Abstract
Four flavonoid glycosides, namely quercetin-3-O-rhamnoside (1), kaempferol-3-O-β-d-glucopyranosyl (2), kaempferol-7-O-α-l-rhamnopyranoside (3), and kaempferol-3-O-β-d-glucopyranosyl-7-O-α-l-rhamnopyranoside (4), from Nephelium lappaceum L. seeds were evaluated for their efficacy against melanin inhibition in B16F10 melanoma cells and tyrosinase inhibition. Among them, kaempferol-7-O-α-l-rhamnopyranoside (3) displayed the highest potency in both activities without any significant cytotoxicity. The combination of compound 3 and arbutin in specific proportions demonstrated a synergistic effect (CI < 1) in inhibiting melanin production in B16F10 cells and tyrosinase inhibition. Additionally, a cosmetic formulation containing compound 3 and arbutin as active ingredients exhibited favorable stability under accelerated storage conditions. Quantitative analysis indicated that compound 3 and arbutin levels in the formulation were above 90% after one month of storage. Determination of the formulation's shelf life using the Q10 method, estimating it to be around 5.2 months from the date of manufacture. The synergy between arbutin and kaempferol-7-O-α-l-rhamnopyranoside (3) extracted from N. lappaceum substantially enhances both the whitening effectiveness and the stability of cosmetic formulations.
Similar content being viewed by others
Introduction
Melanin, a pigment responsible for shielding the skin against ultraviolet (UV) damage and regulating skin pigmentation, plays a pivotal role in maintaining skin integrity1. However, excessive melanin production and accumulation lead to various skin pigmentation disorders, including melasma, freckles, and dark spots2. Hyperpigmentation profoundly affects an individual's physical appearance and self-assurance in social interactions. Presently, the treatment of hyperpigmentation entails the utilization of whitening agents, chemical peels, and laser therapy3. Among these modalities, common topical treatment with effective skin lightening/whitening agents is still necessary due to the resistant and relapsing nature of hyperpigmentation4. Indeed, various commercially available lightening agents, such as kojic acid, hydroquinone, retinoids, have proven to be effective for topical management; however, most of them caused undesirable side effects, including possible carcinogenicity and dermatitis4,5,6. Moreover, hydroquinone and kojic acid are prohibited in most countries. Consequently, the need to discover safer and more effective melanogenic inhibitors persists.
Thailand is renowned for its agricultural prominence, and Nephelium lappaceum L. (known as rambutan), is one of the economically significant crops in Thailand. In addition to commercial fresh fruits, rambutan is industrially processed into can, juice, jam, jelly, and spread7. This processing produces a large amount of seed waste, a yearly average 1900 tons discarded8. There is growing concern about agricultural wastes that contain bioactive compounds. Previous phytochemical investigations demonstrated various phenolic compounds were found in rambutan seed, some of which exhibited antioxidant, antimicrobial, and nitric oxide inhibition9,10,11.
To enhance the economic value of such kind of waste with a growing preference for natural products within the cosmetic industry, rambutan roots-derived phenolics and their melanin and tyrosinase inhibition have been studied. Furthermore, we aimed to explore the synergistic effect of the combination of the potential compounds and melanin-inhibiting whitening agents and/or tyrosinase inhibitors, due to research on synergistic interaction of two or more compounds attracted great interest in recent years12. In addition to greater impact on biological activity, synergy can also influence the physicochemical properties of compounds or mixtures, including solubility and absorption efficiency13. Finally, the chemical stability of both these substances will be examined within cosmetic formulations.
Results and discussion
Anti-melanin activity and cytotoxicity of compounds 1–4
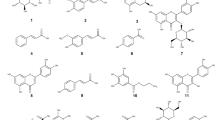
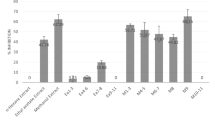
To assess the effectiveness of phytochemicals isolated from the N. lappaceum L. seed extract in inhibiting melanin production, a B16F10 cell-based anti-melanogenic assay was conducted on four specifically chosen compounds (1–4) (Fig. 1). As a positive control, arbutin, a widely recognized tyrosinase inhibitor frequently employed as a cosmetic whitening agent, was utilized14. Among them, compound 3 exhibited the most potent anti-melanogenic activity in a concentration-dependent manner, while no significant toxicity was observed (Fig. 2C). It provided an IC50 value of 23.0 ± 1.3 µM, indicating compound 3 was about 35-fold more potent than positive control arbutin (IC50 = 807.6 ± 2.9 µM) (Table 1). The remaining three compounds 1–2 and 4 could also suppress melanin production in B16F10 cells in a dose-dependent manner, with IC50 values of 134.8 ± 2.1, 190.5 ± 2.2, and > 200 µM, respectively (Fig. 2A,B,D). From these data, some structure–activity relationships could be seen. The position of the rhamnose moiety might play an important role in melanin inhibition and cytotoxicity. Attachment of rhamnose at the C-7 position to kaempferol core skeleton as in 3 exerted a remarkably potent melanin reduction, whereas the C-3 existence of rhamnose in 2 caused much decrease in anti-melanogenic activity but increase in cellular toxicity. In addition, it was implied that the presence of a glucosyl moiety at C-3 position as in 4 made the compound loss of both melanin suppression and toxicity. Surprisingly, Tang et al. previously reported that kaempferol itself did induce melanin production and melanocyte growth15. The results indicated that the existence of the sugar moiety is required for melanin inhibition of the kaempferol core skeleton.
Effect of various concentrations of compounds 1–4 (A–D) on the melanin content in B16F10 melanoma cells. Following exposure, cell viability was then measured by MTT assay. The percentages are plotted as the mean ± SD of triplicate. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with untreated control.
Anti-tyrosinase activity of compound 3
Tyrosinase plays a pivotal role as an essential enzyme, exhibiting multifarious catalytic functionalities within the melanogenesis process16. Hence, inhibiting tyrosinase represents a crucial strategy in addressing disorders associated with pigmentation. In this study, the inhibitory activity of the compound on tyrosinase was evaluated through the employment of a mushroom tyrosinase assay. Based on the anti-melanin activity, compound 3 was chosen for subsequent investigation owing to its pronounced melanin inhibition activity, in addition to the absence of discernible cytotoxicity even after 72 h of treatment with concentrations as high as 1000 µM. Most notably, its anti-melanogenic activity exhibited a 35-fold superiority to that of arbutin. Compound 3 exhibited strong anti-tyrosinase activity, with an IC50 value of 25.3 ± 0.1 µM, comparable to kojic acid (positive control) and much potent than arbutin (active ingredient in commercial skincare products) (Table 2). This heightened activity might be attributed to the hydroxyl groups present in kaempferol, which facilitate binding to the enzymatic site of tyrosinase. Consequently, this binding impedes the catalytic oxidation of L-DOPA to L-DOPA quinone via catecholate activity17.
Synergistic Impact of arbutin and kaempferol-7-O-α-l-rhamnopyranoside (3)
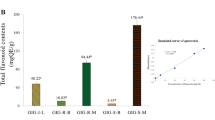
Recent research findings have revealed the application of arbutin, a hydroquinone compound, and kojic acid as potent tyrosinase inhibitors within the realm of cosmetic formulations. Nevertheless, the usage of hydroquinone has been associated with undesirable consequences, including irritative responses, dermatitis, and the impairment of melanin. Furthermore, arbutin derivatives undergo catalysis, leading to the production of benzene metabolites that may possess inherent hematotoxicity18,19. Furthermore, when kojic acid is utilized at concentrations exceeding 1%, it has the potential to elicit skin irritations, contact dermatitis, and increased vulnerability to sunburn, particularly in individuals possessing sensitive skin conditions20. The synergistic effect is a phenomenon that arises when two or more substances collaborate to yield an aggregated impact surpassing the cumulative effect of their individual properties. Moreover, the synergistic effects may extend to influence the physicochemical characteristics of compounds or mixtures, such as solubility12,13. Due to lower side effects, arbutin, a natural β-d-glucopyranoside of hydroquinone, has gained widespread employment as a depigmentation agent within the cosmetic industry. In current study, compound 3 was selected to be synergistically combined with arbutin to investigate their collective impact on suppressing tyrosinase activity and inhibiting melanin production in B16F10 cells. Various combinations of compound 3 and arbutin were prepared, utilizing ratios of 1:5, 1:10, 1:20, and 1:40 to reveal a notable reduction in melanin content within B16F10 cells (Fig. 3). Treatment with compound 3 and arbutin mixtures resulted in reductions of 39.4%, 63.1%, 68.0%, and 71.5% in melanin content, respectively. These sample mixtures were further subjected to evaluation of their anti-tyrosinase activity, which manifested as a reduction in inhibitory percentages of 23.5%, 27.3%, 37.0%, and 49.6%, respectively (Table 3). The calculation of combination index (CI) values revealed that the mixtures comprising compound 3 and arbutin exhibited synergistic effects, as evidenced by CI values below 1.0 (Table 3). Notably, treatment with a 1:40 mixture of compound 3 and arbutin demonstrated the most potent inhibitory effects on melanin production in B16F10 cells, resulting in a remarkable reduction of up to 75.6%. These findings indicated that the combined mixture of compounds exhibits enhanced efficacy as melanin inhibitors compared to their individual extracts. Additionally, Table 3 provides a summary of the percentage inhibition of tyrosinase activity and the corresponding CI values of the mixtures. The results indicated that the mixtures of compound 3 and arbutin also displayed a synergistic effect, as indicated by CI values below 1.0.
Effect of the mixtures of compound 3 and arbutin with various ratio on the melanin content in B16F10 melanoma cells. Following exposure, cell viability was then measured by MTT assay. The percentages are plotted as the mean ± SD of triplicate. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with untreated control.
Stability of cosmetic formulations
Physical stability
The physical attributes of formulation F1 were examined, revealing no discernible alterations in color, odor, or texture. Moreover, F1 demonstrated homogeneity, maintaining its uniformity without any indications of phase separation during centrifugation (Table 4). Additionally, over the duration of the accelerated storage period, F1 displayed consistent pH values, with no significant deviations detected. In contrast, it was observed that formulation F2 experienced a color change after two weeks of the study (Fig. 4). The observed phenomenon can be attributed to the utilization of triethanolamine as a pH-adjusting base in formulation F2, aimed at achieving an optimal pH range for the product. Subsequently, alkaline hydrolysis21 took place specifically with compound 3, leading to a discernible alteration in the product color right from the initiation of the formulation process. Furthermore, during the storage period under accelerated conditions, the color change became increasingly conspicuous and evident.
Chemical stability
Formulation F1 was employed for assessing the chemical stability, as it exhibited consistent physical stability under accelerated conditions without any observable changes. Performing the HPLC analysis using a single peak chromatogram of standard compounds, compound 3 and arbutin to generate the calibration curve (Fig. 5). Calibration curves of compound 3 and arbutin were performed at different concentrations (compound 3: 3.90, 7.81, 15.62, 31.25, 62.50 µg/mL, arbutin: 15.62, 125, 250, 500, 1000 µg/mL) (Fig. 6). The amount of each compound 3 and arbutin was calculated based on a linear equation: Y = 99040X + 352,623, R2 = 0.9964 and Y = 10118X + 566,831, R2 = 0.9992, respectively (Fig. 6). Each calibration point was conducted in triplicate. The formulation F1 contained residual quantities of compound 3 and arbutin, with percentages of 96.00% and 97.44%, respectively (Table 5). Through the calculation of the remaining percentage of both active ingredients in the facial serum, it was established that the quantities of these active ingredients in the formulation remained above 90%22 after 1 month. Additionally, by employing the experimental results and the Q10 method for estimation, it was deduced that the serum possessed a maximum predicted shelf life of 5.2 months, during which no discernible alterations in its chemical and physical properties were observed23,24.
Calculation for predicting the shelf life of a formulation using the Q10 method.
where, T90(T1) = Shelf life at 45 °C (T1); T90(T2) = Estimated shelf life at 30 °C (T2); ∆T = Temperature difference between T2 and T1 (30–45 = − 15); Q10 = Ratio of the rate of reaction with a temperature difference of 10 °C (3 is constant).
Conclusion
Four flavonoid glycosides isolated from N. lappaceum L. seeds were assessed for their anti-melanogenic activity in B16F10 melanoma cells. Among them, kaempferol-7-O-α-l-rhamnopyranoside (3) exhibited the most potent activity without significant cytotoxicity. Moreover, it displayed remarkable anti-tyrosinase activity comparable to that of kojic acid, making it a promising candidate as an active ingredient in skin whitening products. Our study also demonstrated a synergistic compatibility effect when combining compound 3 and arbutin in specific proportions, assessed using the CI value to evaluate synergism. The results revealed a synergistic effect (CI < 1) between compound 3 and arbutin in inhibiting melanin production in B16F10 cells and tyrosinase inhibition using L-tyrosine as a substrate. Consequently, we selected compound 3 and arbutin as active ingredients for inclusion in cosmetic formulations. Furthermore, formulation F1 demonstrated excellent stability under all accelerated storage conditions, and the quantitative analysis confirmed that the levels of compound 3 and arbutin in the formulation remained above 90% after one month of storage. To determine the shelf life of the formulation, we utilized the Q10 method, which estimates a shelf life of approximately 5.2 months from the date of manufacture. In conclusion, the collaborative effect of arbutin and kaempferol-7-O-α-l-rhamnopyranoside (3) derived from N. lappaceum significantly enhances both the whitening efficacy and the stability of cosmetic formulations.
Materials and methods
Materials
Quercetin-3-O-rhamnoside (1), kaempferol-3-O-β-d-glucopyranosyl (2), kaempferol-7-O-α-l-rhamnopyranoside (3), and kaempferol-3-O-β-d-glucopyranosyl-7-O-α-l-rhamnopyranoside (4) were isolated from the air-dried powdered seeds of N. lappaceum L. with several chromatographic techniques as our previous report25. The N. lappaceum (Sapindaceae) seeds were gathered in Amphoe Laem Sing, Chanthaburi Province, Thailand. All methods were carried out in accordance with relevant guidelines. Arbutin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), kojic acid and Tween 20 were purchased from Tokyo Chemical Industry (Japan). L-Tyrosine and mushroom tyrosinase were purchased from Sigma-Aldrich (USA). Dulbecco’s Modified Eagle Medium (DMEM), penicillin/streptomycin, trypan blue solution, trypsin 0.25 (1×) solution were purchased from Hyclone (Austria). The chemicals for cosmetic formulation, which comprised glycerin, propylene glycol, xanthan gum, ActiveProtecTMOX (consisting of Sodium Diethylenetriamine Pentamethylene Phosphonate, Disodium EDTA, Sodium Metabisulfite, and Sodium Gluceptate), arbutin, phenoxyethanol, hydroxyethyl cellulose, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, and triethanolamine, were procured from Chanjao Longevity Co., Ltd. (Thailand).
Methods
Cell culture
Murine melanoma B16F10 cells (obtained from DS Pharma Biomedical, Japan) were cultivated in Dulbecco's Modified Eagle Medium (DMEM), a liquid medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. The cells were incubated at a temperature of 37 °C within a controlled humidified environment containing 5% CO2. To facilitate subculture, the cells were detached from the culture flask using trypsin solution. Subsequently, the cells were subjected to staining with trypan blue solution and quantified using a hemocytometer for further analysis. The final concentration of DMSO was 0.1% for all experiments.
Anti-melanogenic assay
Murine melanoma B16F10 cells were gently rinsed with phosphate-buffered saline (PBS) and detached from the cell tissue culture dish using trypsin. The cells were subsequently seeded in 24-well culture plates at a density of 5 × 104 cells per well and allowed to incubate for 24 h before being subjected to treatment with the experimental samples. Arbutin was used as a positive control. Following the treatment, the cells were further incubated for an additional 72 h, and the absorbance was measured at 510 nm using a microplate reader. Melanin production was expressed as a percentage relative to the control cells treated with DMSO. All experiments were conducted in triplicate. The IC50 value was calculated. The percentage of total melanin content was determined using the following equation:
Cytotoxicity assay
Cytotoxicity was assessed through the utilization of the MTT assay. The cell culture was initiated in 24-well plates, with a seeding density of 5 × 104 cells per well. After treatment with samples for 72 h, 10 µL of MTT solution (5 mg/mL in PBS) were added to each well and incubated for additional 3 h. Then, supernatant was removed and DMSO (250 µL) was added to dissolve the formed formazan crystals. The absorbance was measured at 570 nm using a microplate reader. Cells treated solely with DMSO served as the control. All experiments were conducted in triplicate. The percentage of cell viability was determined using the following calculation:
Anti-tyrosinase assay
The assessment of tyrosinase inhibitory activity was conducted following the method outlined by Saewan et al., with slight modifications26. l-tyrosine served as a substrate and kojic acid was utilized as a positive control. The compounds with various concentrations (50 μL) were added to the 96-well plate, followed by tyrosinase solution (50 μL, 250 units/mL in phosphate buffer, pH 6.8). After the first incubation at 35 °C for 10 min, 50 μL of substrates (2.5 mM l-tyrosine) were added, followed by second incubation at 35 °C for 20 min. The absorbance was measured at 492 nm using a microplate reader. All experiments were replicated in triplicate. The percentage inhibition of tyrosinase was determined using the following equation:
Cosmetic formulation and accelerated stability test
The development of cosmetic formulations has resulted in the creation of two distinct formulations, outlined as follows. Formulation F1 consists of the following ingredients: compound 3 (0.005% w/w), glycerin (3.00% w/w), propylene glycol (3.00% w/w), xanthan gum (0.5% w/w), ActiveProtecTMOX (Sodium Diethylenetriamine Pentamethylene Phosphonate, Disodium EDTA, Sodium Metabisulfite, Sodium Gluceptate) (0.60% w/w), arbutin (0.20% w/w), phenoxyethanol (0.5% w/w), and water (92.195% w/w). Formulation F2 consists of the following ingredients: compound 3 (0.005% w/w), glycerin (2.00% w/w), propylene glycol (1.00% w/w), hydroxyethyl cellulose (0.5% w/w), Acrylates/C10-30 Alkyl Acrylate Crosspolymer (0.10% w/w), triethanolamine (0.05% w/w), phenoxyethanol (0.5% w/w) and water (95.845% w/w). An accelerated stability test was performed by placing the formulation in a 45 °C chamber for 30 days. The physical and chemical stability of the test products were assessed through organoleptic and HPLC analyses.
Determination of kaempferol-7-O-α-l-rhamnopyranoside (3) and arbutin in cosmetic formulation by HPLC
The stock solution was prepared by dissolving 1 mg of the standard compounds in 1 mL of methanol, resulting in a concentration of the stock solutions at 1000 ppm. Subsequently, serial dilutions were conducted using methanol to establish a standard curve. The concentrations varied from 3.9 to 62.5 ppm for kaempferol 7-O-α-l-rhamnopyranoside (3) and from 15.6 to 1000 ppm for arbutin. For the analysis of the sample cosmetic formulations, precisely 200 mg was weighed and placed into a centrifuge tube, then dissolved in 1 mL of methanol. The resulting mixture was vortexed for 1 min, followed by sonication at 25 °C for 15 min and subsequent freezing at − 80 °C for 10 min. The mixture was then subjected to centrifugation at 3000 rpm for 5 min. The sample solution was filtered through a nylon syringe filter with a pore size of 0.45 µm, and 20 μL of the filtrate was injected into a reverse-phase Vertical C18 column (150 mm × 4.6 mm, 5 μm) equipped with a Thermo Scientific UV6000LP detector and Thermo Scientific P200 pump. The ChemQuest version 5.0 software controlled the system. The mobile phase, consisting of water (A) and acetonitrile (B), followed a linear gradient program of 0–100% B over 30 min, with a flow rate of 0.6 mL/min. The UV detector was set at 280 nm, and the injection volume was 20 μL.
Statistical analysis
The 50% inhibition concentration (IC50) value is determined with GraphPad prism software, version 5. Data were presented as mean ± standard deviation (SD) from three independent experiments. Values were evaluated by two-way analysis of variance (ANOVA), followed by Bonferroni correction for multiple comparisons using GraphPad Prism version 5. *p < 0.05, **p < 0.01 and ***p < 0.001 indicate statistical significance (compared with untreated control). The values of combination index (CI) were calculated by following equation:
where IC50a and IC50b are the IC50 value of compound a and compound b, and IC50amix and IC50bmix are the concentration of compound a and compound b in the mixture that cause 50% inhibition. The CI value < 1 indicates synergism, CI = 1 indicates an additive effect, and CI > 1 indicates antagonism15.
Data availability
All data and materials are the result of research and are available from the corresponding author upon reasonable request and are appropriately cited in the manuscript.
References
Brenner, M. & Hearing, V. J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 84, 539–549. https://doi.org/10.1111/j.1751-1097.2007.00226.x (2008).
Nordlund, J. J. et al. The pigmentary system: physiology and pathophysiology. https://doi.org/10.1002/9780470987100 (2006).
Manap, A. et al. Perspective approaches on melanogenesis inhibition. Dermatol. Sin. 39, 1–12. https://doi.org/10.4103/ds.ds_46_20 (2021).
Moolla, S. & Miller-Monthrope, Y. Dermatology: how to manage facial hyperpigmentation in skin of colour. Drugs. Context. 11:2021–11–2. https://doi.org/10.7573/dic.2021-11-2 (2022).
Heilgemeir, G. P. & Balda, B. R. Irreversible toxic depigmentation. Observations following use of hydroquinonemonobenzylether-containing skin bleaching preparation. Munch. Med. Wochenschr. 123, 47–48 (1981).
Ogiwara, Y. et al. Evaluation of the repeated-dose liver, bone marrow and peripheral blood micronucleus and comet assays using kojic acid. Mutat. Res. Genetic Toxicol. Environ. Mutagen. 780, 111–116. https://doi.org/10.1016/j.mrgentox.2015.01.004 (2015).
Lourith, N., Kanlayavattanakul, M., Mongkonpaibool, K., Butsaratrakool, T. & Chinmuang, T. Rambutan seed as a new promising unconventional source of specialty fat for cosmetics. Ind. Crop. Prod. 83, 149–154. https://doi.org/10.1016/j.indcrop.2015.12.045 (2016).
Mahisanut, B., Jom, K. N., Matsukawa, K. N. & Klinkesorn, U. Solvent fractionation of rambutan (Nephelium lappaceum L.) kernel fat for production of non-hydrogenated solid fat: Influence of time and solvent type. J. King Saud. Univ. Sci. 29, 32–46. https://doi.org/10.1016/j.jksus.2016.08.004 (2017).
Rakariyatham, K., Zhou, D., Rakariyatham, N. & Shahidi, F. Sapindaceae (Dimocarpus longan and Nephelium lappaceum) seed and peel by-products: Potential sources for phenholic compounds and use as functional ingredients in food and health applications. J. Funct. Foods. 67, 103846. https://doi.org/10.1016/j.jiff.2020.103846 (2020).
Tan, J., Ma, Q., Li, J., Liu, Q. & Zhuang, Y. Bioavailability and antioxidant activity of rambutan (Nephelium lappaceum) peel polyphenols during in vitro simulated gastrointestinal digestion, Caco-2 monolayer cell model application, and chronic fermentation. J. Agric. Food Chem. 71, 15829–15841. https://doi.org/10.1021/acs.jafc.3c04106 (2023).
Jahurul, M. H. A. et al. Functional and nutritional properties of rambutan (Nephelium lappaceum L.) seed and its industrial application: A review. Trends Food Sci. Technol. 99, 367–374. https://doi.org/10.1016/j.tifs.2020.03.016 (2020).
Yue, G. F. et al. Synergistic anticancer effects of polyphyllin I and evodiamine on freshly-removed human gastric tumors. PLOS ONE 8, e65164. https://doi.org/10.1371/journal.pone.0065164 (2013).
Pezzani, R. et al. Synergistic fffects of plant derivatives and conventional chemotherapeutic agents: An update on the cancer perspective. Medicina 55, 110. https://doi.org/10.3390/medicina55040110 (2019).
Tokiwa, Y., Kitagawa, M., Raku, T., Yanagitani, S. & Yoshino, K. Enzymatic synthesis of arbutin undecylenic acid ester and its inhibitory effect on melanin synthesis. Bioorg. Med. Chem. Lett. 17, 3105–3108. https://doi.org/10.1016/j.bmcl.2007.03.039 (2007).
Tang, H. et al. Kaempferol, the melanogenic component of Sanguisorba officinalis, enhances dendricity and melanosome maturation/transport in melanocytes. J. Pharmacol. Sci. 147, 348–357. https://doi.org/10.1016/j.jphs.2021.08.009 (2021).
Pillaiyar, T., Manickam, M. & Namasivayam, V. Skin whitening agents: medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme. Inhib. Med. Chem. 32, 403–425. https://doi.org/10.1080/14756366.2016.1256882 (2017).
Kubo, I. & Kinst-Hori, I. Flavonols from Saffron Flower: Tyrosinase inhibitory activity and inhibition mechanism. J. Agric. Food Chem. 47, 4121–4125. https://doi.org/10.1021/jf990201q (1999).
Wang, Y. et al. Synergistic promotion on tyrosinase inhibition by antioxidants. Molecules 23, 106. https://doi.org/10.3390/molecules23010106 (2018).
Irfan, M. et al. A mechanistic approach for toxicity and risk assessment of heavy metals, hydroquinone and microorganisms in cosmetic creams. J. Hazard. Mater. 433, 128806. https://doi.org/10.1016/j.jhazmat.2022.128806 (2022).
Liu, J.-K. Natural products in cosmetics. Nat. Prod. Bioprospect. 12, 40. https://doi.org/10.1007/s13659-022-00363-y (2022).
Litvinenko, V. I. & Makarov, V. A. The alkaline hydrolysis of flavonoid glycosides. Chem. Nat. Compd. 5, 305–306. https://doi.org/10.1007/BF00595062 (1969).
Cunniff, P. & International, A. Official methods of analysis of AOAC International. 16th , 3rd rev., 1997 edn, (AOAC International, 1997).
Anderson, G. & Scott, M. Determination of product shelf life and activation energy for five drugs of abuse. Clin. Chem. 37, 398–402. https://doi.org/10.1093/CLINCHEM/37.3.398 (1991).
Magari, R. T. Assessing shelf life using real-time and accelerated stability tests: Although accelerated tests are needed, real-time tests are the ultimate proof. Biopharm. Int. 16, 36–48 (2003).
Wisetsai, A., Choodej, S., Ngamrojanavanich, N. & Pudhom, K. Fatty acid acylated flavonol glycosides from the seeds of Nephelium lappaceum and their nitric oxide suppression activity. Phytochemistry 201, 113262. https://doi.org/10.1016/j.phytochem.2022.113262 (2022).
Saewan, N., Thakam, A., Jintaisong, A. & Kittigowitana, K. Anti-tyrosinase and cytotoxicity activities of curcumin-metal complexes. Int. J. Pharm. Sci. 6, 270–273 (2014).
Acknowledgements
This research is supported by the Second Century Fund (C2F), Chulalongkorn University, and by Thailand Science Research and Innovation Fund, Chulalongkorn University (CU_FRB65_bcg(3)_071_23_01).
Author information
Authors and Affiliations
Contributions
Conceptualization, I.S., K.P., and S.C.; methodology and experimental design, I.S., S.C., and K.P.; Validation, I.S., J.P., S.C., and K.P.; investigation, J.P., and S.C.; writing-original draft preparation, I.S., S.C., and K.P.; writing-review and editing, I.S., S.C., N.N, and K.P.; visualization, I.S. and S.C.; project administration, K.P.; funding acquisition, K.P. All authors have read and agree to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Siridechakorn, I., Pimpa, J., Choodej, S. et al. Synergistic impact of arbutin and kaempferol-7-O-α-l-rhamnopyranoside from Nephelium lappaceum L. on whitening efficacy and stability of cosmetic formulations. Sci Rep 13, 22004 (2023). https://doi.org/10.1038/s41598-023-49351-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-49351-3
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.