Abstract
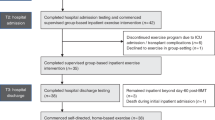
Physical capacity prior allogeneic stem cell transplantation (allo-HCT) has been shown as a relevant prognostic factor for survival after transplant. Therefore, we evaluated feasibility and preliminary efficacy of a high-intensity interval training (HIIT) and moderate to high-intensity resistance exercise (RE) to increase physical capacity in patient’s prior allo-HCT. In this multicentre single arm pilot study, a supervised exercise program was performed twice weekly for 4–12 weeks prior allo-HCT, depending on the individual time remaining. Outcomes were feasibility (recruitment, adherence, safety), physical capacity (cardiorespiratory fitness [VO2peak], muscle strength) and patient reported outcomes (physical functioning, fatigue). Thirty patients were intended, 16 could be included, and 14 completed post intervention assessment (75% male, 55 ± 11 years). The study was stopped early due to a low recruitment rate. Nine patients (64%) reached the initial minimum planned number of eight exercise sessions. Individual adherence was high with 92% for HIIT and 85% for RE. 87% of all performed exercise sessions were completed without complaints and VO2peak increased significantly from 20.4 to 23.4 ml/kg/min. The low recruitment rate suggests that initiation of the intervention concept immediately before allo-HCT is feasible only in a small number of patients. In particular, the timeframe directly prior allo-HCT seems too short for exercise interventions, although the exercise program was designed to improve outcomes in a very short time frame. HIIT and RE were feasible, effective and well accepted by the included patients.
Similar content being viewed by others
Introduction
Allogeneic stem cell transplantation (allo-HCT) is an important treatment option for many hematological malignancies, e.g. leukemia or lymphoma. Although five-year survival rate is increasing due to advances in the management of complications e.g. graft versus host disease (GvHD) or infections1,2, physical capacity after allo-HCT remains low3,4,5, and patients suffer from reduced quality of life (QOL)6,7. Almost half of the patients show moderate to severe impairments in exercise capacity one year after allo-HCT resulting in disability, fatigue und reduced QOL4.
Evidence suggests that exercise interventions in patients with haematological cancer are safe and beneficial. Positive effects range from improved physical capacity to psychosocial effects, resulting in enhanced QOL8,9,10.
However, patients experience significant reductions in physical capacity11,12,13,14,15 already prior allo-HCT. First observational studies demonstrate that objectively assessed cardio-respiratory fitness (CRF) through a cardiopulmonary exercise test at exhaustion (CPET, with VO2peak outcome measure) was a significant predictor for overall survival and non-relapse mortality16,17. Retrospective data support these findings18.
Therefore, it seems to be important to implement exercise interventions prior allo-HCT to achieve a higher level of physical function prior treatment. This concept of prehabilitation has been already examined in cancer pre-surgical settings and proved to be safe and feasible19,20,21. However, only one small pilot study proved the concept of prehabilitation in allo-HCT. The intervention (individually tailored home-based exercise) was safe and feasible and after at least six weeks of intervention, patients significantly improve their CRF22.
In summary, the pre-transplant period seems to be an important and insufficiently investigated research area with the prospect to improve clinical outcomes and prognosis. However, the time frame from scheduling of planned allo-HCT to admission is usually relatively short. It thus seems pivotal to choose an exercise intervention that is able to improve physical capacity in a very short time such as high-intensity interval training (HIIT). HIIT is characterized by relatively short bouts of high-intensity workloads interspersed by periods of low-intensity workloads for recovery. Hereby, the total accumulated time of vigorous exercise is higher than what could be achieved during continuous exercise (usually moderate intensity)23. A recent meta-analysis of HIIT-effects in the prehabilitation of cancer treatment include seven RCTs and show a significant improvement in CRF and positive effects for health-related outcomes24.
Therefore, this pilot-study evaluates safety and feasibility of a combined moderate-to HIIT and RE program in patients with haematological cancer prior allo-HCT. Preliminary efficacy data on CRF, muscle strength, QOL and fatigue were also assessed.
Methods
The study was a multicentre, prospective, single-arm study to investigate safety and feasibility of moderate to high-intensity HIIT and RE in patients with haematological cancer prior allo-HCT. The study (PRESENT-P trial [Pre-Exercise for allogeneic stem cell transplant patients: a pilot-study]) was conducted at the National Center for Tumor Diseases (NCT) Heidelberg and University Hospital Heidelberg, National Center for Tumor Diseases Dresden (NCT/UCC), the Department of Hematology, Oncology, and Stem Cell Transplantation University Medical Center Freiburg and the University Center for Tumor Diseases (UCT) Frankfurt, Germany, between November 2016 and February 2019. The study was approved by the ethics committee (leading committee) of the University Hospital Heidelberg and validated by the local committees of the clinical partners and is registered at ClinicalTrials.gov (NCT03080792; 15/03/2017). All participants provided written informed consent prior initiating any study activity.
Patients were eligible to participate when they met the following inclusion criteria: hematological cancer with allo-HCT scheduled within the next four to 12 weeks, ≥ 18 years of age, sufficient German language skills, willing/ able to train at the provided exercise facilities twice weekly. Exclusion criteria were: heart insufficiency > NYHA III or uncertain arrhythmia, uncontrolled hypertension, severe renal dysfunction (GFR < 30%, Creatinine > 3 mg/dl), insufficient hematological capacity (either haemoglobin value below 8 g/dl or thrombocytes below 30.000/ µL, reduced standing or walking ability, further comorbidities that preclude participation in the exercise program, and engaging in systematic intense exercise training (at least 1h twice weekly). Patients were screened and recruited 4–12 weeks prior admission for allo-HCT. When allo-HCT was scheduled, patients were informed about the study by their treating hematologist. Further information they received from the study personnel at each center.
Intervention
The prehabilitation intervention comprised of individually tailored supervised exercise sessions twice weekly for 4 to 12 weeks. The total duration of the individual intervention length depended on the final scheduling of transplantation. The exercise program consisted of a supervised HIIT on a bicycle ergometer and RE.
The HIIT-protocol was individualized based on the peak power output (PPO) achieved in the cardiopulmonary exercise test (CPET) prior intervention (t0) and comprised eight one-minute high-intensity bouts at 90–100% PPO, interrupted by one-minute rest periods at 50% PPO. Each session started with a 5–10 min warm up at 50% PPO and ended with a 5-min cool-down at 50% PPO. The first two exercise sessions were familiarization sessions at slightly lower intensities of 70% PPO. The selection of exercise intensities was based on own findings where we defined exercise intensities in a sample of patients prior allo-HCT25 and developed based on findings in patients with chronic heart failure26.
HIIT was followed by a 30 min moderate to intense RE program that include four to six exercises targeting the whole body (legs, arms, shoulders, back, and chest) at resistance training machines or free weights. RE was performed at an intensity of 12 repetition maximum, based on a one repetition maximum test (1-RM, only for resistance training machines). The 1-RM was applied after one familiarization session according to approved methods27. Two sets of each exercise were performed (2 × 12 repetitions) at 60–80% of 1 RM.
All exercise sessions were supervised by exercise specialists and performed at one of the recruiting centres or at certified exercise facilities provided by the OnkoAktiv network. The OnkoActive network is a German network with which we link health-oriented exercise facilities to enable training close to patients home. The exercise program complies with the American College of Sports Medicine (ACSM) exercise guidelines for cancer survivors28. When patients were not able to exercise at this high intensity, exercise intensity was reduced to a tolerable intensity (Rating of perceived exertion (RPE) < 18,29). Exercise was contraindicated during fever and acute infections, when thrombocytes dropped below 15.000/µl and when haemoglobin values were < 8 g/dl. Exercise intensity was reduced to 60% PPO when thrombocytes dropped under 20.000/µl and for RE to 40% 1 RM.
Measures
All participants underwent assessment prior intervention (t0) and post intervention/ prior admission for allo-HCT (t1). CRF was measured via CPET on an electronically braked cycle ergometer (Ergoselect 100; Ergoline, Bitz, Germany). We measured peak oxygen uptake (VO2peak) and PPO for exercise prescription. During the graded exercise test (starting with 2-min unloaded warm-up, the load was increased by 10 W every minute, starting at 20 W) respiratory gas exchange was measured continuously using a breath-by-breath system (Ergostik; Geratherm Respiratory, Bad Kissingen, Germany). Gas exchange data and HR were stationary time averaged over 30s. VO2peak and HRmax were considered as the highest 30-s average value during or immediately post exercise. For safety reasons, a 12-lead electrocardiogram (ECG) was recorded continuously. Patients were encouraged to spend maximal effort until voluntary exhaustion.
Additionally, all patients underwent a 6-min walk test according to published guidelines30. For isometric muscle strength assessment, hand grip strength (HGS) was assessed on both sides using JAMAR handgrip dynamometer (Patterson Medical, Warrenville, IL, USA). The test was performed in a sitting position with the elbow attached to the trunk and elbow angle of 90º. The best out of three attempts for both sides was analysed. Additionally, maximum voluntary isometric contraction (MVIC) was measured with IsoMed 2000-system (D&R Ferstl GmbH, Hemau, Germany) for knee extension, elbow flexion and hip flexion at both sides.
QOL was assessed with the validated questionnaire of the European Organisation for Research and Treatment of Cancer (EORTC QOL-C30)31 and fatigue with the Multidimensional Fatigue Inventory (MFI)32. Sociodemographic and medical information were partly obtained from medical records and partly self-reported. Shortly before and directly after each exercise sessions patients reported on actual symptoms, based on the Edmonton Symptom Assessment Scale (ESAS, VAS 0–10)33 and provide a general feedback of the exercise session. The satisfaction of the patients with the intervention was assessed at the end of the intervention on a visual analogue scale (VAS 1–7).
Statistical methods
Since PRESENT-P is a pilot-study that will provide information for a larger confirmatory RCT, no power calculation has been conducted and data were mainly of descriptive nature. We planned to recruit n = 30 patients in four centres. Due to the short time period between indication of allo-HCT and the start of treatment, our feasibility goal was to enable all included patients to perform at least eight prehabilitative exercise sessions before admission for transplantation. Further, we calculated exercise adherence for each patient individually, based on the individual number of available weeks. For this feasibility outcome, we considered a rate of 75% (at least eight exercise sessions possible and adherence to the sessions) as adequate. Regarding safety considerations, we reported on exercise related side effects (e.g. pain, bleeding signs). For preliminary efficacy, we did pre-post comparisons using Wilcoxon rank-sum test, Cohen’s d for VO2peak and present individual responses. All analyses were conducted in SPSS statistical software package (V26).
Ethical approval
This study was performed in line with the principles of Declaration of Helsinki. Approval was granted by the Ethics Committee of the University Hospital Heidelberg (S-030/2016).
Results
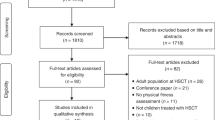
During the recruitment period between 11/2016 until 02/2019, 16 patients were included (n = 10 Heidelberg; n = 3 Dresden; n = 2 Freiburg, n = 1 Frankfurt). Enrolment stopped before the anticipated number of participants was reached because of low recruitment rate due to various reasons. Two patients dropped out (n = 1 allo-HCT postponed, n = 1 reason unknown). In total, 16 patients underwent baseline testing, n = 14 patients post-intervention testing (attrition rate 87.5%). Patient characteristics are presented in Table 1.
Feasibility, adherence and safety
In total, 133 exercise sessions were performed. Intervention length was 5.1 weeks (median 4.4, ranging 1.4–11.6 weeks). Nine patients (64.3%) reached the initial minimum planned number of eight exercise sessions. The mean number of completed exercise sessions was 9.5 (median 8.5, range 3–19). Figure 1 displays the individual adherence to HIIT and RE. Adherence to HIIT was 92% with overall mean interval per exercise of 7.4 from 8 intervals. Adherence to RE was 85.4% with overall mean of 11.7 repetitions (from 12 repetitions per 2 sets).
Exercise adherence. Individual amount of exercise sessions categorized in HIIT and RE (133 sessions in total). HIIT, high intensity interval training; RE, resistance exercise. [Example: Patient no 5 exercised 7 sessions in total, 6 sessions were combined sessions (HIIT + RE) and one session only HIIT].
We received direct feedback of 128 exercise sessions. One-hundred-twelve exercise sessions (87.5%) were without complaints. Session with complaints include the following: four (3.1%) sessions had a premature ending (2 × during HIIT because of exhaustion and palpitations; 2 × during RE because of shoulder pain and lack of energy/weakness; in five (3.9%) sessions patients were unable to perform certain exercises (e.g. reduced intensity or termination of HIIT, termination of certain RE exercises; in seven (5.5%) sessions patients felt discomfort (e.g. 2 × vertigo directly after strain, 2 × exhaustion). At the beginning of 8 sessions (6.3%) patients reported on pain (e.g. muscle soreness, knee pain) associated with the last training session. Further reported complaints like dyspnoea, nausea, infections and bleeding signs were not related to exercise. No adverse events were reported. One-hundred-twelve (87.5%) sessions were rated with “just the right intensity”, eight sessions (6.3%) were rated with “overstraining” (5 × same patient) and seven sessions (5.5%) were rated with “underdemanding”. Enjoyment after each session was rated with 6.4 (MW, median 7) out of 7 points.
After intervention program, likeability (6.64), helpfulness (6.79), enjoyment (6.64), and recommendation to others (6.79) were rated on average with 6.65 out of 7 points. Patients rated the positive effect on the individual physical condition with 6.2 and the positive effect for the psychological effect with 5.4 out of 7.
Preliminary efficacy
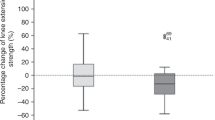
After the intervention program, VO2peak increased significantly from 20.4 to 23.4 ml/kg/min (median improvement 2.15 ml/kg/min, z = − 2.34, p = 0.019, CI 95% 0.67; 5.35, d = 1.6). The individual changes of VO2peak in ml/kg/min are presented in Fig. 2, including individual baseline level of all patients. PPO increased significantly from 117 to 131.2 W (median improvement 15 W, z = − 2.56, p = 0.010, CI 95% 5.04; 23.53).
Completed distance in the 6-min walk test increased significantly from 558.8 to 608.6 m (median improvement 30.5 m, z = − 2.03, p = 0.043). HGS (n = 9) increased significantly from 37.8 to 39.6 kg at the dominant side (median improvement 2 kg, z = − 2.06, p = 0.040) and from 35.4 to 39.5kg at the non-dominant side (median improvement 2 kg, z = − 2.38, p = 0.018). The MVIC test (n = 11) showed no significant changes in muscle strength of knee-extension (change of the dominant side: + 11.25 Nm), elbow-flexion (change of the dominant side: + 2.13 Nm) and the hip-flexion (change of the dominant side: − 14.00 Nm) at the dominant and non-dominant side.
Table 2 displays changes in patient reported outcomes (PROs). Significant improvements could be shown in physical functioning, role functioning, emotional functioning, fatigue and dyspnoea. Results of the MFI show a significant reduction of physical fatigue and reduced activity after intervention period.
To assess a single session effect we compared the results from the ESAS Scale from directly prior to directly after every session. Patients had a significant reduction of pain (z = − 2.19, p = 0.028), anxiety (z = − 3.07, p = 0.002) and general well-being (z = − 2.82, p = 0.0.005 [lower values belongs to better general well-being]) directly after the exercise sessions. For nausea, depression, drowsiness, appetite and dyspnoea no changes could be found.
Discussion
In this pilot-trial, we investigated a supervised HIIT and RE exercise program in patients prior allo-HCT with the aim to improve CRF and muscle strength in a very short timeframe. Results show that our prehabilitation exercise program was feasible, safe, effective, and well accepted by participating patients. However, due to low recruitment rate, the study was stopped before we reached the anticipated number of n = 30 patients.
The initial minimum planned number of eight exercise sessions prior allo-HCT was achieved by nine patients (64.3%). However, only 5.1 (range 1.4–11.6) weeks of training were possible due to scheduling for allo-HCT. Hence, it seems that adherence to exercise was not problematic (adherence depending on individual intervention length was 92% for HIIT and 85.4% for RE), but timing of the intervention, respectively the short time-period available after scheduling for allo-HCT.
All study centres had problems recruiting the patients within our planned recruitment period due to the tight time frame of the patients directly prior allo-HCT. For example, in Heidelberg (Germany) the recruiting study personnel attended the weekly transplant board to screen for eligible patients. Almost all patients received their schedule for allo-HCT only 2 weeks in advanced and were therefore not eligible for this study (inclusion criteria: 4–12 weeks prior planned admission/conditioning for allo-HCT). Main reasons for the short-term planning were the changing clinical status of patients and donor availability. However, this is an organisational problem which cannot be changed by the given procedures in the participating transplant clinics. Further challenges included personal resources, e.g. lack of work force and no capacity by physicians to screen for eligible patients. Therefore, recruitment was very challenging, and for this prehabilitation study concept is was not feasible. We thus stopped recruitment before we reached the anticipated number of n = 30 patients due to the longer recruitment period than anticipated and lack of manpower. Thus, a better collaboration between clinicians and exercise professionals should be sought for future projects.
Another, recently published pilot RCT in patients prior allo-HCT from the US faced similar problems (including insufficient time for exercise before allo-HCT) and the study was stopped because of low recruitment rate as well34. Given these similar implementation barriers of studies in this setting it needs to be questioned, whether the chosen approaches fit with the clinical patient flow as wells as with the demands of allo-HCT patients and their medical treaters. Scientifically, it seems to be indisputable that patients should be at their individual best physical constitution and function level, when they are entering the transplant process. If the medical team is also convinced by this evidence, there is a need to offer an extend time frame to enrol patients in a prehabilitation program prior allo-HCT which requires a modified transplant preparation procedure. Additionally, by incorporating exercise prehabilitation as a routine procedure in the allo-HCT preparation this might led to improved transplant eligibility. Facing the older and partly multimorbid/frailpatient populations scheduled for allo-HCT, future comprehensive transplant programs will not be able to ignore the potential of prehabilitation measures when preparing the patients for this challenging treatment procedure.
Despite problems in recruitment, our prehabilitation concept was safe, feasible and effective for the included patients. Our attrition rate was 87.5% and adherence was with 92% to HIIT and 85.4% to RE relatively high in comparison to two pilot studies prior autologous and allo-HCT22,35 and to our RCTs in allo-HCT during and after transplantation36,37. In comparison to the concept of Wood et al. our intervention program was supervised, more standardised in terms of the exercise contend (free chosen activity vs HIIT protocol on a bicycle ergometer of 8 × 1 min high-intensity at 90–100% PPO) and included moderate to high-intensity resistance exercise34. A recent review of HIIT in cancer patients reported an adherence between 71.2 and 95.6%38. We think that the combination of short intervention period, supervised training (including appointments with the exercise facility) and diverse exercise content have led to the high adherence rate in our study. The very good feedback on likeability, helpfulness and enjoyment underline the feasibility of the exercise program. However, for future studies it would be helpful to have additionally an exercise facility integrated in the transplant unit so that patients could also train directly in the clinic. Particularly in the view of the limited time available, this would make the implementation of exercise much easier.
In our study, only 11 sessions (6.3%) were rated with “overstraining” indicating that our demanding high to moderate intensity exercise program was feasible and acceptable. However, four exercise sessions had a premature ending because of exhaustion and lack of energy and in five sessions, the intensity needed to be reduced or single exercises (RE) needed to be terminated. Since patient’s prior allo-HCT have already limitations in physical capacity caused by previous treatments14 and the clinical status can vary, a good monitoring of training load during every exercise session is mandatory.
Evidence showed that the objectively measured physical capacity prior allo-HCT has an impact on outcomes, such as prognosis and symptom severity11,15,16,17,18. Therefore, it is of high clinical importance to increase CRF and functionality already prior allo-HCT. Furthermore, it is assumed that building up physiological reserve will lead to better treatment tolerance due to higher level of physiologic function throughout treatment and may mitigate further decline after allo-HCT due to complications e.g. GvHD. GvHD after allo-HCT is associated with a further decline in functional performance and reduced QOL39. Despite the short intervention period of 5.5 weeks available and 9.5 exercise sessions performed, we observe significant effects on physical capacity measures.
VO2peak increased significantly from 20.4 to 23.4 ml/kg/min and PPO increased significantly from 117 to 131 W. This VO2 peak improvement correspond to a very large effect (Choen’s d = 1.6). However, these results are in line with the results of another pilot study prior transplantation, where 3.7 ml/kg/min of VO2peak improvement in the allo-HCT patients could be observed22. It could be shown that a VO2peak decrease of 1 ml/kg/min was associated with higher mortality rates after allo-HCT (HR 1.13)17. Interestingly, there was a large variance in the initial performance level and the response of the patients seemed not to depend on number of exercise sessions performed or initial value. However, in 5 patients the VO2peak value prior allo-HCT lay below the threshold of 18 ml/kg/min, which was proposed to be a threshold for physically independence in older adults40. Though, after intervention only 2 patients lay below the threshold. Interestingly, these 2 patients were the ones who did not improve their VO2peak but deteriorated. We can speculate, if for those two patients the intervention program did not fit. However, another patient that started on a very low level could clearly increase his VO2peak, but had more exercise sessions available. Therefore, it could be that patients with a very low performance need more sessions to achieve a clear training effect on VO2peak.
We can speculate on further influencing factors that may play a role for the response. For example, clinical factors, e.g. the pre-treatment including cardiotoxic argents, blood counts (e.g. hemoglobin value) may play a role here41. In or previous work we performed an analysis on influencing clinical factors on the VO2peak prior allo-HCT14, however, it would be interesting to perform an subgroup analysis on the influence of different factors on the exercise response prior allo-HCT in future studies.
The 6-min walking distance improved significantly by 30.5 m which is comparable to other studies in this setting22. In clinical populations, the minimum clinically important difference is defined as 14.0–30.5 m42. In allo-HCT it could be shown that each 100 m decrease was associated with a higher risk of mortality (HR 2.98)17. In our previous RCT we demonstrated that a higher baseline fitness (meters in 6 min walking test) were associated with lower total mortality rates in a multivariate model18. This study could also demonstrate that a partly supervised exercise program may reduce mortality rates after allo-HCT18.
Since not only a great CRF seems to improve outcome after allo-HCT, but also sarcopenia was associated with higher non-relapse mortality and shorter overall survival after allo-HCT15,43, we included RE in our exercise intervention. Our previous RCTs in patients during and after allo-HCT demonstrated the feasibility and effectiveness of combined interventions18,36,37. A recent study revealed the HGS as a significant predictor for non-relapse mortality (HR 2.56)44. Our results showed a significant improvement of HGS of about 2 kg, indicating a positive effect of our RE on HGS.
So far, very few prehabilitation studies focused on PROs. However, not only physical capacity but also a better QOL prior allo-HCT was associated with reduced risk of mortality45. A reduced symptom severity, e.g. reduced fatigue and physical functioning will lead to better general QOL throughout treatment. Our intervention had a significant positive impact on physical functioning, role functioning, emotional functioning, fatigue (physical fatigue, reduced activity) and dyspnoea. Previous interventions with moderate intensity training during or after allo-HCT demonstrated the positive effect of exercise on various PROs9.
Strengths of our study included a sound intervention concept, including highly standardised HIIT and RE and individualized exercise prescription using percentages from the individual maximal capacity for both, HIIT and RE. Additionally, a highly standardised measurement protocol was applied, including gold-standard methods to assess CRF. Furthermore, our exercise intervention was supervised to ensure a safe completion and good adherence to the exercise protocol. Our study also used a multicentre approach to gain results regarding recruiting structures from different study centres. However, our study has several limitations and our findings must be interpreted cautiously. First of all, we had a small sample size due to a low recruitment rate and most of the participants were being recruited in one study centre (Heidelberg). Furthermore, our sample seems not to fully represent patient’s diversity according disease distribution prior allo-HCT because most participants were diagnosed with MDS or Lymphoma. Normally, AML is the most frequently diagnosis for allo-HCT. Finally, this was a single arm pilot study with a small sample size and larger randomized studies are necessary to confirm our results.
Conclusion
Our study showed that HIIT and high-to moderate RE is feasible for patients prior allo-HCT and may improve CRF and PROs in a very short timeframe. This is clinically highly relevant since a higher physical capacity prior allo-HCT may improve symptoms and survival. However, recruitment rates were much too low and recruitment needed to be terminated. Therefore, we conclude that the time-point in the weeks prior allo-HCT need to be expanded and include also patients with planned allo-HCT but without further scheduling. Especially patients with high-risk profiles and low physical capacity should be offered a structured and individually tailored exercise program as supportive care option to enhance physical capacity/CRF and ultimately improve outcomes such as survival.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on a reasonable request.
References
Penack, O. et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 7, e157–e167. https://doi.org/10.1016/s2352-3026(19)30256-x (2020).
Copelan, E. A. Hematopoietic stem-cell transplantation. N. Engl. J. Med. 354, 1813–1826 (2006).
Ishikawa, A. et al. Factors affecting lower limb muscle strength and cardiopulmonary fitness after allogeneic hematopoietic stem cell transplantation. Support Care Cancer 27, 1793–1800. https://doi.org/10.1007/s00520-018-4433-5 (2019).
Dirou, S. et al. Deconditioning, fatigue and impaired quality of life in long-term survivors after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transpl. 53, 281–290. https://doi.org/10.1038/s41409-017-0057-5 (2018).
Fiuza-Luces, C., Simpson, R. J., Ramirez, M., Lucia, A. & Berger, N. A. Physical function and quality of life in patients with chronic GvHD: A summary of preclinical and clinical studies and a call for exercise intervention trials in patients. Bone Marrow Transpl. 51, 13–26. https://doi.org/10.1038/bmt.2015.195 (2016).
Devins, G. M. et al. Quality of life trajectories during the first year following hematopoietic cell transplantation: An inception cohort study. Support Care Cancer 26, 2379–2386. https://doi.org/10.1007/s00520-018-4059-7 (2018).
Mosher, C. E., Redd, W. H., Rini, C. M., Burkhalter, J. E. & DuHamel, K. N. Physical, psychological, and social sequelae following hematopoietic stem cell transplantation: A review of the literature. Psychooncology 18, 113–127. https://doi.org/10.1002/pon.1399 (2009).
Persoon, S. et al. Effects of exercise in patients treated with stem cell transplantation for a hematologic malignancy: A systematic review and meta-analysis. Cancer Treat. Rev. 39, 682–690. https://doi.org/10.1016/j.ctrv.2013.01.001 (2013).
Prins, M. C. et al. The effect of exercise and nutrition interventions on physical functioning in patients undergoing haematopoietic stem cell transplantation: A systematic review and meta-analysis. Support Care Cancer 29, 7111–7126. https://doi.org/10.1007/s00520-021-06334-2 (2021).
Morales-Rodriguez, E., Pérez-Bilbao, T., San Juan, A. F. & Calvo, J. L. Effects of exercise programs on physical factors and safety in adult patients with cancer and haematopoietic stem cell transplantation: A systematic review. Int. J. Environ. Res. Public Health 19, 1288. https://doi.org/10.3390/ijerph19031288 (2022).
Jones, L. W. et al. Prognostic importance of pretransplant functional capacity after allogeneic hematopoietic cell transplantation. Oncologist https://doi.org/10.1634/theoncologist.2015-0200 (2015).
Yoshida, S., Someya, F. & Yahata, T. Cardiac output response to exercise in patients before allogenic hematopoietic stem cell transplantation. Int. J. Clin. Oncol. 23, 1173–1177. https://doi.org/10.1007/s10147-018-1315-2 (2018).
Morishita, S. et al. Impaired physiological function and health-related QOL in patients before hematopoietic stem-cell transplantation. Support Care Cancer 20, 821–829. https://doi.org/10.1007/s00520-011-1156-2 (2012).
Limbach, M. et al. Influencing factors of cardiorespiratory fitness in allogeneic stem cell transplant candidates prior to transplantation. Support Care Cancer 29, 359–367. https://doi.org/10.1007/s00520-020-05485-y (2021).
Kirsten, J. et al. Sarcopenia screening allows identifying high-risk patients for allogenic stem cell transplantation. Cancers 13(8), 1771 (2021).
Kelsey, C. R. et al. Cardiopulmonary exercise testing prior to myeloablative allo-SCT: A feasibility study. Bone Marrow Transpl. 49, 1330–1336. https://doi.org/10.1038/bmt.2014.159 (2014).
Wood, W. A. et al. Cardiopulmonary fitness in patients undergoing hematopoietic SCT: A pilot study. Bone Marrow Transpl. 48, 1342–1349. https://doi.org/10.1038/bmt.2013.58 (2013).
Wiskemann, J. et al. Effects of physical exercise on survival after allogeneic stem cell transplantation. Int. J. Cancer 137, 2749–2756. https://doi.org/10.1002/ijc.29633 (2015).
Michael, C. M., Lehrer, E. J., Schmitz, K. H. & Zaorsky, N. G. Prehabilitation exercise therapy for cancer: A systematic review and meta-analysis. Cancer Med. 10, 4195–4205. https://doi.org/10.1002/cam4.4021 (2021).
Heger, P. et al. A systematic review and meta-analysis of physical exercise prehabilitation in major abdominal surgery (PROSPERO 2017 CRD42017080366). J. Gastrointest. Surg. 24, 1375–1385. https://doi.org/10.1007/s11605-019-04287-w (2020).
Avancini, A. et al. Exercise prehabilitation in lung cancer: Getting stronger to recover faster. Eur. J. Surg. Oncol. 47, 1847–1855. https://doi.org/10.1016/j.ejso.2021.03.231 (2021).
Wood, W. A. et al. Personalized home-based interval exercise training may improve cardiorespiratory fitness in cancer patients preparing to undergo hematopoietic cell transplantation. Bone Marrow Transpl. 51, 967–972. https://doi.org/10.1038/bmt.2016.73 (2016).
Meyer, P., Gayda, M., Juneau, M. & Nigam, A. High-intensity aerobic interval exercise in chronic heart failure. Curr. Heart Fail. Rep. 10, 130–138. https://doi.org/10.1007/s11897-013-0130-3 (2013).
Palma, S., Hasenoehrl, T., Jordakieva, G., Ramazanova, D. & Crevenna, R. High-intensity interval training in the prehabilitation of cancer patients-a systematic review and meta-analysis. Support Care Cancer 29, 1781–1794. https://doi.org/10.1007/s00520-020-05834-x (2021).
Kuehl, R. et al. Exercise intensity classification in cancer patients undergoing allogeneic HCT. Med. Sci. Sports Exerc. 47, 889–895. https://doi.org/10.1249/MSS.0000000000000498 (2015).
Guiraud, T. et al. High-intensity interval training in cardiac rehabilitation. Sports Med. 42, 587–605. https://doi.org/10.2165/11631910-000000000-00000 (2012).
Reynolds, J. M., Gordon, T. J. & Robergs, R. A. Prediction of one repetition maximum strength from multiple repetition maximum testing and anthropometry. J. Strength Cond. Res. 20, 584–592. https://doi.org/10.1519/r-15304.1 (2006).
Campbell, K. L. et al. Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 51, 2375–2390. https://doi.org/10.1249/MSS.0000000000002116 (2019).
Löllgen, H. Das Anstrengungsempfinden (RPE, Borg-Skala). Dtsch. Z. Sportmed. 55, 299–300 (2004).
Crapo, R. et al. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 166, 111–117 (2002).
Aaronson, N. K. et al. The European-Organization-for-Research-and-Treatment-of-Cancer Qlq-C30: A quality-of-life instrument for use in international clinical-trials in oncology. J. Natl. Cancer Inst. 85, 365–376. https://doi.org/10.1093/jnci/85.5.365 (1993).
Smets, E. M., Garssen, B., Bonke, B. & De Haes, J. C. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 39, 315–325 (1995).
Bruera, E., Kuehn, N., Miller, M. J., Selmser, P. & Macmillan, K. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J. Palliat. Care 7, 6–9 (1991).
Wood, W. A. et al. Lessons learned from a pilot randomized clinical trial of home-based exercise prescription before allogeneic hematopoietic cell transplantation. Support Care Cancer 28, 5291–5298. https://doi.org/10.1007/s00520-020-05369-1 (2020).
van Haren, I. et al. Physical exercise prior to hematopoietic stem cell transplantation: A feasibility study. Physiother. Theory Pract. https://doi.org/10.1080/09593985.2018.1423655 (2018).
Kuehl, R. et al. Determinants of exercise adherence and contamination in a randomized controlled trial in cancer patients during and after allogeneic HCT. Support Care Cancer 24, 4327–4337. https://doi.org/10.1007/s00520-016-3271-6 (2016).
Wiskemann, J. et al. Effects of a partly self-administered exercise program before, during, and after allogeneic stem cell transplantation. Blood 117, 2604–2613 (2011).
Wallen, M. P. et al. High-intensity interval training improves cardiorespiratory fitness in cancer patients and survivors: A meta-analysis. Eur. J. Cancer Care 29, e13267. https://doi.org/10.1111/ecc.13267 (2020).
Mitchell, S. A. et al. Determinants of functional performance in long-term survivors of allogeneic hematopoietic stem cell transplantation with chronic graft-versus-host disease (cGVHD). Bone Marrow Transpl. 45, 762–769 (2010).
Morey, M. C., Pieper, C. F. & Cornoni-Huntley, J. Is there a threshold between peak oxygen uptake and self-reported physical functioning in older adults?. Med. Sci. Sports Exerc. 30(8), 1223–1229. https://doi.org/10.1097/00005768-199808000-00007 (1998).
Howden, E. J. et al. Standing up to the cardiometabolic consequences of hematological cancers. Blood Rev. https://doi.org/10.1016/j.blre.2018.02.005 (2018).
Bohannon, R. W. & Crouch, R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: A systematic review. J. Eval. Clin. Pract. https://doi.org/10.1111/jep.12629 (2016).
Jia, S. et al. Prognostic value of sarcopenia in survivors of hematological malignances undergoing a hematopoietic stem cell transplantation: A systematic review and meta-analysis. Support Care Cancer 28, 3533–3542. https://doi.org/10.1007/s00520-020-05359-3 (2020).
Salas, M. Q. et al. Pilot prospective study of Frailty and Functionality in routine clinical assessment in allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 56, 60–69. https://doi.org/10.1038/s41409-020-0979-1 (2021).
Hamilton, B. K. et al. Prognostic significance of pre-transplant quality of life in allogeneic hematopoietic cell transplantation recipients. Bone Marrow Transpl. 50, 1235–1240. https://doi.org/10.1038/bmt.2015.122 (2015).
Acknowledgements
We thank all patients who participated in this study. B. Friedmann-Bette and her team for CPET assessment in Heidelberg. A. Wehrle for assessment and administration in Freiburg. Nadja Knauthe, Christine Pawandenat, Katja Prate and the department of Sports Medicine in Dresden. For the publication fee we acknowledge financial support by Deutsche Forschungsgemeinschaft within the funding programme “Open Access Publikationskosten” as well as by Heidelberg University.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
R.K.: study design, data collection, statistical analysis, writing the manuscript. J.F.: contribution to statistical analysis. M.L.: data collection, critical review of the manuscript, A.P., F.S., H.B., A.W.: data collection, critical review of the manuscript. F.R.: contribution to the study design, critical review of the manuscript. P.D., T.L.: critical review of the manuscript. J.W.: study design, critical review of the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuehl, R., Feyer, J., Limbach, M. et al. Prehabilitative high-intensity interval training and resistance exercise in patients prior allogeneic stem cell transplantation. Sci Rep 13, 22069 (2023). https://doi.org/10.1038/s41598-023-49420-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-49420-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.