Abstract
It is known that the rate of caesarean section (C-section) has been increasing among preterm births. However, the relationship between C-section and long-term neurological outcomes is unclear. In this study, we utilized diffusion tensor imaging (DTI) to characterize the association of delivery method with brain white matter (WM) microstructural integrity in preterm infants. We retrospectively analyzed the DTI scans and health records of preterm infants without neuroimaging abnormality on pre-discharge term-equivalent MRI. We applied both voxel-wise and tract-based analyses to evaluate the association between delivery method and DTI metrics across WM tracts while controlling for numerous covariates. We included 68 preterm infants in this study (23 delivered vaginally, 45 delivered via C-section). Voxel-wise and tract-based analyses revealed significantly lower fractional anisotropy values and significantly higher diffusivity values across major WM tracts in preterm infants delivered via C-section when compared to those delivered vaginally. These results may be partially, but not entirely, mediated by lower birth weight among infants delivered by C-section. Nevertheless, these infants may be at risk for delayed neurodevelopment and could benefit from close neurological follow up for early intervention and mitigation of adverse long-term outcomes.
Similar content being viewed by others
Introduction
Preterm birth (< 37 weeks gestation) accounts for more than 10% of live births worldwide1. Prematurity is also the leading cause of infant mortality, contributing to approximately one million infant deaths each year2,3. These infants suffer from a range of pathologies in the neonatal period including respiratory distress, intraventricular hemorrhage, and necrotizing enterocolitis among others4. Numerous studies have also linked prematurity to adverse long-term neurodevelopmental outcomes, including increased rates of cerebral palsy, cognitive impairment, and behavioral disorders in preterm infants when compared to term births5,6,7. The degree of prematurity has also been associated with increased risk of such adverse neurological outcomes8,9. Notably, while C-sections account for more than a fifth of all live births globally, the rate of C-section in preterm births has risen from around 25% to over 35% in the past three decades10,11. Thus given the increasing prevalence of C-section delivery, particularly in prematurity, a particular area of recent interest has been the relationship between delivery method and infant outcomes.
Multiple studies have found significant associations of C-section delivery with adverse cognitive outcomes, emotional problems, sleep problems, and increased risk of developing autism spectrum disorder and attention deficit hyperactivity disorder later in life12,13,14,15. Some of these adverse outcomes may—at least partially—be mediated by confounding maternal and infant factors such as birthweight, maternal age, maternal substance use and infant sex16,17. In contrast, some studies report no association between delivery method and infant mortality, neurodevelopmental delay, or rate of neurological disorder development among term, extremely preterm or very low birth weight infants18,19,20,21,22. Given these conflicting reports, we aimed to examine the association of delivery method with preterm infants’ brain white matter (WM) microstructure, a biomarker of developmental disorders.
Although conventional brain MRIs can identify drastic structural and pathologic abnormalities, advanced MR techniques such as diffusion tensor imaging (DTI) can further characterize brain development by evaluating WM microstructural integrity. Prior studies have utilized DTI to characterize WM microstructure in infants across a range of gestational ages, identifying specific WM regions that are microstructurally delayed in infants of lower gestational age23,24. We have recently shown the associations of gestational age at birth and corrected age at the time of scan on infants’ brain WM microstructural development23. Deoni et al. have also utilized DTI to study how delivery method is associated with infant brain development in full-term, healthy infants, finding that those born via C-section displayed signs of lower WM development at two weeks. However, they noted that such differences seemed to be less apparent later in life25.
In this study, we compared the early WM microstructural integrity of radiologically normal singleton preterm infants born via C-section versus vaginal delivery after correction for gestational age at birth and time of scan, weight z-scores at birth, and other pertinent clinical variables. Our results elucidate neurobiological correlates of delivery method and explain the possible microstructural underpinnings of prior studies reporting adverse neurological outcomes in association with C-section delivery.
Methods
Subject selection
We reviewed the imaging and electronic health records of all infants who had brain MRI performed within 3 months of birth between January 2013 and March 2021 at our center. Data access and retrieval were performed on March 31, 2021. The authors had access to information that could identify individual participants during and after data collection. Preterm infants at our institution routinely undergo brain MRI at term equivalent age or prior to discharge. This predischarge MRI scan includes DTI series at the end, if tolerated by the infant. We included infants who (1) were born between 24- and 37-weeks gestational age at birth, (2) had no structural or signal abnormalities (gross distortion or severe motion artifact) on conventional brain MRI series, (3) had DTI performed as part of their MRI study, and (4) had sufficient information in the health record regarding: delivery methods, indication of early delivery, gestational age at birth and time of scan, birth weight, and 1- and 5-min APGAR scores. All birth weights were converted to Z-scores using the 2013 Fenton growth chart for preterm infants 26. In addition, we excluded infants whose mothers engaged in documented substance use during pregnancy and those who were the product of a multiple gestation. We recorded the incidence of maternal preeclampsia, chorioamnionitis, premature rupture of membranes, need for infant intubation, need for infant therapeutic hypothermia, pertinent postnatal pathologies, and indications for premature delivery for our included subjects.
As prior studies have identified low birth weight as a risk factor for delayed neurodevelopment and adverse neurological outcomes, a sub-cohort excluding infants born small for gestational age (SGA, birthweight z-score < − 1.28) was identified to conduct additional analyses27,28. This study was approved by our institutional review board and the need for consent was waived by the Yale Human Research Protection Program Institutional Review Boards due to the retrospective nature of the study. Additionally, all research was conducted in accordance with the Declaration of Helsinki to ensure ethical respect to human subjects.
Image acquisition and pre-processing
MRI scans were performed on a Siemens 3 T Skyra scanner. DTI series were obtained using a single-shot echoplanar image sequence with the following parameters: repeat time = 10,200 ms, echo time = 94 ms, flip angle = 90°, field of view = 18 × 18 cm, slice thickness = 2.5 mm, matrix = 128 × 128, including a single b = 0 and 30 noncolinear direction b = 1000 s/mm2 acquisitions. Motion artifact for neonatal MRI is reduced at our institution preferentially using the “feed-and-swaddle” technique with anesthetic sedation being used as a second line option29. Preprocessing of DTI scans was performed using FMRIB’s Diffusion Toolbox (FDT) in FMRIB’s Software Library (FSL). This including corrections for susceptibility distortions using the TOPUP tool, and correction for eddy currents and subject movement using the EDDY tool. Then, the DTIFIT tool was used to fit diffusion tensors and generate fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD) maps. All DTI metric maps were visually inspected for quality control prior to further analysis.
Tract based spatial statistics (TBSS) and voxel-wise general linear model (GLM) analysis
The TBSS toolbox in FSL was used for voxel-wise statistical analysis of DTI metrics along WM tracts. As a part of this process, the most representative FA map of the cohort was first identified by non-linearly co-registering each FA map to all other FA maps. The TBSS script then identifies the most “typical” or representative FA map among the cohort. This representative FA map was then co-registered using affine alignment registration to the MNI-152 standard brain space. The FA maps of all other subjects were then similarly co-registered to MNI-152 by combining the non-linear co-registration to the representative FA map and the affine alignment co-registration of the representative map to MNI-152. This process was repeated to bring MD, RD, and AD maps to the MNI-152 space. A skeletonized WM tract map across all subjects was generated by averaging the aligned FA maps using a threshold value of 0.1. Then, we used the “randomise” tool in FSL for GLM analyses to examine the association of delivery method with DTI metric values across WM tracts while controlling for gestational age at birth, gestational age at scan, 5-min APGAR, birth weight Z-score, and presence of preeclampsia as covariates. Supplemental Fig. S1 displays a visual representation of the GLM design and contrast files that were used to conduct the “randomise” function. The 5-min APGAR was chosen due to more prevalent availability in our dataset compared to 10-min APGAR and its established association with outcomes30. As birth weight Z-score and presence of preeclampsia differed significantly between infants delivered vaginally and via C-section (Table 1), we performed additional analyses to examine the association of birth weight Z-score and presence of preeclampsia with DTI metrics after controlling for delivery method, gestational age at birth, gestational age at scan, and 5-min APGAR as covariates. Additionally, as prior reports have described the association between general anesthesia use during C-section delivery and infant neurodevelopment, particularly with the use of inhaled anesthetics, we also analyzed the association between general anesthesia use during delivery and DTI metrics after controlling for delivery method, gestational age at birth, gestational age at scan, 5-min APGAR, birth weight Z-score, and presence of preeclampsia as covariates31,32. We applied 5000 permutations and threshold-free cluster enhancements while correcting for multiple comparisons in voxel-wise analysis. Results of the GLM analyses were visualized by overlaying a color-coded map of p-values corrected for multiple comparisons (windowed for p-values < 0.05) onto the MNI-152 brain space template. Similar TBSS and GLM analyses were repeated for the sub-cohort excluding infants born small for gestational age.
Tract-specific linear regression analysis
The results of our GLM analyses were further verified by conducting additional linear regression analyses across 48 WM tracts based on the John’s Hopkins University (JHU) atlas23. For every subject, the means of non-zero DTI metric values were calculated in each of the 48 WM tracts from JHU atlas using FSL. Then, we applied linear regression analyses to determine the association of delivery method with mean DTI metric values in each WM tract while controlling for gestational age at birth, gestational age at scan, 5-min APGAR, birth weight Z-score, and presence of preeclampsia as covariates. Similar tract-specific linear regression analyses were repeated for the sub-cohort excluding infants born small for gestational age. All p-values were also adjusted for multiple comparisons using the Benjamini–Hochberg procedure33.
Statistics
The data are presented as mean ± standard deviation, median (interquartile range) or frequency (percentage) as appropriate. The “stats” package in R was used to determine if significant differences existed with regards to clinical variables between infants delivered vaginally and via C-section. A two-sample t-test was used for continuous variables, a Wilcoxon rank-sum test was used for APGAR scores, and a chi-squared test was used for binary variables. R was also used to conduct tract-specific linear regression analyses.
Results
Subject characteristics
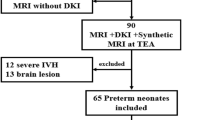
Figure 1 depicts the flow chart for the inclusion of 68 preterm infants for this study as well as the 59 infants included in sub-cohort analyses. Our primary cohort consisted of 23 infants delivered vaginally and 45 infants delivered via C-section. Table 1 summarizes the clinical demographics of these infants as well as the occurrence of pertinent postnatal conditions experienced by the infants. Among the infants in whom a patent ductus arteriosus was noted, only one infant (born by C-section) underwent ductus closure with a closure device. Of note, birth weight Z-score and presence of preeclampsia differed significantly between infants delivered vaginally and via C-section. The most common reason for preterm delivery in infants delivered vaginally was preterm labor (n = 18). Other reasons for preterm vaginal delivery included preterm labor with premature rupture of membranes (n = 4) or induction for chorioamnionitis (n = 1). The most common indication for preterm delivery via C-section were breech presentation (n = 13). Among those requiring C-section delivery due to breech presentation, preterm delivery was needed due to premature rupture of membranes (n = 7), preterm labor (n = 5), Hemolysis, Elevated Liver enzymes, and Low Platelets (HELLP) syndrome (n = 1), and failed external version (n = 1). Other indications for preterm C-section delivery included fetal heart rate abnormalities (n = 9), severe or worsening preeclampsia or HELLP syndrome (n = 11), placenta previa or accreta (n = 4), repeat C-section (n = 3), cord prolapse (n = 2), arrested growth and absent or reversed umbilical artery doppler flow (n = 1), or preterm labor with history of prior uterine surgery (n = 1). In 13 of the 45 C-section deliveries, general anesthesia was used. For all infants whose maternal charts contained detailed anesthesia information (n = 11), sevoflurane was used as the inhaled anesthetic of choice and in 4 deliveries nitrous oxide was used as an additional inhaled anesthetic.
Relationship of preterm infants’ delivery method with WM microstructure
Figure 2 depicts the results of the voxel-wise GLM analyses for the association of delivery method with FA, MD, and RD values after controlling for gestational age at birth, gestational age at scan, 5-min APGAR, birth weight Z-score, and presence of preeclampsia as covariates. These results were supported by tract-specific analyses (Supplemental Tables S1–S3). FA values in the corpus callosum, left internal capsule, right coronal radiata, and right tapetum tended to be lower in infants delivered via C-section compared to those delivered vaginally (Fig. 2A and Supplemental Table S1). MD values in the corpus callosum, cerebellar peduncle, left retrolenticular internal capsule, superior corona radiata, and fornix tended to be higher in infants delivered via C-section than those delivered vaginally (Fig. 2B and Supplemental Table S2). RD values in the corpus callosum, cerebellar peduncle, internal capsule, superior corona radiata, left cingulum, fornix, and left superior fronto-occipital fasciculus tended to be higher in infants delivered via C-section than those delivered vaginally (Fig. 2C and Supplemental Table S3). Of note, statistically significant associations between delivery method and DTI metrics were observed in the aforementioned regions on voxel-wise GLM analyses after correcting for multiple comparisons (Fig. 2). However, while results of our tract-specific analyses similarly trended toward significance, significant associations between delivery method and DTI metrics were not observed on tract-specific linear regressions analyses after correction for multiple comparisons (Supplemental Tables S1–S3). There was no significant association of delivery method with AD values on voxel-wise or tract-specific analyses.
Voxel-wise general linear model analyses of the association of delivery method with (A) fractional anisotropy (FA), (B) mean diffusivity (MD), (C) and radial diffusivity (RD) values after controlling for gestational age at birth, gestational age at scan, 5-min APGAR, birth weight Z-score, presence of preeclampsia, and presence of chorioamnionitis as covariates. Green areas depict white matter FA skeletons derived from tract based spatial statistics; the color bar and corresponding red–orange areas depicts regions where delivery method was significantly (1 − p > 0.95) associated with corresponding diffusion tensor metrics.
Relationship of preterm infants’ birth weight, maternal preeclampsia, and general anesthesia use during delivery with WM microstructure
Despite differing significantly between the two experimental groups, neither birth weight Z-score nor presence of maternal preeclampsia were significantly associated with any DTI metrics on voxel-wise GLM analysis or tract-specific regression after adjusting for delivery mode and other covariates listed above. Use of general anesthesia during delivery was also not significantly associated with any DTI metrics on voxel-wise GLM analysis or tract-specific regression after adjusting for delivery mode and additional covariates.
Relationship of delivery method with WM microstructure after excluding small-for-gestational-age preterm infants
Nine infants born SGA (8 delivered by C-section and 1 delivered vaginally) were excluded from the sub-cohort. Table 2 summarizes the clinical demographics of the infants included in the sub-cohort as well as the occurrence of pertinent postnatal conditions experienced by the infants. Of note, birth weight Z-score differed significantly between infants delivered vaginally and via C-section in the sub-cohort. The excluded infant delivered vaginally required preterm delivery due to preterm labor. Indications for preterm C-section delivery among the excluded infants included severe or worsening preeclampsia or HELLP syndrome (n = 5), fetal heart rate abnormalities (n = 2), and breech presentation with failed external version (n = 1). Of the excluded C-section deliveries, one required the use of general anesthesia.
Figure 3 depicts the results of the voxel-wise GLM analyses for the association of delivery method with FA, MDRD values in the sub-cohort after controlling for gestational age at birth, gestational age at scan, 5-min APGAR, birth weight Z-score, and presence of preeclampsia as covariates. These results were supported by tract-specific analyses (Supplemental Tables S4–S6). FA values in the corpus callosum, corona radiata, and right tapetum tended to be lower in infants delivered via C-section compared to those delivered vaginally (Fig. 3A and Supplemental Table S4). MD values in the pontine crossing tract, cerebellar peduncle, left retrolenticular internal capsule, right posterior corona radiata, fornix, stria terminalis, and right superior longitudinal fasciculus tended to be higher in infants delivered via C-section than those delivered vaginally (Fig. 3B and Supplemental Table S5). RD values in the pontine crossing tract, corpus callosum, cerebellar peduncle, corona radiata, fornix, stria terminalis, and right superior longitudinal fasciculus tended to be higher in infants delivered via C-section than those delivered vaginally (Fig. 3C and Supplemental Table S6). Of note, statistically significant associations between delivery method and DTI metrics were observed in the aforementioned regions on voxel-wise GLM analyses after correcting for multiple comparisons (Fig. 3). However, while results of our tract-specific analyses similarly trended toward significance, significant associations between delivery method and DTI metrics were not observed on tract-specific linear regressions analyses after correction for multiple comparisons (Supplemental Tables S4–S6). There was no significant association of delivery method with AD values on voxel-wise or tract-specific analyses. Birth weight z-score, maternal preeclampsia, and use of general anesthesia during delivery were not significantly associated with any DTI metrics in the sub-cohort after adjusting for delivery method and additional covariates.
Voxel-wise general linear model analyses of the association of delivery method with (A) fractional anisotropy (FA) and (B) radial diffusivity (RD) values in sub-cohort excluding infants born small for gestational age after controlling for gestational age at birth, gestational age at scan, 5-min APGAR, birth weight Z-score, presence of preeclampsia, and presence of chorioamnionitis as covariates. Green areas depict white matter FA skeletons derived from tract based spatial statistics; the color bar and corresponding red–orange areas depict regions where delivery method was significantly (1 − p > 0.95) associated with corresponding diffusion tensor metrics.
Discussion
In voxel-wise GLM analyses, preterm infants requiring C-section delivery had reduced WM microstructural integrity compared to those delivered vaginally, most prominently in the corpus callosum, internal capsule, and corona radiata. Notably, the difference between delivery mode of preterm infants was found after adjusting for gestational age at birth, gestational age at scan, 5-min APGAR, birth weight Z-score, and presence of preeclampsia as covariates—and after excluding infants from multiple gestations and those with any maternal history of drug use. While tract-specific linear regression analyses supported the results of our voxel-wise GLM analyses, significant associations between delivery method and DTI metrics were not observed after correcting for multiple corrections in these analyses. Although birthweight Z-score and presence of maternal preeclampsia differed significantly between infants delivered vaginally and via C-section, additional voxel-wise GLM analyses found no significant association between these risk factors and DTI metrics after controlling for delivery method and other clinical variables. In the sub-cohort excluding SGA infants, significant association between delivery method and DTI metrics was noted in similar regions to the overall cohort, including the the corpus callosum, internal capsule, and corona radiata.
Our results provide the neurobiological indications and early WM microstructural correlates for clinical studies reporting that preterm infants who require C-section are at risk for adverse neurodevelopmental outcome. The difference in early WM microstructural development may be a consequence of multiple factors necessitating C-section delivery; nevertheless, our findings highlight the higher risks of neurodevelopmental delay among preterm infants delivered via C-section and the need for close follow-up and potential intervention to mitigate these risks in this vulnerable cohort.
The myelination of WM tracts as infants age reduces the random diffusion of water molecules and results in alignment of diffusion along WM tracts. Thus, changes in DTI metrics such as increased FA and reduced MD and RD are corollaries for WM maturation34. DTI metrics have also been shown to predict neurological outcome, with studies reporting associations between DTI metrics measured during term-equivalent scans and cognitive, motor, and visual outcomes in childhood and adolescence35,36. Of note, some have reported associations between the development of cerebral palsy and other motor abnormalities and term equivalent DTI metrics in the same WM regions where we identified significant association between delivery method and DTI metrics such as the corpus callosum and internal capsule37,38. Thus, term-equivalent DTI can potentially help identify infants at risk for adverse neurological outcomes with regards to their method of delivery, especially when neurological exam and standard neuroimaging might otherwise be unremarkable.
The relationship between delivery method and infant brain development has not been extensively studied. Deoni et al. have compared the DTI metrics between term infants delivered via C-section (n = 11) versus vaginally (n = 32)25. Similar to our results, they reported higher FA values in WM tracts of term infants delivered vaginally compared to those delivered via C-section. We found higher MD and RD in addition to reduced FA in preterm infants delivered via C-section. Deoni et al. also noted slight variations in results with the inclusion of additional covariates, suggesting that factors beyond delivery method could be contributing to perceived differences in WM maturation. We attempted to address this by controlling for clinical variables known to be independently associated with WM maturation in infants (gestational age) in addition to those we found differed significantly between our two experimental groups (birthweight z-score and maternal preeclampsia), among others23. As prior reports have raised concern that exposure to general anesthesia, particularly inhaled agents such as sevoflurane, may contribute to delayed neurodevelopment and adverse long-term neurological outcomes, we also confirmed that exposure to general anesthesia during delivery was not independently associated with WM maturation in our cohort31,32,39,40. Of note, Deoni et. al found no difference in WM tract DTI metrics when comparing separate cohorts of eight-year-old children delivered via C-section (n = 23) versus vaginally (n = 37). Thus, they proposed that WM microstructural differences in association with delivery mode may only be present in early (pre-discharge) MRIs and not resolve at older age—although they analyzed different children’s cohorts from infants.
As we demonstrate the relationship between delivery method and WM maturation on neuroimaging, the question of how delivery method may influence early brain development arises. It has been suggested that vaginal delivery is an important contributor to the normal development of infants’ gut microbiome and that alterations in this process (due to C-section) can disturb normal brain development41,42. The impact of differential infant hormonal expression between vaginal and C-section deliveries on early brain development has also been proposed43. However, these explanations are complicated by studies reporting contrasting results regarding the association of delivery method with neurodevelopmental disorders19,44,45. Even among the numerous studies suggesting that delivery method may be associated with neurological outcomes, differences in methodology (such as additional clinical covariates that were controlled) and characteristics of experimental cohorts among prior reports complicate interpretation. Additionally, some investigators report that delivery method may be associated with neurological outcome only in the context of additional factors such as infant gender, APGAR scores, multiple gestations, and use of general anesthesia during C-section12,16,17,32. Given the many maternal and infant factors that may influence need for C-section versus vaginal delivery, it is challenging to control for all covariates that may influence brain development independently or cooperatively with delivery method. Nevertheless, our study provides WM microstructural correlates for prior clinical studies reporting that preterm infants requiring C-section are at higher risk of neurodevelopmental delay. While our findings may be related to multifactorial circumstances necessitating C-section delivery, they highlight the need for close neurological follow up and timely interventions in these vulnerable children.
Exclusion of infants born SGA revealed a significant difference in birth weight z-score between infants delivered by C-section and those delivered vaginally. Of note, prior reports have described the association between birth weight and DTI metrics as well as long-term neurological outcomes46,47,48. However, we address this by controlling for birth weight z-score as a covariate in analyses of the primary and sub-cohorts and also demonstrating a lack of significant independent association between birth-weight Z-score and DTI metrics in the primary and sub-cohorts.
Our study is limited by its retrospective, single center design and small sample size. Our small sample size also limits the power of our study in including multiple covariates, which may complicate the interpretations of our analyses on the independent associations of birthweight z-score, maternal preeclampsia, and general anesthesia use during delivery with DTI metrics. DTI metric analysis can be influenced by acquisition protocol and timing, as well as processing methods used to generate DTI metric maps. Although we controlled for several demographic and clinical variables, including those that differed significantly between experimental groups, we could not account for all possible covariates that may influence delivery method and explain observed differences in DTI metrics between the experimental groups in this study. Of note, recent work has suggested that intrauterine inflammation due to chorioamnionitis and other pathologies impacts fetal and infant neurodevelopment49,50. Additional postnatal pathologies such as bronchopulmonary dysplasia, necrotizing enterocolitis and sepsis have also been described to influence neurodevelopmental outcome in preterm and low birth weight infants51,52,53. However, given that the rate of such pathologies did not differ significantly between infants born by C-section or vaginally in our primary or sub-cohort, we elected not to include these pathologies as covariates in our analyses. We also described various indications for respective delivery method but could not control for all such indications given the widely varying delivery indications in our cohort. Finally, long-term neurological outcomes were not available in our dataset to corroborate clinical correlates of DTI findings.
Conclusion
Preterm infants requiring C-section section delivery, compared to those delivered vaginally, have neuroimaging markers of delayed WM maturation on predischarge MRI scans, most prominently in the corpus callosum, internal capsule, and corona radiata independent of gestational age at birth, gestational age at scan, 5-min APGAR, birth weight Z-score, maternal preeclampsia, and chorioamnionitis. Possible confounders of birth weight z-score, rates of maternal preeclampsia, and use of general anesthesia during C-section delivery are not independently associated with WM maturation in this cohort. These findings suggest that preterm infants requiring C-section are at risk for delays in WM maturation and thus may require close neurologic follow up to identify and mitigate adverse long-term neurological outcomes.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Walani, S. R. Global burden of preterm birth. Int. J. Gynaecol. Obstet. 150(1), 31–33 (2020).
da Fonseca, E. B., Damião, R. & Moreira, D. A. Preterm birth prevention. Best Pract. Res. Clin. Obstet. Gynaecol. 69, 40–49 (2020).
Purisch, S. E. & Gyamfi-Bannerman, C. Epidemiology of preterm birth. Semin. Perinatol. 41(7), 387–391 (2017).
Moore, T. A., Berger, A. M. & Wilson, M. E. A new way of thinking about complications of prematurity. Biol. Res. Nurs. 16(1), 72–82 (2014).
Odding, E., Roebroeck, M. E. & Stam, H. J. The epidemiology of cerebral palsy: Incidence, impairments and risk factors. Disab. Rehab. 28(4), 183–191 (2006).
Bhutta, A. T., Cleves, M. A., Casey, P. H., Cradock, M. M. & Anand, K. J. Cognitive and behavioral outcomes of school-aged children who were born preterm: A meta-analysis. JAMA. 288(6), 728–737 (2002).
Delobel-Ayoub, M. et al. Behavioral problems and cognitive performance at 5 years of age after very preterm birth: The EPIPAGE Study. Pediatrics. 123(6), 1485–1492 (2009).
Sommer, C., Urlesberger, B., Maurer-Fellbaum, U., Kutschera, J. & Müller, W. Neurodevelopmental outcome at 2 years in 23–26 weeks old gestation infants. Klinische Pädiatrie. 1, 23–29 (2006).
Robertson, C. M., Watt, M.-J. & Yasui, Y. Changes in the prevalence of cerebral palsy for children born very prematurely within a population-based program over 30 years. JAMA. 297(24), 2733–2740 (2007).
Lantos, J. D. & Lauderdale, D. S. What is behind the rising rates of preterm birth in the United States?. Rambam Maimonides Med. J. 2(4), e0065 (2011).
Ana Pilar, B., Jiangfeng, Y., Ann-Beth, M., João Paulo, S. & Jun, Z. Trends and projections of caesarean section rates: Global and regional estimates. BMJ Global Health. 6(6), e005671 (2021).
González-Valenzuela, M. J., González-Mesa, E., Cazorla-Granados, O. & López-Montiel, D. Type of delivery, neuropsychological development and intelligence in twin births. Front. Psychol. 10, 972 (2019).
Polidano, C., Zhu, A. & Bornstein, J. C. The relation between cesarean birth and child cognitive development. Sci. Rep. 7(1), 11483 (2017).
Kelmanson, I. A. Emotional and behavioural features of preschool children born by Caesarean deliveries at maternal request. Eur. J. Dev. Psychol. 10(6), 676–690 (2013).
Zhang, T. et al. Association of cesarean delivery with risk of neurodevelopmental and psychiatric disorders in the offspring: A systematic review and meta-analysis. JAMA Netw. Open. 2(8), e1910236 (2019).
Grace, T., Bulsara, M., Robinson, M. & Hands, B. Early life events and motor development in childhood and adolescence: A longitudinal study. Acta Paediatr. 105(5), e219–e227 (2016).
Sucksdorff, M. et al. Lower Apgar scores and Caesarean sections are related to attention-deficit/hyperactivity disorder. Acta Paediatr. 107(10), 1750–1758 (2018).
Macharey, G. et al. Neurodevelopmental outcome at the age of 4 years according to the planned mode of delivery in term breech presentation: a nationwide, population-based record linkage study. J. Perinatal Med. 46(3), 333–339 (2018).
Curran, E. A. et al. Obstetrical mode of delivery and childhood behavior and psychological development in a British cohort. J. Autism Dev. Disord. 46(2), 603–614 (2016).
Leung, C. Y., Leung, G. M. & Schooling, C. M. Mode of delivery and child and adolescent psychological well-being: Evidence from Hong Kong’s “Children of 1997” birth cohort. Sci. Rep. 7(1), 1–8 (2017).
Zhu, J.-J., Bao, Y.-Y., Zhang, G.-L., Ma, L.-X. & Wu, M.-Y. No relationship between mode of delivery and neonatal mortality and neurodevelopment in very low birth weight infants aged two years. World J. Pediatr. 10(3), 227–231 (2014).
Yamamoto, R., Ikeda, M., Hayashi, S., Mitsuda, N. & Ishii, K. Infantile survival and neurodevelopment at three years of age on delivery by the intended delivery mode in extremely preterm infants. Taiwan. J. Obstet. Gynecol. 61(2), 312–316 (2022).
Bobba, P. S. et al. Age-related topographic map of magnetic resonance diffusion metrics in neonatal brains. Hum. Brain Map. 43(14), 4326–4334 (2022).
Arzoumanian, Y. et al. Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. AJNR Am. J. Neuroradiol. 24(8), 1646–1653 (2003).
Deoni, S. C. et al. Cesarean delivery impacts infant brain development. Am. J. Neuroradiol. 40(1), 169 (2019).
Fenton, T. R. & Kim, J. H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 13(1), 1–13 (2013).
Hack, M., Klein, N. K. & Taylor, H. G. Long-term developmental outcomes of low birth weight infants. Future Child. 1, 176–196 (1995).
Lugli, L. et al. Neuroprem: The Neuro-developmental outcome of very low birth weight infants in an Italian region. Ital. J. Pediatr. 46(1), 26 (2020).
Barkovich, M. J., Xu, D., Desikan, R. S., Williams, C. & Barkovich, A. J. Pediatric neuro MRI: Tricks to minimize sedation. Pediatr. Radiol. 48(1), 50–55 (2018).
Persson, M., Razaz, N., Tedroff, K., Joseph, K. S. & Cnattingius, S. Five and 10 minute Apgar scores and risks of cerebral palsy and epilepsy: Population based cohort study in Sweden. BMJ. 360, k207 (2018).
Chien, L.-N., Lin, H.-C., Shao, Y.-H.J., Chiou, S.-T. & Chiou, H.-Y. Risk of autism associated with general anesthesia during cesarean delivery: A population-based birth-cohort analysis. J. Autism Dev. Disord. 45(4), 932–942 (2015).
Huberman Samuel, M. et al. Exposure to general anesthesia may contribute to the association between cesarean delivery and autism spectrum disorder. J. Aut. Dev. Disord. 49(8), 3127–3135 (2019).
Yoav, B. Controlling the false discovery rate: A practical and powerful approach to multiple testing. JR Stat. Soc. B. 57, 289–300 (1995).
Hüppi, P. S. et al. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr. Res. 44(4), 584–590 (1998).
Balakrishnan, U. et al. MRI at term equivalent age for predicting long-term neurodevelopmental outcome in preterm infants—a cohort study. J. Maternal Fetal Neonatal Med. 33(11), 1867–1873 (2020).
Skranes, J. et al. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain J. Neurol. 130(Pt 3), 654–666 (2007).
Rose, J. et al. Neonatal microstructural development of the internal capsule on diffusion tensor imaging correlates with severity of gait and motor deficits. Dev. Med. Child Neurol. 49(10), 745–750 (2007).
Kim, D.-Y., Park, H.-K., Kim, N.-S., Hwang, S.-J. & Lee, H. J. Neonatal diffusion tensor brain imaging predicts later motor outcome in preterm neonates with white matter abnormalities. Ital. J. Pediatr. 42(1), 104 (2016).
Wu, Z., Xue, H., Gao, Q. & Zhao, P. Effects of early postnatal sevoflurane exposure on oligodendrocyte maturation and myelination in cerebral white matter of the rat. Biomed. Pharmacother. 131, 110733 (2020).
Block, R. I. et al. Are anesthesia and surgery during infancy associated with decreased white matter integrity and volume during childhood?. Anesthesiology. 127(5), 788–799 (2017).
Shaterian, N., Abdi, F., Ghavidel, N. & Alidost, F. Role of cesarean section in the development of neonatal gut microbiota: A systematic review. 16(1), 624–639 (2021).
Diaz, H. R. Fetal, neonatal, and infant microbiome: Perturbations and subsequent effects on brain development and behavior. Semin. Fetal Neonatal Med. 21(6), 410–417 (2016).
Castillo-Ruiz, A., Mosley, M., Jacobs, A. J., Hoffiz, Y. C. & Forger, N. G. Birth delivery mode alters perinatal cell death in the mouse brain. Proc. Natl. Acad. Sci. 115(46), 11826–11831 (2018).
Običan, S. G. et al. Mode of delivery at periviability and early childhood neurodevelopment. Am. J. Obstet. Gynecol. 213(4), 578 (2015).
Smithers, L. G., Mol, B. W., Wilkinson, C. & Lynch, J. W. Implications of caesarean section for children’s school achievement: A population-based study. Aust. N. Zeal. J. Obstet. Gynaecol. 56(4), 374–380 (2016).
Lepomäki, V. et al. Effect of antenatal growth and prematurity on brain white matter: diffusion tensor study. Pediatr. Radiol. 42(6), 692–698 (2012).
Constable, R. T. et al. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: An investigation of group and gender effects. Pediatrics. 121(2), 306–316 (2008).
Hollund, I. M. H. et al. White matter alterations and their associations with motor function in young adults born preterm with very low birth weight. NeuroImage Clin. 17, 241–250 (2018).
Ganguli, S. & Chavali, P. L. Intrauterine viral infections: Impact of inflammation on fetal neurodevelopment. Front. Neurosci. 15, 771557 (2021).
Tomlinson, M. S. et al. Microorganisms in the placenta: links to early-life inflammation and neurodevelopment in children. Clin. Microbiol. Rev. 32(3), 1. https://doi.org/10.1128/cmr.00103-18 (2019).
Ferreira, R. C., Mello, R. R. & Silva, K. S. Neonatal sepsis as a risk factor for neurodevelopmental changes in preterm infants with very low birth weight. J. Pediatr. 90, 293–299 (2014).
Katz, T. A. et al. Severity of bronchopulmonary dysplasia and neurodevelopmental outcome at 2 and 5 years corrected age. J. Pediatr. 243(40–6), e2 (2022).
Salhab, W. A., Perlman, J. M., Silver, L. & Sue, B. R. Necrotizing enterocolitis and neurodevelopmental outcome in extremely low birth weight infants< 1000 g. J. Perinatol. 24(9), 534–540 (2004).
Funding
P.B. received support from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (T35DK104689). S.P. received grant support from National Institutes of Health (K23NS118056), Doris Duke Charitable Foundation (2020097) and Foundation of American Society of Neuroradiology.
Author information
Authors and Affiliations
Contributions
P.B. conducted primary analyses, wrote the main manuscript text, and generated all figures. S.P. generated the idea for the project and provided access to necessary data and resources in the completion of the analyses presented in the manuscript. C.W., A.M., M.B., J.C., S.T., and L.R. all ensured integrity of the data and initial analyses conducted during this project and provided valuable insight into the revision of analyses to the final forms presented in the manuscript. All authors contributed to review of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bobba, P.S., Weber, C.F., Malhotra, A. et al. Early brain microstructural development among preterm infants requiring caesarean section versus those delivered vaginally. Sci Rep 13, 21514 (2023). https://doi.org/10.1038/s41598-023-48963-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48963-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.