negative correlation between the lines and their HMPs evidently indicated that additive and dominant QTLs acted independently in the testcross populations as previously reported17. In our study, the correlations between the lines and HMPs for yield traits were different according to genetic background and location, which suggested that the role of heterotic loci for yield was affected by genetic background and environment8,41,47,48. Actually, a lot of epistasis between two random loci were detected for the four traits under the two locations (data not shown). So, complexity of heterosis in rice, reflected by a large number of loci involved, complex epistatic relationships, and genetic background- and environment-dependent gene actions on heterosis, suggested that marker-assisted selection for significantly improving heterosis of yield traits in hybrid rice breeding programs may be very challenging.
A few already reported genes in rice were co-located with the heterotic QTLs identified in this study such as qGYP10.1 (OsPQT3, increases grain yield in the field)49, qPN9.2 and qTGW9 (OsCCC1 for cell elongation and panicle number50, OsTb2 for tillering and grain yield per panicle51, and Oshox4 affecting bushy tillers52, OsZHD1 for tiller number53, and OsEATB, ERF protein associated with tillering and panicle branching54, qTGW1.1 (GW5L, negatively regulates grain width and weight)55, qTGW2.2 (OsGS1, growth rate and grain filling)56, qTGW3.3 (GS3, grain length and weight)57, qTGW3.6 (OsMADS34, grain size and yield58, Pho1, the size of mature seeds and the starch content)59, and qTGW6.1 (OsKASI, 1000-grain weight and tiller number)60. All the above mentioned genes provided heterotic effects with heterozygous alleles in UD-type or D-type M-QTLs. However, some M-QTLs detected in this study exhibited a positive overdominance heterosis such as qPN1.2, qPN1.5 and qPN4.3 for increased AHMPs of PN in TCF1s; qGYP9 and qGYP12.1 for positive overdominance effect of GYP in both TCF1s and AHMPs. They enhanced the performance of TCF1s hybrid by increasing PN and GYP. Similarly, qTGW3.4 and qTGW8.2 enhanced TGW and showed a positive overdominance effect in AHMPs. These overdominance heterotic QTLs identified in this study across different populations would be highly valuable for breeding to enhance grain yield of hybrid rice by marker-assisted selection.
Materials and methods
Experimental materials
The rice populations used in this study included a set of 209 F2:10 RILs derived from single-seed descent from a cross between a photosensitive late japonica variety XS09 developed in China and an indica inbred line IR2061 developed at IRRI, and a set of 222 BC2F8 XSILs under XS09 background with IR2061 as a donor. Then two testcross F1 populations were developed by crossing the RILs and XSILs to a common maternal tester line PA64S, which is a stable indica thermo-sensitive genic male sterile line with excellent wide compatibility to both indica and japonica cultivars61 and has been extensively used as a sterile line in two-line hybrid rice breeding programs in China. The first testcross population consisting of 209 RILTCF1s from crosses between the RILs (used as male) and PA64S. The second one consisted of 222 XSILTCF1s from crosses between the XSILs (used as male) and PA64S. In addition, the parents XS09 and IR2061, PA64 (the recurrent parent of PA64S, an isogenic line of PA64S), two F1s (XS09 × IR2061 and PA64S × XS09) were used as checks in the phenotyping experiments.
Field experiment and trait evaluation
Field experiments were conducted at two experimental stations of Wenzhou Academy of Agricultural Sciences, including LS (18.5° N, 110° E) of Hainan Province and WZ (27.3° N, 119.4° E) of Zhejiang Province. In the LS experiment, the RILs, XSILs, the two testcross populations (RILTCF1s and XSILTCF1s), the parents (XS09, IR2061 and PA64S) and their F1s (XS09 × IR2061 and PA64S × XS09) were sowed in the seedling nursery on November 25, 2014. The 25-day-old seedlings were transplanted into four-row plots each consisting of a single row of the male RIL, XSIL and the two testcross hybrids (RILTCF1 and XSILTCF1). The plots were arranged in a randomized complete block design with three replications. Each row within a plot consisted of 12 plants with a spacing of 17 cm between the plants and 25 cm between rows. Five check plots consisting of XS09, IR2061, PA64S, and XS09 × IR2061 F1 and PA64S × XS09 F1 were randomly arranged in each replication. In the WZ experiment, materials were sowed in the seedling nursery on June 15, 2015, and the 25-day-old seedlings were transplanted into four-row plots each consisting of a single row of a RIL, XSIL and the two testcross hybrids. The field arrangement in WZ was the same as the LS experiment. In addition, five check plots consisting of XS09, IR2061, PA64, XS09 × IR2061 F1, and PA64S × XS09 F1 were included in each replication. Crop management followed local field production practices in the two sites. At maturity, PN was investigated from the middle 10 plants. Total grain number per panicle was measured from 10 main panicles from the middle 10 plants (one main panicle each plant) each plot. The grain yield each plot was obtained after weighing all grains collected from the rest panicles and the 10 main panicles of the middle 10 plants. Then GYP (g) was calculated by the ratio of grain yield each plot to 10. FGNP was calculated by the ratio filled grain number each plot to 10. An estimate of the TGW (g) was made by weighing three lots of 100 grains per entry.
For each testcross F1, AHMP and the relative HMP was calculated as AHMP = F1 − MP and HMP (%) = (F1 − MP)/MP × 100, respectively, where F1 is the trait value of a testcross F1 and MP is the mean value of the corresponding paternal RIL or XSIL and the common maternal tester line PA64S in LS and PA64 (an isogenic line of PA64S) in WZ. PA64S was replaced by PA64 for trait measurement because the former shows sterility in later season in WZ, where the temperature is over 23.5 °C at the panicle differentiation stage (a crucial stage of fertility transformation of two-line sterile lines.
SNP genotyping
Genomic DNA for SNP genotyping was isolated from approximately 100 mg fresh leaf samples of 5-week-old seedlings for the 209 RILs, 222 XSILs, the parents XS09 and IR2061, and a tester line PA64S using a modified cetyltrimethylammonium bromide (CTAB) method62. Genotyping was performed using a customized rice 56 K SNP array containing 56,897 SNP screened from the 3 K Rice Genome Project63,64. Target DNA preparation, chip hybridization, and array processing were conducted by CapitalBio Technology (Beijing, China) according to the Affymetrix Axiom 2.0 assay protocol. A total of 39,070 high-quality SNPs was screened based on polymorphism between XS09 and IR2061. Among them, 25,296 high-quality non-redundant SNPs were finally selected for genotype analysis (Fig. S1).
Genotypes of TCF1s at 25,296 SNPs were determined based on the SNP genotypes of the corresponding RILs or XSILs and PA64S. Specifically, if the two parents (RIL or XSIL and PA64S) have the same homozygous genotype, their TCF1s shared the same genotype, and if the two parents have different homozygous genotypes, the genotypes of their TCF1s were deduced as heterozygotes.
Genetic linkage map construction and QTL mapping
Filtered and high-quality SNPs with less than 10% missing were used for the construction of bin maps for each population using the BIN function in QTL IciMapping Version 4.265. For non-redundancy, only one SNP was retained to represent each bin, either one with a minimum missing rate, or a random one when the missing rate was equal. The SNPs which displayed a unique pattern of segregation and did not fall into a bin were removed. We then constructed the linkage map of each population using these bins by the MAP function in QTL IciMapping Version 4.265. The values obtained for the recombination frequencies were converted into map distance by the Kosambi mapping function66. A total of 1756 bins were used to construct two high-density linkage maps with 2017.1 cM and 1082.9 cM for RIL and XSIL populations, respectively. The genotypes for each cross in the RILTCF1s and XSILTCF1s were deduced from the RILs/XSILs and the original parents that were used as the parents for the crosses.
QTL mapping for four yield-related traits was performed separately for the RIL, XSIL, RILTCF1, and XSILTCF1 populations. For the RIL and XSIL datasets, the mean trait values from three replications at each location were used as input data. For RILTCF1 and XSILTCF1 populations, the mean trait values and AHMPs of the TCF1s were used as input data. All datasets were analyzed using the biparental populations (BIP) function in QTL IciMapping Version 4.265. The inclusive composite interval mapping of additive (ICIM-ADD) QTL method was performed to identify M-QTLs by using default settings. The analyses of M-QTLs were performed with pre-adjusted IciMapping parameters, in which the P values for entering a variable (PIN) were set at 0.001 and the scanning step was set at 1.0 cM. The inclusive composite interval mapping of the digenic epistatic (ICIM-EPI) QTL method was used to find possible digenic E-QTLs by using default settings. The corresponding scan step and PIN for E-QTLs mapping were set at 5 cM and 0.0001, respectively. The LOD threshold values 3.0 and 5.0 were used to declare significant M-QTLs and E-QTLs, respectively. The physical position of a QTL was retrieved based on the left and right markers of the detected interval. The known genes underlying the related traits within an identified QTL interval were considered as candidate genes based on the Nipponbare reference genome (IRGSP 1.0)67. The QTLs were named as “q + trait abbreviation + chromosome number + QTL number” following the rules recommended by McCouch and CGSN68. The type of digenic epistasis without M-QTL was ignored.
Inference of M-QTL actions
The detected M-QTLs can be divided into four types by estimating additive effect and dominance effect in RILs or XSILs and corresponding TCF1s according to the Mei et al.’s and Kim et al.’s methods18,69: additive (detected only in lines or TCF1s) QTLs, heterotic (D-type, OD-type, and UD-type) M-QTLs. D-type and OD-type QTLs were determined using the values of d/a and a + d. QTLs with 0 <|d/a|< 1 or |2d/(a + d)|≤ 1 or |2a/(a + d)|≥ 1 were designated as D-type QTLs. QTLs with 0 <|d/a|< 1 or |2d/(a + d)|> 1 or |2a/(a + d)|< 1, or those detected only in AHMP datasets, were designated as OD-type QTLs. QTLs detected in AHMP datasets but showing negative ‘d’ values were defined as UD-type QTLs. Of the detected QTLs, only D-type, OD-type, and UD-type M-QTLs were used for subsequent comparative studies and direct effect analysis. In this study, all these heterotic QTLs are genetic loci underlying heterosis of yield-related traits in rice.
Statistical analysis
Analyses of variance were performed to determine significant variation between locations and genotypes for all measured traits by the Agricolae Package in R. Significant phenotypic differences among the check parents and the relative hybrids using Duncan’s multiple comparison test, and among RILs, XSILs and the TCF1s were statistically assessed using Student’s t-test by the agricolae package in R. Here, RILs and XSILs when combined were termed “lines”. HMP was tested with a Student’s t-test based on the contrast between F1 hybrid mean and average performance of corresponding parental lines70. Pearson’s correlation analyses among the phenotypic traits measured were performed by the Hmisc Package in R.
Legislation statement
The experimental research and field studies on plant materials comply with relevant institutional, national, and international guidelines and legislation.
Abbreviations
- LS:
-
Lingshui
- WZ:
-
Wenzhou
- XS09:
-
Xiushui09
- PA64S:
-
Peiai64S
- PN:
-
Panicle number per plant
- FGNP:
-
Filled grain number per panicle
- TGW:
-
1000-Grain weight
- GYP:
-
Grain yield per plant
- RIL:
-
Recombinant inbred line
- XSIL:
-
Xiushui09 background introgression line
- TCF1 :
-
Testcross F1
- RILTCF1s:
-
Testcross F1s of RILs with PA64S
- XSILTCF1s:
-
Testcross F1s of XSILs with PA64S
- H MP :
-
Mid-parent heterosis
- AH MP :
-
Absolute mid-parent heterosis
- QTL:
-
Quantitative trait locus
- M-QTL:
-
Main-effect QTL
- E-QTL:
-
Epistatic QTL
- D-type:
-
Dominance-type
- OD-type:
-
Overdominance-type
- UD-type:
-
Underdominance-type
- PVE:
-
Phenotypic variation explained
- SNP:
-
Single nucleotide polymorphism
- LOD:
-
Logarithm of the odds
References
Tao, Y. et al. Exploitation of heterosis loci for yield and yield components in rice using chromosome segment substitution lines. Sci. Rep. 6, 36802–36802. https://doi.org/10.1038/srep36802 (2016).
Chen, L. et al. Marker-assisted breeding of a photoperiod-sensitive male sterile japonica rice with high cross-compatibility with indica rice. Mol. Breed. 27, 247–258. https://doi.org/10.1007/s11032-010-9427-z (2011).
Zhou, G. et al. Genetic composition of yield heterosis in an elite rice hybrid. Proc. Natl. Acad. Sci. U.S.A. 109, 15847–15852 (2012).
Fu, D. et al. What is crop heterosis: New insights into an old topic. J. Appl. Genet. 56, 1–13. https://doi.org/10.1007/s13353-014-0231-z (2015).
Fujimoto, R. et al. Recent research on the mechanism of heterosis is important for crop and vegetable breeding systems. Breed. Sci. 68, 145–158. https://doi.org/10.1270/jsbbs.17155 (2018).
Jones, W. Hybrid vigor in rice. J. Amer. Soc. Agron 18, 423–428 (1926).
Cheng, S.-H. et al. Super hybrid rice breeding in China: Achievements and prospects. J. Integr. Plant Biol. 49, 805–810. https://doi.org/10.1111/j.1744-7909.2007.00514.x (2007).
Li, Z. K. et al. Overdominant epistatic loci are the primary genetic basis of inbreeding depression and heterosis in rice. I. Biomass and grain yield. Genetics 158, 1737–1753 (2001).
Wu, X. Prospects of developing hybrid rice with super high yield. Agron. J. 101, 688. https://doi.org/10.2134/agronj2008.0128f (2009).
Bagheri, N. & Jelodar, N. Heterosis and combining ability analysis for yield and related-yield traits in hybrid rice. Int. J. Biol. https://doi.org/10.5539/ijb.v2n2p222 (2010).
Yuan, L. P. Development of hybrid rice to ensure food security. Rice Sci. 21, 1–2 (2014).
Shukla, S. K. & Pandey, M. P. Combining ability and heterosis over environments for yield and yield components in two-line hybrids involving thermosensitive genic male sterile lines in rice (Oryza sativa L.). Plant Breed. 127, 28–32. https://doi.org/10.1111/j.1439-0523.2007.01432.x (2008).
Chin, J. H. et al. Identification of QTLs for hybrid fertility in inter-subspecific crosses of rice (Oryza sativa L.). Genes Genom. 33, 39–48. https://doi.org/10.1007/s13258-010-0100-z (2011).
Chu, S.-H., Jiang, W., Lee, J., Chin, J. H. & Koh, H.-J. QTL analyses of heterosis for grain yield and yield-related traits in indica-japonica crosses of rice (Oryza sativa L.). Genes Genom. 34, 367–377. https://doi.org/10.1007/s13258-011-0223-x (2012).
Dan, Z. et al. Balance between a higher degree of heterosis and increased reproductive isolation: A strategic design for breeding inter-subspecific hybrid rice. PLoS ONE 9, e93122. https://doi.org/10.1371/journal.pone.0093122 (2014).
Thalapati, S. et al. Heterosis and combining ability in rice as influenced by introgressions from wild species Oryza rufipogon including qyld2.1 sub-QTL into the restorer line KMR3. Euphytica 202, 81–95. https://doi.org/10.1007/s10681-014-1222-1 (2014).
Xin, X. Y., Wang, W. X., Yang, J. S. & Luo, X. J. Genetic analysis of heterotic loci detected in a cross between indica and japonica rice (Oryza sativa L.). Breed. Sci. 61, 380–388. https://doi.org/10.1270/jsbbs.61.380 (2011).
Kim, C.-K. et al. Identification of heterosis QTLs for yield and yield-related traits in indica-japonica recombinant inbred lines of rice (Oryza sativa L.). Plant Breed. Biotechnol. 5, 371–389. https://doi.org/10.9787/PBB.2017.5.4.371 (2017).
Kim, M.-S. et al. Breeding hybrid rice with genes resistant to diseases and insects using marker-assisted selection and evaluation of biological assay. Plant Breed. Biotechnol. 7, 272–286. https://doi.org/10.9787/PBB.2019.7.3.272 (2019).
Rongrong, M. et al. Heterosis on plant morphology of yongyou 6, an indica-japonica inter-subspecific super high-yielding hybrid rice. Chin. J. Rice Sci. 21, 281–286 (2007).
Lin, J.S.X.-W., Wu, M. & Cheng, S. Breeding technology innovation of indica-japonica super hybrid rice and varietal breeding. Sci. Agric. Sin 49, 207–218 (2016).
Huang, X. et al. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat. Genet. 42, 961–967. https://doi.org/10.1038/ng.695 (2010).
Huang, X. et al. Genome-wide association study of flowering time and grain yield traits in a worldwide collection of rice germplasm. Nat. Genet. 44, 32–39. https://doi.org/10.1038/ng.1018 (2012).
Huang, X. et al. Genomic architecture of heterosis for yield traits in rice. Nature 537, 629–633. https://doi.org/10.1038/nature19760 (2016).
Zhen, G. et al. Genome-wide dissection of heterosis for yield traits in two-line hybrid rice populations. Sci. Rep. 7, 7635–7635. https://doi.org/10.1038/s41598-017-06742-7 (2017).
Shen, G., Zhan, W., Chen, H. & Xing, Y. Dominance and epistasis are the main contributors to heterosis for plant height in rice. Plant Sci. 215–216, 11–18. https://doi.org/10.1016/j.plantsci.2013.10.004 (2014).
Bian, J. et al. Identification of japonica chromosome segments associated with heterosis for yield in indica × japonica rice hybrids. Crop Sci. 50, 2328–2337. https://doi.org/10.2135/cropsci2010.05.0242 (2010).
Luo, X.-J., Xin, X.-Y. & Yang, J.-S. Genetic-basis analysis of heterotic loci in Dongxiang common wild rice (Oryza rufipogon Griff.). Genet. Res. 94, 57–61. https://doi.org/10.1017/S0016672312000250 (2012).
Yu, S. et al. Importance of epistasis as the genetic basis of heterosis in an elite rice hybrid. Proc. Natl. Acad. Sci. U.S.A. 94, 9226–9231. https://doi.org/10.1073/pnas.94.17.9226 (1997).
Li, L. et al. Dominance, overdominance and epistasis condition the heterosis in two heterotic rice hybrids. Genetics 180, 1725. https://doi.org/10.1534/genetics.108.091942 (2008).
Zhu, Y.-J. et al. Detection of QTLs for yield heterosis in rice using a RIL population and its testcross population. Int. J. Genom. 1–9, 2016. https://doi.org/10.1155/2016/2587823 (2016).
Mei, H. W. et al. Gene actions of QTLs affecting several agronomic traits resolved in a recombinant inbred rice population and two testcross populations. Theor. Appl. Genet. 107, 89–101. https://doi.org/10.1007/s00122-003-1192-5 (2003).
Xiang, C. et al. Dissection of heterosis for yield and related traits using populations derived from introgression lines in rice. Crop J. 4, 468–478. https://doi.org/10.1016/j.cj.2016.05.001 (2016).
Latha, S., Deepak, S. & Gulzar, S. S. Combining ability and heterosis for grain yield and its component traits in rice (Oryza sativa L.). Not. Sci. Biol. 5, 90. https://doi.org/10.15835/nsb519006 (2013).
Xu, S., Zhu, D. & Zhang, Q. Predicting hybrid performance in rice using genomic best linear unbiased prediction. Proc. Natl. Acad. Sci. U.S.A. 111, 12456–12461. https://doi.org/10.1073/pnas.1413750111 (2014).
Lin, Z. et al. Divergent selection and genetic introgression shape the genome landscape of heterosis in hybrid rice. Proc. Natl. Acad. Sci. U.S.A. 117, 4623. https://doi.org/10.1073/pnas.1919086117 (2020).
Li, D. et al. Integrated analysis of phenome, genome, and transcriptome of hybrid rice uncovered multiple heterosis-related loci for yield increase. Proc. Natl. Acad. Sci. U.S.A. 113, E6026. https://doi.org/10.1073/pnas.1610115113 (2016).
Wang, Z. Q. J. L. et al. QTL Mapping of heterotic loci of yield-related traits in rice. Chin. J. Rice Sci. 27, 569–576 (2013).
Li, R. H. X., He, Y. Q., Yuan, L. P. & Wang, X. K. Relationship between the exent of genetic differentiation in parental lines and heterosis of indica-japonica hybrid rice. Acta Agron. Sin. 24, 564–576 (1998).
Chen Shen-guang, C.L.-Y., Hua-min, S. I. & Shi-hua, C. Subspecific differentiation of male sterile lines of rice derived from indica/japonica cross and geterosis performance of the F1 hybrids. Chin. J. Rice Sci 24, 667–671 (2010).
Hua, J. et al. Single-locus heterotic effects and dominance by dominance interactions can adequately explain the genetic basis of heterosis in an elite rice hybrid. Proc. Natl. Acad. Sci. 100, 2574. https://doi.org/10.1073/pnas.0437907100 (2003).
Wen, J. et al. Genetic dissection of heterosis using epistatic association mapping in a partial NCII mating design. Sci. Rep. 5, 18376–18376. https://doi.org/10.1038/srep18376 (2015).
Luo, L. J. et al. Overdominant epistatic loci are the primary genetic basis of inbreeding depression and heterosis in rice. II. Grain yield components. Genetics 158, 1755–1771 (2001).
Li, L. et al. Genomewide mapping reveals a combination of different genetic effects causing the genetic basis of heterosis in two elite rice hybrids. J. Genet. 94, 261–270. https://doi.org/10.1007/s12041-015-0527-8 (2015).
Melchinger, A. E., Utz, H. F., Piepho, H. P., Zeng, Z. B. & Schön, C. C. The role of epistasis in the manifestation of heterosis: A systems-oriented approach. Genetics 177, 1815–1825. https://doi.org/10.1534/genetics.107.077537 (2007).
He, X., Hu, Z. & Zhang, Y.-M. Genome-wide mapping of QTL associated with heterosis in the RIL-based NCIII design. Chin Sci. Bull. 57, 2655–2665. https://doi.org/10.1007/s11434-012-5127-x (2012).
Zou, Y., Zhou, S. & Tang, Q. Status and prospect of high yielding cultivation researches on China super hybrid rice. J. Hunan Agric. Univ. 29, 78–84 (2003).
Haque, M. M., Pramanik, H. R., Biswas, J. K., Iftekharuddaula, K. M. & Hasanuzzaman, M. Comparative performance of hybrid and elite inbred rice varieties with respect to their source-sink relationship. Sci. World J. 2015, 326802. https://doi.org/10.1155/2015/326802 (2015).
Alfatih, A. et al. Loss of rice paraquat tolerance 3 confers enhanced resistance to abiotic stresses and increases grain yield in field. Plant Cell Environ. 43, 2743–2754. https://doi.org/10.1111/pce.13856 (2020).
Chen, Z. C., Yamaji, N., Fujii-Kashino, M. & Ma, J. F. A Cation-chloride cotransporter gene is required for cell elongation and osmoregulation in rice. Plant Physiol. 171, 494–507. https://doi.org/10.1104/pp.16.00017 (2016).
Lyu, J. et al. Neo-functionalization of a teosinte branched 1 homologue mediates adaptations of upland rice. Nat. Commun. 11, 725. https://doi.org/10.1038/s41467-019-14264-1 (2020).
Zhou, W., Malabanan, P. B. & Abrigo, E. OsHox4 regulates GA signaling by interacting with DELLA-like genes and GA oxidase genes in rice. Euphytica 201, 97–107. https://doi.org/10.1007/s10681-014-1191-4 (2015).
Xu, Y. et al. Overexpression of OsZHD1, a zinc finger homeodomain class homeobox transcription factor, induces abaxially curled and drooping leaf in rice. Planta 239, 803–816. https://doi.org/10.1007/s00425-013-2009-7 (2014).
Qi, W. et al. Rice ethylene-response AP2/ERF factor OsEATB restricts internode elongation by down-regulating a gibberellin biosynthetic gene. Plant Physiol. 157, 216. https://doi.org/10.1104/pp.111.179945 (2011).
Tian, P. et al. GW5-Like, a homolog of GW5, negatively regulates grain width, weight and salt resistance in rice. J. Integr. Plant Biol. 61, 1171–1185. https://doi.org/10.1111/jipb.12745 (2019).
Tabuchi, M. et al. Severe reduction in growth rate and grain filling of rice mutants lacking OsGS1;1, a cytosolic glutamine synthetase1;1. Plant J. 42, 641–651. https://doi.org/10.1111/j.1365-313X.2005.02406.x (2005).
Fan, C. et al. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 112, 1164–1171. https://doi.org/10.1007/s00122-006-0218-1 (2006).
Zhang, Y. et al. Loss of function of OsMADS34 leads to large sterile lemma and low grain yield in rice (Oryza sativa L.). Mol. Breed. 36, 147. https://doi.org/10.1007/s11032-016-0578-4 (2016).
Satoh, H. et al. Mutation of the plastidial alpha-glucan phosphorylase gene in rice affects the synthesis and structure of starch in the endosperm. Plant Cell 20, 1833–1849. https://doi.org/10.1105/tpc.107.054007 (2008).
Ding, W. et al. OsKASI, a β-ketoacyl-[acyl carrier protein] synthase I, is involved in root development in rice (Oryza sativa L.). Planta 242, 203–213. https://doi.org/10.1007/s00425-015-2296-2 (2015).
Zhang, X. C. Y. et al. Close linkage SSR markers for wide compatibility locus S-5 in rice TGMS line Peiai64. Mol. Plant Breed. 4, 506–512 (2006).
Murray, M. G. & Thompson, W. F. Rapid isolation of high molecular weight plant DNA. Nucl. Acid Res. 8, 4321–4325. https://doi.org/10.1093/nar/8.19.4321 (1980).
Meng, L. et al. Association mapping of ferrous, zinc, and aluminum tolerance at the seedling stage in Indica rice using magic populations. Front. Plant Sci. https://doi.org/10.3389/fpls.2017.01822 (2017).
Wang, W. et al. Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature 557, 43–49. https://doi.org/10.1038/s41586-018-0063-9 (2018).
Meng, L., Li, H., Zhang, L. & Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 3, 269–283. https://doi.org/10.1016/j.cj.2015.01.001 (2015).
Kosambi, D. D. The estimation of map distances from recombination values. Ann. Eugen. 12, 172–175. https://doi.org/10.1111/j.1469-1809.1943.tb02321.x (1943).
Kawahara, Y. et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 6, 4. https://doi.org/10.1186/1939-8433-6-4 (2013).
McCouch, S. & Cooperative, C. Gene nomenclature system for rice. Rice 1, 72–84. https://doi.org/10.1007/s12284-008-9004-9 (2008).
Mei, H. W. et al. Gene actions of QTLs affecting several agronomic traits resolved in a recombinant inbred rice population and two backcross populations. Theor. Appl. Genet. 110, 649–659. https://doi.org/10.1007/s00122-004-1890-7 (2005).
Larièpe, A. et al. The genetic basis of heterosis: Multiparental quantitative trait loci mapping reveals contrasted levels of apparent overdominance among traits of agronomical interest in maize (Zea mays L.). Genetics 190, 795–811. https://doi.org/10.1534/genetics.111.133447 (2012).
Funding
This work was funded by the Key Project (2016c02050-1 and 2016c02050-3) of New Variety Development from Zhejiang Province Science & Technology, the Project (2019zx006-3) of New Variety Development Wenzhou Collaboration Program, and the Agricultural Science and Technology Innovation Program and the Cooperation and Innovation Mission (CAAS-ZDXT202001).
Author information
Authors and Affiliations
Contributions
J.X. and J.L. designed the experiment; W.Y., J.W., H.L. and S.N. performed all the phenotypic evaluation; F.Z. performed analysis and interpretation of the data; S.Z. and F.Z. drafted the manuscript; J.X. and J.L. revised the MS; all authors revised the paper and approved the final version to be published.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, W., Zhang, F., Zafar, S. et al. Genetic dissection of heterosis of indica–japonica by introgression line, recombinant inbred line and their testcross populations. Sci Rep 11, 10265 (2021). https://doi.org/10.1038/s41598-021-89691-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-89691-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.