Abstract
The prognosis of advanced biliary tract cancer (BTC) patients remains poor due to limited efficacy of chemotherapy and difficulties in management. Thus, prediction of survival is crucial for the clinical management of advanced BTC. The aim was to develop and validate a nomogram to predict 6-month and 12-month survival in advanced BTC patients treated with chemotherapy. A multivariable Cox regression model was used to construct a nomogram in a training set (JCOG1113, a phase III trial comparing gemcitabine plus S-1 [GS] and gemcitabine plus cisplatin, n = 351). External validity of the nomogram was assessed using a test set (JCOG0805, a randomized, phase II trial comparing GS and S-1 alone, n = 100). Predictive performance was assessed in terms of discrimination and calibration. The constructed nomogram included lymph node metastasis, liver metastasis, carbohydrate antigen 19-9, carcinoembryonic antigen, albumin, and C-reactive protein. Uno’s concordance index was 0.661 (95% confidence interval [CI] 0.629–0.696) in the training set and 0.640 (95% CI 0.566–0.715) in the test set. The calibration plots for 6-month and 12-month survival showed good agreement in the two analysis sets. The present nomogram can facilitate prediction of the prognosis of advanced BTC patients treated with chemotherapy and help clinicians’ prognosis-based decision-making.
Similar content being viewed by others
Introduction
Biliary tract cancer (BTC), including intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, gallbladder cancer, and ampullary cancer, is relatively rare worldwide. However, it is estimated that, in 2017, approximately 210,000 new patients were diagnosed with this disease worldwide1,2. Despite differences in anatomical sites and geographical regions, the incidence of BTC has increased1,3, and one epidemiological study reported that it increased by 76% between 1990 and 20171. Moreover, the majority of the patients presented in an advanced stage at the time of diagnosis, and such patients are basically treated with chemotherapy4. Since the gemcitabine plus cisplatin (GC) regimen was shown to prolong survival in patients with advanced BTC5, various chemotherapeutic regimens have been developed. Above all, gemcitabine, platinum, and fluorouracil are widely used as key drugs for advanced BTC6,7,8,9,10,11. However, the patients’ prognosis remains poor, with a median overall survival (OS) of about 1 year3.
One possible reason for the poor prognosis is the difficulty in the management of BTC. Differences in anatomical site, clinical presentation, comorbidity, and surgical therapy lead to further challenges in treatment for BTC for many physicians4,12. In such situations, prediction of survival is crucial for the clinical management of advanced BTC. However, there are few reports of prognostic models for advanced BTC13, and the majority of these studies were single-center, retrospective designs14,15,16. The major drawback of these studies was that the number of censored subjects accounted for 25% to 50%15,17,18, which could attenuate the predictability of the built prognostic models for the external datasets. In fact, despite the heterogeneity of BTC, the external validity of these models has not been fully evaluated19. Therefore, to develop the prognostic model for advanced BTC, it is necessary to construct a model based on prospectively collected, high-quality data and evaluate the validity of the model. The Japan Clinical Oncology Group (JCOG) previously reported the results of two multicenter, randomized trials that evaluated gemcitabine, cisplatin, and the oral fluorouracil agent S-1. The first one was JCOG0805 (a randomized phase II trial, UMIN Clinical Trials Registry [http://www.umin.ac.jp/ctr/index.htm] number, UMIN000001685), comparing gemcitabine plus S-1 (GS) and S-1 alone, which reported more promising efficacy of GS in the 1-year OS20. The second one was JCOG1113 (a phase III trial after JCOG0805, UMIN Clinical Trials Registry number, UMIN000010667), comparing GS and GC in patients with advanced BTC, which demonstrated non-inferiority of GS to GC in OS, but superiority was not shown9. Consequently, the GS regimen, as well as the GC regimen, was positioned as one of the standard treatments for advanced BTC in Japan.
Using the two trials’ datasets, an ancillary analysis (termed JCOG1917A) was conducted to develop a nomogram to predict 6-month and 12-month survival proportions.
Results
Patients’ characteristics
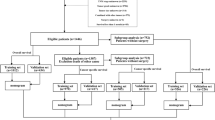
The patient flow chart is shown in Fig. 1. The training set consisted of 351 advanced BTC patients who were enrolled in JCOG1113 between May 2013 and March 2016 (174 in arm GC, and 177 in arm GS). The test set consisted of 100 advanced BTC patients who were enrolled in JCOG0805 between February 2009 and April 2010 (50 in arm GS, and 50 in arm S-1) (Fig. 1). Their baseline characteristics are summarized in Table 1.
Survival
The median follow-up was 13.8 months in the training set and 10.5 months in the test set. The Kaplan–Meier curves of OS are shown in Fig. 2A,B. In the training set, the 6-month and 12-month OS for all patients were 87.5% and 58.7%, respectively (GS arm: 6-month OS, 88.7%; 12-month OS, 59.3%; GC arm: 6-month OS, 86.2%; 12-month OS, 58.0%). In the test set, the 6-month and 12-month OS were 77.0% and 46.0%, respectively (GS arm: 6-month OS, 80.0%; 12-month OS, 52.0%; S-1 arm: 6-month OS, 74.0%; 12-month OS, 40.0%).
Development and validation of the nomogram
The backward elimination procedure in the Cox regression analysis identified 6 factors (lymph node metastasis, liver metastasis, carbohydrate antigen 19-9 [CA19-9; log scale], carcinoembryonic antigen [CEA; log scale], albumin, and C-reactive protein [CRP; log scale]) in the training set. The final model is summarized in the left panel of Table 2, and the nomogram for 6-month and 12-month survivals is shown in Fig. 3.
Nomogram for predicting 6-month and 12-month survival in patients with biliary tract cancer. To use the nomogram, an individual patient’s value is located on each variable axis, and a line is drawn upward to determine the number of points received for each variable value. The sum of these numbers is located on the Total Points axis, and a line is drawn downward to the survival axes to determine the probability of 6-month or 12-month survival.
In the training set, the time-dependent receiver-operating characteristic (ROC) is shown in Supplemental Fig. 1, and the AUC was approximately 0.614–0.656 for the thresholds examined. Uno’s concordance index was 0.661 (95% confidence interval [CI] 0.629–0.696). The optimism-corrected value calculated from a bootstrap sample was 0.644. The calibration plots comparing the nomogram-predicted 6-month and 12-month survivals with their corresponding Kaplan–Meier estimates showed good agreement (Fig. 4A,B). In the test set, Uno’s concordance index was 0.640 (95% CI 0.566–0.715), and the optimism-corrected value was 0.640. The calibration plots also showed good agreement (Fig. 4C,D).
Calibration plots of overall survival probabilities at 6 months (A) and 12 months (B) in the training set, and at 6 months (C) and 12 months (D) in the test set. The nomogram-predicted probability of overall survival is plotted on the X-axis, with actual overall survival on the Y-axis. Dashed lines along the diagonal line through the origin point represent the perfect calibration models in which the predicted probabilities are identical to the observed probabilities. CA19-9 carbohydrate antigen 19-9, CEA carcinoembryonic antigen, CRP C-reactive protein, OS overall survival.
Validation and risk stratification in each treatment arm
Discrimination and calibration were further assessed in each treatment arm. In the training set, Uno’s concordance indices were 0.662 (GC arm) and 0.661 (GS arm). The indices in the test set were 0.613 (GS arm) and 0.612 (S-1 arm). On the calibration plots by treatment arm for predicting 12-month OS, the calibration plots showed good agreement (Fig. 5A–D). However, slight upper deviation was observed at higher predicted probabilities in the GS arm in the training set.
In addition, patients in the training and test sets were stratified into two groups (high-risk vs. low-risk) according to the median value of the nomogram-predicted score. The Kaplan–Meier curves stratified by treatment regimen indicated that the nomogram could discriminate patients into high-risk and low-risk groups in the training set (Fig. 6A,B), but it did so poorly in the test set (Fig. 6C,D).
Kaplan–Meier curves comparing overall survival in high-risk and low-risk groups of advanced BTC patients treated with GC in the training set (A), in high-risk and low-risk groups of advanced BTC patients treated with GS in the training set (B), in high-risk and low-risk groups of advanced BTC patients treated with GS in the test set (C), and in high-risk and low-risk groups of advanced BTC patients treated with S-1 in the test set (D). GC gemcitabine plus cisplatin, GS gemcitabine plus S-1.
Discussion
A nomogram for survival was constructed and validated in patients treated with chemotherapy for advanced BTC using data derived from two randomized trials. The nomogram included lymph node metastasis, liver metastasis, CA19-9, CEA, albumin, and CRP, and its external validity was not problematic in the test set. The use of this nomogram will facilitate prognosis prediction for patients with advanced BTC and help inform patients in the clinical setting. Furthermore, the nomogram also showed calibration abilities in 2 gemcitabine-based chemotherapeutic regimens (GC and GS), as well as S-1 monotherapy. The derived prognostic score based on the nomogram modestly stratified patients into high-risk and low-risk groups in these different regimens for advanced BTC, and these findings suggest that the nomogram can potentially be generalized to other cytotoxic chemotherapeutic regimens.
One strength of the nomogram is its simplicity. A nomogram is a simple-to-use graphical tool and intuitive representation of the statistical model. In recent years, it has been applied in the medical field and used for personalized assessment of risk in patients with various types of cancer21. The present nomogram contains 6 variables (lymph nodes metastasis, liver metastasis, CA19-9, CEA, albumin, and CRP), all of which are commonly used in daily medical practice. Thus, this nomogram can be easily applied in daily clinical use. Other strengths of this study include external validation of the findings and use of prospectively collected clinical trial data. The American Joint Committee on Cancer (AJCC)/TNM staging system is the most widely used classification for prognostic estimates, but the prognostic performance of AJCC/TNM staging has mainly been validated in surgical cases and rarely in unresectable cases22. Therefore, the AJCC/TNM staging system may not be suitable for risk stratification of patients with unresectable disease. A previous study by Wu et al. developed a nomogram for advanced BTC patients treated with gemcitabine-based chemotherapy using retrospectively collected data14. Their nomogram-predicted 6-month survival outcomes were well correlated with the actual observed ones in the analysis set for model construction. Generally, the median OS of advanced BTC patients treated with gemcitabine-based chemotherapy reaches about 1 year, and the long-term predictability of their proposed nomogram was uncertain. The present nomogram was developed using prospectively collected data from multicenter clinical trials and validated externally. Therefore, the results should be more applicable to the general population than models whose external validity has not been assessed. However, the credibility and validity of this nomogram for patients who do not meet the inclusion criteria or who meet the exclusion criteria for the two trials have not been established.
The factors constituting the present nomogram are categorized mainly into two groups: tumor-related factors and inflammatory-related factors. Tumor-related factors, lymph node metastasis, liver metastasis, CA19-9, and CEA, represent tumor burden, and their importance as prognostic factors is widely accepted16,23,24,25,26. On the other hand, the inflammatory-related factors have recently been highlighted. Cancer-related inflammation leads to alterations of the tumor microenvironment and contributes to cancer cell proliferation, invasion, and metastatic spread27. Furthermore, this inflammation also affects cancer development by increasing catabolism and impairing nutrient absorption. Conversely, malnutrition promotes the exacerbation of inflammation28. Thus, inflammation and malnutrition are interrelated and promote cancer progression. Albumin and CRP are well-known inflammation-related prognostic factors, and their prognostic values have been confirmed in a variety of solid tumors29. However, their utilities for advanced BTC have been not well examined up to now. In the present study, the addition of inflammatory-related factors strengthened the prognostic capabilities of the nomogram, and this fact indicates that systemic inflammation plays a major prognostic role in advanced BTC. Furthermore, it is noteworthy that our model comprehensively predicts the prognosis of BTC from both tumor and inflammation aspects compared to previous reports18,30,31.
The present nomogram not only enables simple application to predict survival, but it also helps clinicians’ prognosis-based decision-making. If poor outcomes are predicted by the nomogram, early comprehensive genomic profiling is recommended for precision medicine. After first-line gemcitabine-based chemotherapy, fluorouracil-based chemotherapy is commonly used for advanced BTC, but its efficacy is modest32. However, novel biomarker-based therapies33,34,35, such as isocitrate dehydrogenase 1 (IDH1) inhibitors for IDH1 mutations36, fibroblast growth factor receptor (FGFR) inhibitors for FGFR2 fusion/rearrangement37,38,39,40,41, and immune checkpoint inhibitors (ICIs) for microsatellite instability-high/mismatch repair-deficiency42, have been emerging for advanced BTC, and they have shown promising results in these patients with a poor prognosis. Thus, early genomic profiling opens up the possibility of new treatments for patients who are expected to have a poor prognosis with standard treatment. Prognostic prediction by the present nomogram may accelerate the application of precision medicine and contribute to prognosis-based decision-making in the therapeutic strategy for advanced BTC patients.
The present study had some limitations. The first limitation is the fact that the nomogram was developed using integrated data with limited sample size. However, the predictive performance of the present nomogram was good in the validation set. The second limitation is that this nomogram was developed based on data of patients treated only with cytotoxic chemotherapy. Recently, a phase III trial showed that a combination of ICI and cytotoxic chemotherapy (durvalumab plus GC) improved OS compared with cytotoxic chemotherapy alone (GC)11. It is unclear whether the present nomogram can be used for patients treated with ICIs. The third limitation is that the discriminative ability of the nomogram was relatively modest. This could be due to the heterogeneity exhibited by BTCs. BTCs are subclassified into important subtypes, such as intrahepatic cholangiocarcinoma and gallbladder cancer, each with its own unique characteristics including prognosis. The test set in this study had a higher proportion of intrahepatic cholangiocarcinoma than the train set. This fact may contribute to the relatively modest discriminative ability. The fourth limitation is that, due to the sample size, our model can only classify patients into two risk groups: high-risk and low-risk. Previous reports have classified patients into three groups: high-risk, intermediate-risk, and low-risk18, which may be more appropriate for practical use.
In conclusion, a nomogram for predicting survival in patients treated with chemotherapy for advanced BTC was developed using data derived from two randomized trials. The nomogram successfully demonstrated external validity and also showed calibration abilities for three different chemotherapeutic regimens. This nomogram will facilitate prognosis prediction for patients with advanced BTC, help clinicians’ prognosis-based decision-making, and inform patients in the clinical setting.
Methods
A total of 455 patients were enrolled in JCOG0805 (101 patients) and JCOG1113 (354 patients). Details of the study design, inclusion/exclusion criteria, and efficacy and safety results have been described previously9,20. Briefly, the primary endpoint of JCOG1113 was OS, and that of JCOG0805 was 1-year OS. The protocol of this ancillary study (JCOG1917A) was approved by the institutional review board of National Cancer Center Hospital East. Written informed consent, including secondary use of data, was obtained from each patient before enrollment in JCOG1113 or JCOG0805. This study was performed in accordance with the international ethical recommendations of the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects.
Of all 455 randomized patients, four were excluded, because two patients in JCOG1113 had indistinguishable primary sites and the other two patients (one in JCOG1113 and one in JCOG0805) had missing data for factors needed to construct the nomogram. Training and test sets consisted of 351 and 100 patients, respectively.
The endpoint of interest was OS, defined as the duration between the date of randomization and the date of death or censored on the last date they were known to be alive. In the nomogram, prediction performance of survival proportions at 6 and 12 months was also evaluated, since the median OS in first-line chemotherapy for advanced BTC is about 1 year5,9,11.
Statistical considerations
Multivariable Cox regression analyses were performed to assess the associations between various factors and OS. The factors included were age (continuous)17,43,44, sex (male or female)3,45,46, Eastern Cooperative Oncology Group (ECOG) performance status (0 or 1)15,16,23, body mass index (BMI; continuous)47,48, primary tumor location (intrahepatic bile duct, gallbladder, extrahepatic bile duct, or ampulla of Vater)3,49, extent of disease (locally advanced or metastatic), lymph node metastasis (present or absent)50, liver metastasis (present or absent)16, lung metastasis (present or absent), unresectability due to extensive biliary involvement (yes or no)51, unresectability due to vascular invasion (yes or no)50,51, CA19-9 (continuous)24, CEA (continuous)23, albumin (continuous)52, CRP (continuous)24,52, neutrophils (continuous)13,15, and alkaline phosphatase (continuous)16. Logarithmic transformations were performed for continuous variables, except age, BMI, and albumin, because their empirical distributions were highly skewed with a heavy tail. For CA19-9 and CRP, a small value of 0.01 was added to handle the logarithmic transformation of zero. A non-linear association of age and neutrophils (logarithmic scale) with the log hazard ratio was observed on univariable Cox regression analysis, and thus the two factors were modeled as restricted cubic spline functions with 4 knots. The final model was selected following a backward elimination procedure using Akaike’s information criterion as a stopping rule. The nomogram for 6-month and 12-month OS was formulated with rms package version 6.2 in R.
Predictive performance of the constructed nomogram was assessed in two ways (discrimination and calibration) with the training set53. Discrimination was first graphically assessed using time-dependent ROC curves for every six months until 48 months54. Uno’s concordance index was calculated, and its optimistic-corrected value was estimated by bootstrapping with 1000 resamples55. Calibration was next evaluated by comparing nomogram-predicted survival with their Kaplan–Meier estimates in 4 groups classified based on a linear predictor of the final model. Calibration was assessed by plotting the predicted values against the actual estimates, which should be distributed near a 45-degree line for a well-calibrated model.
Finally, external validation was performed with the test set in the same manner as with the training set. Considering the limited sample size, calibration was done in 3 groups classified based on a linear predictor. Calibration was further assessed by stratifying by treatment arm in the training and test sets. All analyses were performed with R version 4.1.2. All reported P-values are two-sided.
Data availability
The data underlying this article cannot be shared publicly due to protection of privacy of individuals who participated in the study. The data will be shared with investigators whose proposed use of the data has been approved by investigators from the JCOG Hepatobiliary and Pancreatic Oncology Group. Proposals should be directed to the corresponding author.
References
Ouyang, G. et al. The global, regional, and national burden of gallbladder and biliary tract cancer and its attributable risk factors in 195 countries and territories, 1990 to 2017: A systematic analysis for the Global Burden of Disease Study 2017. Cancer 127, 2238–2250. https://doi.org/10.1002/cncr.33476 (2021).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. https://doi.org/10.3322/caac.21492 (2018).
Flemming, J. A., Zhang-Salomons, J., Nanji, S. & Booth, C. M. Increased incidence but improved median overall survival for biliary tract cancers diagnosed in Ontario from 1994 through 2012: A population-based study. Cancer 122, 2534–2543. https://doi.org/10.1002/cncr.30074 (2016).
Valle, J. W., Kelley, R. K., Nervi, B., Oh, D.-Y. & Zhu, A. X. Biliary tract cancer. The Lancet 397, 428–444. https://doi.org/10.1016/s0140-6736(21)00153-7 (2021).
Valle, J. et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 362, 1273–1281. https://doi.org/10.1056/NEJMoa0908721 (2010).
Fiteni, F. et al. Cisplatin/gemcitabine or oxaliplatin/gemcitabine in the treatment of advanced biliary tract cancer: A systematic review. Cancer Med. 3, 1502–1511. https://doi.org/10.1002/cam4.299 (2014).
Kim, S. T. et al. Capecitabine plus oxaliplatin versus gemcitabine plus oxaliplatin as first-line therapy for advanced biliary tract cancers: A multicenter, open-label, randomized, phase III, noninferiority trial. Ann. Oncol. 30, 788–795. https://doi.org/10.1093/annonc/mdz058 (2019).
Lee, J. et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 13, 181–188. https://doi.org/10.1016/s1470-2045(11)70301-1 (2012).
Morizane, C. et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: The FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann. Oncol. 30, 1950–1958. https://doi.org/10.1093/annonc/mdz402 (2019).
Ioka, T. et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 (GCS) versus gemcitabine, cisplatin (GC) for advanced biliary tract cancer (KHBO1401-MITSUBA). J. Hepatobiliary Pancreat. Sci. https://doi.org/10.1002/jhbp.1219 (2022).
Oh, D.-Y. et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. https://doi.org/10.1056/EVIDoa2200015 (2022).
Kim, D., Konyn, P., Cholankeril, G., Bonham, C. A. & Ahmed, A. Trends in the mortality of biliary tract cancers based on their anatomical site in the United States from 2009 to 2018. Am. J. Gastroenterol. 116, 1053–1062. https://doi.org/10.14309/ajg.0000000000001151 (2021).
Grenader, T. et al. Derived neutrophil lymphocyte ratio may predict benefit from cisplatin in the advanced biliary cancer: The ABC-02 and BT-22 studies. Ann. Oncol. 26, 1910–1916. https://doi.org/10.1093/annonc/mdv253 (2015).
Wu, C. E. et al. Establishment of a pretreatment nomogram to predict the 6-month mortality rate of patients with advanced biliary tract cancers undergoing gemcitabine-based chemotherapy. Cancers 13, 133139. https://doi.org/10.3390/cancers13133139 (2021).
Suzuki, Y. et al. Predictive factors of the treatment outcome in patients with advanced biliary tract cancer receiving gemcitabine plus cisplatin as first-line chemotherapy. J. Gastroenterol. 54, 281–290. https://doi.org/10.1007/s00535-018-1518-3 (2019).
Park, I. et al. Prognostic factors and predictive model in patients with advanced biliary tract adenocarcinoma receiving first-line palliative chemotherapy. Cancer 115, 4148–4155. https://doi.org/10.1002/cncr.24472 (2009).
Sasaki, T. et al. Prognostic factors in patients with advanced biliary tract cancer receiving chemotherapy. Cancer Chemother. Pharmacol. 67, 847–853. https://doi.org/10.1007/s00280-010-1360-7 (2011).
Salati, M. et al. The ALAN score identifies prognostic classes in advanced biliary cancer patients receiving first-line chemotherapy. Eur. J. Cancer 117, 84–90. https://doi.org/10.1016/j.ejca.2019.05.030 (2019).
Harrell, F. E. Jr. Regression Modeling Strategies (Springer, 2015).
Morizane, C. et al. Randomized phase II study of gemcitabine plus S-1 versus S-1 in advanced biliary tract cancer: A Japan Clinical Oncology Group trial (JCOG 0805). Cancer Sci. 104, 1211–1216. https://doi.org/10.1111/cas.12218 (2013).
Tosoian, J. J. et al. Prediction of pathological stage based on clinical stage, serum prostate-specific antigen, and biopsy Gleason score: Partin Tables in the contemporary era. BJU Int. 119, 676–683. https://doi.org/10.1111/bju.13573 (2017).
Spolverato, G. et al. Comparative performances of the 7th and the 8th editions of the American Joint Committee on Cancer staging systems for intrahepatic cholangiocarcinoma. J. Surg. Oncol. 115, 696–703. https://doi.org/10.1002/jso.24569 (2017).
Park, H. S. et al. Prognostic factors and scoring model for survival in metastatic biliary tract cancer. Cancer Res. Treat. 49, 1127–1139. https://doi.org/10.4143/crt.2016.538 (2017).
Briggs, C. D. et al. Prognostic molecular markers in cholangiocarcinoma: A systematic review. Eur. J. Cancer 45, 33–47. https://doi.org/10.1016/j.ejca.2008.08.024 (2009).
Izquierdo-Sanchez, L. et al. Cholangiocarcinoma landscape in Europe: Diagnostic, prognostic and therapeutic insights from the ENSCCA registry. J. Hepatol. 76, 1109–1121. https://doi.org/10.1016/j.jhep.2021.12.010 (2022).
Bridgewater, J. et al. Long-term outcomes and exploratory analyses of the randomized phase III BILCAP study. J. Clin. Oncol. 40, 2048–2057. https://doi.org/10.1200/JCO.21.02568 (2022).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437. https://doi.org/10.1038/nm.3394 (2013).
DeBerardinis, R. J. & Chandel, N. S. Fundamentals of cancer metabolism. Sci. Adv. 2, e1600200. https://doi.org/10.1126/sciadv.1600200 (2016).
McMillan, D. C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 39, 534–540. https://doi.org/10.1016/j.ctrv.2012.08.003 (2013).
Du, J. H. & Lu, J. Circulating CEA-dNLR score predicts clinical outcome of metastatic gallbladder cancer patient. J. Clin. Lab. Anal. 33, e22684. https://doi.org/10.1002/jcla.22684 (2019).
Rovesti, G. et al. Prognostic role of a new index tested in European and Korean advanced biliary tract cancer patients: The PECS index. J. Gastrointest. Cancer 53, 289–298. https://doi.org/10.1007/s12029-021-00596-z (2022).
Lamarca, A. et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 22, 690–701. https://doi.org/10.1016/s1470-2045(21)00027-9 (2021).
Subbiah, V. et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 21, 1234–1243. https://doi.org/10.1016/s1470-2045(20)30321-1 (2020).
Javle, M. et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 22, 1290–1300. https://doi.org/10.1016/s1470-2045(21)00336-3 (2021).
Ohba, A. et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): An investigator-initiated multicenter phase 2 study (HERB trial). J. Clin. Oncol. 40, 4006–4006. https://doi.org/10.1200/JCO.2022.40.16_suppl.4006 (2022).
Abou-Alfa, G. K. et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. https://doi.org/10.1016/s1470-2045(20)30157-1 (2020).
Abou-Alfa, G. K. et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. https://doi.org/10.1016/s1470-2045(20)30109-1 (2020).
Meric-Bernstam, F. et al. Futibatinib, an irreversible FGFR1-4 inhibitor, in patients with advanced solid tumors harboring FGF/FGFR aberrations: A phase I dose-expansion study. Cancer Discov. 12, 402–415. https://doi.org/10.1158/2159-8290.CD-21-0697 (2022).
Javle, M. et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: Mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol. Hepatol. 6, 803–815. https://doi.org/10.1016/s2468-1253(21)00196-5 (2021).
Mazzaferro, V. et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer 120, 165–171. https://doi.org/10.1038/s41416-018-0334-0 (2019).
Bahleda, R. et al. Multicenter phase I study of erdafitinib (JNJ-42756493), oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced or refractory solid tumors. Clin. Cancer Res. 25, 4888–4897. https://doi.org/10.1158/1078-0432.CCR-18-3334 (2019).
Marabelle, A. et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair–deficient cancer: Results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 38, 1–10. https://doi.org/10.1200/jco.19.02105 (2020).
Kayahara, M., Nagakawa, T., Nakagawara, H., Kitagawa, H. & Ohta, T. Prognostic factors for gallbladder cancer in Japan. Ann. Surg. 248, 807–814. https://doi.org/10.1097/SLA.0b013e31818a1561 (2008).
Cho, S. Y. et al. Survival analysis of intrahepatic cholangiocarcinoma after resection. Ann. Surg. Oncol. 17, 1823–1830. https://doi.org/10.1245/s10434-010-0938-y (2010).
He, X. D. et al. Gender-specific prognostic markers of patients with gallbladder cancer after surgical resection. Am. Surg. 76, 1269–1274 (2010).
Gupta, V. et al. Sex-based differences in gallbladder cancer: An analysis of 257 patients. J. Clin. Oncol. 32, e15095. https://doi.org/10.1200/jco.2014.32.15_suppl.e15095 (2014).
Calle, E. E., Rodriguez, C., Walker-Thurmond, K. & Thun, M. J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N. Engl. J. Med. 348, 1625–1638. https://doi.org/10.1056/NEJMoa021423 (2003).
Kang, J. et al. Body mass index and weight change during initial period of chemotherapy affect survival outcome in advanced biliary tract cancer patients. PLoS ONE 13, e0195118. https://doi.org/10.1371/journal.pone.0195118 (2018).
Ishihara, S. et al. Biliary tract cancer registry in Japan from 2008 to 2013. J. Hepatobiliary Pancreat. Sci. 23, 149–157. https://doi.org/10.1002/jhbp.314 (2016).
Mavros, M. N., Economopoulos, K. P., Alexiou, V. G. & Pawlik, T. M. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: Systematic review and meta-analysis. JAMA Surg. 149, 565–574. https://doi.org/10.1001/jamasurg.2013.5137 (2014).
Sakamoto, Y. et al. Proposal of a new staging system for intrahepatic cholangiocarcinoma: Analysis of surgical patients from a nationwide survey of the Liver Cancer Study Group of Japan. Cancer 122, 61–70. https://doi.org/10.1002/cncr.29686 (2016).
Proctor, M. J. et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: A Glasgow inflammation outcome study. Br. J. Cancer 104, 726–734. https://doi.org/10.1038/sj.bjc.6606087 (2011).
Moons, K. G. et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): Explanation and elaboration. Ann. Intern. Med. 162, W1–W73. https://doi.org/10.7326/M14-0698 (2015).
Heagerty, P. J. & Zheng, Y. Survival model predictive accuracy and ROC curves. Biometrics 61, 92–105. https://doi.org/10.1111/j.0006-341X.2005.030814.x (2005).
Uno, H., Cai, T., Pencina, M. J., D’Agostino, R. B. & Wei, L. J. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat. Med. 30, 1105–1117. https://doi.org/10.1002/sim.4154 (2011).
Acknowledgements
JCOG 1917A was the conducted by the JCOG Hepatobiliary and Pancreatic Oncology Group. The authors would like to express their sincere appreciation to all participating patients and families, and the investigators, research nurses, study coordinators in JCOG1113 and JCOG0805. The authors would also like to express their sincere appreciation to the members of the JCOG Data Center and JCOG Operations Office for their support.
Funding
This work was supported by National Cancer Center Research and Development Funds [Grant Numbers 29-A-3 and 2020-J-3].
Author information
Authors and Affiliations
Contributions
Conceptualization: H.I. Collection and assembly of data: H.I., M.I., C.M., T.O., M.O., S.S., K.Y., N.O., K.S., H.S., N.M., S.S., H.Y., R.S., K.G., K.S., A.A., K.N., M.U. Data curation: S.N. Formal analysis: S.N. Methodology: H.I., S.N. Supervision: M.I., C.M., M.U. Writing—original draft: H.I. Writing—review & editing: M.I., S.N., C.M., T.O., M.U.
Corresponding author
Ethics declarations
Competing interests
HI has received research funding from Ono Pharmaceutical; honoraria from Nihon Servier, Yakult Honsha, AstraZeneca, Kaneka Medix, and Medico's Hirata; and is a member of advisory board for Nihon Servier. MI has received research funding from Eisai, Merck biopharma, Eli Lilly Japan, Yakult, Ono Pharmaceutical, ASLAN, J-Pharma, AstraZeneca, Pfizer, Merus N.V., Nihon Servier, Delta-Fly Pharma, Chiome Bioscience, Chugai, Bristol-Myers Squibb, Novartis, Bayer, Takeda, MSD, and Syneos Health; and honoraria from Eisai, MSD, Eli Lilly Japan, Yakult, Teijin Pharma, Astellas, Sumitomo Dainippon, Otsuka, Nihon Servier, Taiho, Chugai, Bristol-Myers Squibb, Novartis, Bayer, Takeda, EA Pharma, AstraZeneca, AbbVie, Abbott Japan, Fujifilm Toyama Chemical, and Incyte Biosciences Japan. CM has received research funding from Eisai, Yakult Honsha, Ono Pharmaceutical, Taiho Pharmaceutical, J-Pharma, AstraZeneca, Merck biopharma, Daiichi Sankyo, and HITACHI; consulting fees from Yakult Honsha, MSD K.K., and Servier; and honoraria from Novartis, Yakult Honsha, Teijin Pharma, Taiho Pharmaceutical, Eisai, and MSD K.K. TO has received research funding from AstraZeneca, Syneos Health, EP clude, Eisai, MSD, and Insight Japan; consulting fees from AstraZeneca, Eisai, Nihon Servier, Dainippon Sumitomo Pharma, Bristol-Myers, and FUJIFILM Toyama Chemical; and honoraria from AstraZeneca, Insight Japan, Eisai, Ono Pharmaceutical, Yakult Honsha, Johnson & Johnson, Daiichi Sankyo, Taiho Pharmaceutical, Chugai Pharmaceutical, Nihon Servier, Nippon Shinyaku, Eli Lilly Japan, Pfizer Japan, and Novartis Pharma. MO has received honoraria from Taiho Pharmaceutical, MSD, Nihon Servier, Pfizer, Yakult Honsha, Ono Pharmaceutical, and Bayer. SS has received research funding from AstraZeneca, Incyte Corporation, and Delta-Fly Pharma. NO has received honoraria from Taiho Pharmaceutical, li Lilly Japan, Eisai, Bayer Yakuhin, Chugai Pharma, Ono Pharmaceutical, Takeda, and Daiichi Sankyo; and is a member of advisory board for GlaxoSmithKline. NM has received research funding from Yakult Honsha, Novartis, MSD, ASLAN Pharmaceuticals, Incyte, Ono Pharmaceutical, Seagen, Taiho Pharmaceutical, and Dainippon Sumitomo Pharma; and honoraria from Yakult Honsha, AstraZeneca, Novartis, FUJIFILM Toyama Chemical, and MSD; and is a member of advisory board for AstraZeneca. SS has received research funding from Nihon Servier; and honoraria from Nihon Servier. HY has received honoraria from Bristol-Myers Squib, Ono Pharmaceutical, Taiho Pharmaceutical, Chugai Pharmaceutical, and Eli Lilly Japan. KN has received honoraria from Yakult Honsha and Daiich Sankyo. MU has received research funding from Taiho Pharmaceutical, AstraZeneca, Merck Biopharma, MSD, Astellas Pharma, Eisai, Ono Pharmaceutical, Incyte, Chugai Pharmaceutical, DFP, Daiichi Sankyo, Novartis, and Boehringer Ingelheim; and honoraria from Taiho Pharmaceutical, AstraZeneca, Yakult Honsha, MSD, Nihon Servier, Ono Pharmaceutical, Incyte, Chugai Pharmaceutical, and Boehringer Ingelheim. The other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Imaoka, H., Ikeda, M., Nomura, S. et al. Development of a nomogram to predict survival in advanced biliary tract cancer. Sci Rep 13, 21548 (2023). https://doi.org/10.1038/s41598-023-48889-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48889-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.