Abstract
Previous surveys suggests that body mass index (BMI) may be positively related to development of chronic kidney disease (CKD). However, this association might be altered by metabolic syndrome. Therefore, we aimed to evaluate the association of metabolic health status with CKD. The present cross-sectional study was carried out on 3322 representative sample of Iranian adults. Metabolic syndrome was identified based on the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) and BMI was assessed by anthropometric measurements. Estimated glomerular filtration rate (eGFR) was calculated by modification of diet in renal disease-Chronic Kidney Disease Epidemiology Collaboration (MDRD-EPI) formula. Subjects were categorized into four phenotypes: metabolically healthy normal weight (MHNW), metabolically healthy overweight and obesity (MHO), metabolically unhealthy normal weight (MUHNW), and metabolically unhealthy overweight and obesity (MUHO). Based on multivariate-adjusted models, the risk of CKD was significantly higher in MUHO compared with MHNW (OR: 1.48; p < 0.05). Although MUHNW and MUHO were associated with lower eGFR and albuminuria, the significant association was not observed in case of hematuria. Furthermore, subjects with kidney stones tended to be in MHO (OR: 1.42; p < 0.05) and MUHO phenotypes (OR: 1.64; p < 0.05), in comparison to the MHNW phenotype. The odds of kidney disorders were higher in adults with metabolic syndrome, regardless of BMI. However, this relationship might be strengthened by the concomitance of metabolic syndrome and obesity. To verify our findings, clarify the causality, and elucidate the underlying mechanisms, further research are warranted.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) has been recognized as a gradual and progressive loss of renal function, present for at least 3 months, and is considered as a leading cause of end-stage renal disease (ESRD), as well as increasing the risk of morbidity and mortality from cardiovascular diseases1,2. CKD may be defined by abnormal urinary albumin excretion (at least 30 mg per 24 h or albumin to creatinine ratio at least 30 mg/g creatinine), decreased glomerular filtration rate (GFR) less than 60 mL/min/1.73 m2, and markers of kidney damage, including hematuria or structural abnormalities by imaging persisting for more than 3 months1,3.
The prevalence of CKD varies between 8–16% in populations from different parts of the world4,5,6. The major complications of impaired kidney functions are early CVD, anemia, metabolic acidosis, and bone diseases5,7,8; whilst the leading risk factors and predictors for CKD are impaired fasting plasma glucose, hypertension, and high body mass index (BMI)6,9,10.
Accumulating evidence has suggested that overweight and obesity may be implicated in development of CKD11,12,13,14. However, the association between BMI and the prevalence of CKD could be modified by metabolic health status, including presence or absence of insulin resistance, dyslipidemia, hypertension, as well as metabolic syndrome13,15. A study by Hanks et al. reported that the risk of mortality among adults with CKD was lower in the subgroup of overweight and obese individuals with normal metabolic status, called metabolically healthy overweight and obesity (MHO), compared with metabolically healthy normal weight (MHNW)16. In addition, Panwar et al. showed that the association between BMI and ESRD could be significantly modified by metabolic profiles; thereby indicating the higher BMI reduced the risk of ESRD in subjects without metabolic syndrome17. However, several studies have suggested that overweight and obese patients are not protected from CKD risk by healthy metabolic profiles13,15. Indeed, one study identified that the risk of CKD was higher in unhealthy metabolic patients, whether overweight and obese or normal-weight, compared with subjects with healthy metabolic status18. Furthermore, other studies did not found any significant association between MHO and CKD compared with normal-weight counterparts19,20.
Some systematic review and meta-analyses have reported that metabolically unhealthy subjects have an elevated risk for CKD, regardless of BMI and in subjects with healthy metabolic status, higher BMI is accompanied by an increased risk of kidney disorders21,22,23. However, this point should be taken into account that previous meta-analyses and their included studies that investigated the association between phenotypes of metabolic health status and renal disorders mostly focused on measuring eGFR to define CKD and renal disorders, and did not consider other markers of kidney damage, including hematuria, albuminuria or structural abnormalities.
Because of the contradictory results and dearth of studies that have evaluated the association between CKD and other types of kidney disorder in subtypes of BMI and metabolic profiles, we sought to conduct a cross-sectional studies to clarify the association of CKD and other kidney disorders with subtypes of metabolic health status and BMI.
Materials and methods
Study design and participants
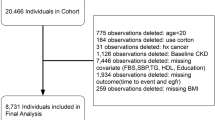
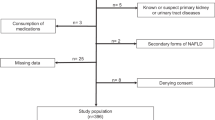
The current cross-sectional population-based study was designed to be conducted in cooperation with Isfahan University of Medical Sciences. Participants were recruited from those visiting the health care centers within Isfahan city. The eligibility criteria included adults 18 years and above, living in Isfahan, willing to participate in study, lack of fever and common cold at the time of laboratory tests, refrained engaging in heavy exercise 48 h before laboratory tests, and not fasting. Those with incomplete questionnaires or not willing to participate in the tests were excluded. Also, pregnant women or those in their menstruation period were excluded. Finally, a total of 3322 eligible adults were included in the current study. This study was carried out in accordance with the Declaration of Helsinki and with the approval of the local Ethics Committee of Isfahan University of Medical Sciences (IR.MUI.REC.1396.1.086). All studied individuals provided written informed consent prior to study commencement.
Data collection
Anthropometric measurement
Weight was measured by a mechanical scale (Zyklusmed ZYK-MS01, China), with 0.01 kg accuracy, with participants wearing minimal clothing and unshod. Also, a non-stretch tape (Seca) was applied for stature measurement, to the nearest 1 mm, and participants unshod. Body Mass Index (BMI) was computed as weight (kg)/height2 (m2).
Blood pressure and laboratory measurement
Blood pressure (BP) was evaluated within the medical examination, with participants in a sitting position, after a resting phase of at least 5 min. The subjects were asked to refrain from consuming tea and food, smoking, and exercise, for at least half an hour before BP measurement, and if the bladder is full, it should be emptied. Systolic and diastolic BPs (SBPs and DBPs) were measured using a digital sphygmomanometer (Omron BF511 (Omron Corp, Kyoto, Japan)) on the right arm. If the first BP was above 140/90 mmHg, a second measurement was carried out with a break of 15 min between measurements, and BP was defined as the mean of first and second measurements24.
Peripheral blood was obtained by means of venipuncture, after an overnight fast of at least 12 h from each subject. Albumin and creatinine were measured from a spot morning urine sample. Urinary Albumin was measured by using sulfosalicylic acid procedure (MN (analyticon) kit), whilst serum creatinine (mg/dl) and urine creatinine were determined by means of the Jaffè calorimetric method using a Hitachi-917 auto-analyzer (Pars Azmun kit). The urine ACR (UACR) was calculated by dividing urine albumin by urine creatinine and expressed as milligrams per gram. eGFR was calculated by Modification of Diet in Renal Disease (MDRD) study equation—Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation25.
Other laboratory parameters were measured by enzymatic colorimetric method using Pars-Azmun kits on Hitachi-917 auto-analyzer, including fasting blood glucose (GOD-PAP), serum levels of total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol (CHOD), and serum triglyceride (DOD-PAP). Blood Urea Nitrogen (BUN) was also measured using the Urease GLDH method on a Hitachi-917 analyzer (Adit kit).
Definitions
A BMI equivalent to or higher than 25 was considered as overweight and obese and lower than 25 considered as non-obese, based on Asian-specific BMI criteria26.
The National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) was applied for determination of metabolic syndrome. Subjects who met at least three of the following conditions were considered as metabolic syndrome: (1) elevated waist circumference (≥ 102 cm (≥ 40 inches) in men and ≥ 88 cm (≥ 35 inches) in women), (2) Elevated triglycerides (≥ 150 mg/dL (1.7 mmol/L) or on drug treatment for elevated triglycerides), (3) hypertension (≥ 130 mmHg systolic blood pressure or ≥ 85 mmHg diastolic blood pressure or on antihypertensive drug treatment in a patient with a history of hypertension), (4) hyperglycemia (≥ 100 mg/dL or on drug treatment for elevated glucose), and (5) Reduced HDL-C (< 40 mg/dL (1.03 mmol/L) in men and < 50 mg/dL (1.3 mmol/L) in women or On drug treatment for reduced HDL-C)27,28.
Based on BMI and metabolic health status, subjects were categorized into four groups: metabolically healthy overweight and obesity (MHO), metabolically healthy normal weight (MHNW), metabolically unhealthy overweight and obesity (MUHO), and metabolically unhealthy normal weight (MUHNW).
We defined CKD as decreased renal function for more than 3 months, determined by the eGFR based on the following criteria. The presence of less than 30 mg/g albumin, 30–299 mg/g creatinine, and equivalent to or greater than 300 mg/g creatinine in a random urine sample were classified as normal, moderately, and severely increased albuminuria, respectively3. Imaging techniques were performed for diagnosis of any unnamed disorder. Whole urine analysis was performed for urinary RBC count and hematuria diagnosis, and an RBC count > 2 per high power field was considered as positive29.
Other assessments
By using an electronic questionnaire (http://www.ckd-epidemiology.ir), additional predictors of interest were assessed, including; gender, age, marital status, literacy, residence, smoking, opium and substance abuse, alcohol use, medications, self-reported history of cardiovascular diseases, diabetes, and hyperlipidemia, use of herbal medicines or preparations, and analgesics. In addition, habitual physical activity was evaluated by the Baecke questionnaire30.
Statistical analysis
To evaluate the normality of quantitative variables, the Kolmogorov–Smirnov test was applied. The quantitative variables were illustrated as mean ± SD and qualitative variables as frequency (percentage). To compare quantitative variables across four categories of BMI and metabolic health status, one-way analysis of variance (ANOVA) was applied, while for categorical variables, Chi-square was used. The correlation of eGFR and UACR with anthropometric and biochemical variables across BMI and metabolic phenotypes was evaluated by Pearson correlation coefficients. To determine the association between phenotypes of BMI and metabolic status with CKD and related disorders, multivariable logistic regression was applied. The odds ratios (ORs) and their 95% confidence intervals (95% CIs) were calculated in crude and adjusted models. The MHNW phenotype was considered as the reference category in all analyses. SPSS software version 16 (IBM, Chicago, IL) was used to perform analysis and a p-value < 0.05 (two-sided) was, a priori, considered statistically significant.
Results
Demographic and biochemical characteristics of the study participants across BMI and metabolic status phenotypes are demonstrated in Table 1. The percent of females in MUHNW was higher than other groups. Individuals with unhealthy metabolic status, whether normal weight or overweight and obese, were older than healthy metabolic subjects. Body weight, BMI, and waist circumference were lower in MHNW in comparison to other groups. The lipid profiles, blood pressure, and FBS tended to be abnormal in unhealthy metabolic subjects compared to metabolically healthy individuals, whether normal or abnormal in weight. In ACR, the highest levels were associated with the MUHO phenotype. Furthermore, eGFR in MHNW and MHO was greater in comparison to MUHNW and MUHO, respectively. The prevalence of albuminuria, CKD, and kidney stones were higher in metabolically unhealthy phenotypes compared to metabolically healthy counterparts. However, no significant differences in prevalence of hematuria were observed among phenotypes of BMI and metabolic status.
The correlation coefficients of eGFR and ACR with anthropometric and biochemical variables across BMI and metabolic phenotypes, and in the total study population, are shown in Table 2. In case of eGFR, a negative correlations were seen between eGFR and age, BMI, WC, ACR, blood pressure, FBS, and lipid profiles. In MUHNW group, the significant association was limited to age and FBS. The results for the ACR demonstrated positive significant correlations between ACR and age, BMI, WC, blood pressure, eGFR, and FBS, as well as a negative correlation with HDL-C.
In Table 3, Multivariate adjusted odds ratio’s (OR) and 95% confidence interval’s (CI) for the association of BMI and metabolic health status with CKD and related disorders are shown. In CKD, the odds of having CKD were higher in MUHNW (OR: 1.78; 95% CI: 1.15, 2.75) and MUHO (OR: 2.02; 95% CI: 1.60, 2.55) in the crude model. After adjustment for potential confounders, the association remained significant only in the MUHO phenotype (OR: 1.48; 95% CI: 1.15, 1.89). No significant association was observed between hematuria and phenotypes of BMI and metabolic syndrome both in crude and adjusted models. In both crude and adjusted models, MUHNW and MUHO were associated with lower eGFR and albuminuria; however, no significant association was shown between MHO phenotype and eGFR and albuminuria. In addition, subjects with kidney stones tended to be in the MHO (OR: 1.45; 95% CI: 1.15, 1.84) and MUHO groups (OR: 1.81; 95% CI: 1.41, 2.34). Adjustment for confounding variables attenuated the association of kidney stones with MHO (OR: 1.42; 95% CI: 1.12, 1.81) and MUHO phenotypes (OR: 1.64; 95% CI: 1.26, 2.15).
Discussion
In the present cross-sectional study, a significant positive association was found between prevalence of CKD and MUHO phenotypes. In addition, decreased eGFR and albuminuria were associated with MUHNW and MUHO. The present study provides further evidence that MHO and MUHO individuals are at higher odds for developing kidney stones. However, no significant association was found between hematuria and phenotypes of combined BMI and metabolic syndrome.
Although the association of overweight and obesity with CKD has been reported in previous studies31,32, this association could be notably affected by metabolic syndrome33. Indeed, previous studies have suggested that the association of obesity with CKD might be attenuated by components of metabolic syndrome34,35. The Framingham Offspring Study, that was conducted on subjects without history of stage III CKD at baseline, suggested that the significant association between obesity and higher risk of KD could be rendered statistically insignificant after adjustment for CVD risk factors14. On the other hand, individuals with both high BMI and metabolic syndrome purportedly have a synergist effect on the development and progression of CKD, which is in line with our study36.
The inconsistent results in the literature for the association between MHO and MUHNW, as intermediate subgroups, have been reported by previous studies. In contrast to the current study, a prospective cohort study on young and middle-aged men and women reported that MHO phenotypes increased risk of CKD, and a lack of metabolic abnormities could not protect subjects from incidence of CKD15. In addition, two cohort studies demonstrated that both MHO and MUHNW were associated with elevated risk of CKD incidence13,37. Concordant with our findings, the results of several studies has illustrated that metabolic syndrome, regardless of BMI, is related to an elevated CKD risk18,38. In addition, the results of a cross-sectional study showed that MUHO, but not MHO, elevated the risk of CKD in middle-aged Chinese population39. To interpret these results the results of previous studies and the present analysis be considered; indeed, although the total body fat and BMI was the same in both MHO and MUHO, the values of waist circumference as visceral fat measurement as well as waist to hip ration was lower in MHO phenotypes compared with MUHO counterparts40,41. In addition, previous studies have showed that insulin resistance appeared to be lower in MHO in comparison to MUHNW42.
Numerous, multifaceted, biological mechanisms are involved in the association of obesity and metabolic syndrome with CKD and kidney damage. Previous studies have suggested that in individuals with obesity, abnormal lipid profiles43, alteration in inflammatory status, as well as hormonal factors, might be implicated in initiation and development of CKD. Empirical evidence from previous studies supports that inflammatory status, including high levels of IL-6, TNF-α, and CRP, may be associated with lower eGFR and initiation of kidney injury44,45. Hormonal factors, such as insulin resistance and increased levels of insulin46, higher levels of leptin47, as well as decreased levels of adiponectin, might play important roles in proteinuria and incidence of CKD47,48.
In nephrolithiasis, a cohort study reported that obesity, as an independent risk factor, was associated with renal stone incidence, regardless of metabolic health status49. The up-regulation of inflammatory cytokines, such as TNF-α and IL-650, oxidative stress51,52, as well as alteration in chemistry of urine53, might be considered as the underlying mechanism for the association between obesity and nephrolithiasis.
Several limitations should be taken into account in interpreting the results of this study. We are unable to draw a causal relationship between indicators of CKD and associated factors due to the cross-sectional nature of the study. Another limitation of this study is the difficultly in generalizing the results, since it was conducted in Isfahan, Iran. Despite these limitations, the present study included a large representative sample of the adult population in Iran using multi-stage cluster random sampling approach and estimated the prevalence of CKD based on its stages. We have also provided novel, and supportive, evidence to advocate the identification of metabolic phenotypes.
Conclusion
A significant positive association was found between prevalence of CKD and MUHO phenotype. In addition, decreased eGFR and albuminuria were associated with metabolic syndrome, regardless of BMI. The present study provides further evidence that MHO and MUHO individuals may be at higher odds for developing kidney stones. However, no significant association was found between hematuria and phenotypes of combined BMI and metabolic syndrome. To verify our findings, clarify the causality, and elucidate the underlying mechanisms, further research must be conducted.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 39(2 Suppl 1), S1–S266 (2002).
Eckardt, K.-U. et al. Evolving importance of kidney disease: From subspecialty to global health burden. Lancet 382(9887), 158–169 (2013).
Eknoyan, G. et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 3(1), 5–14 (2013).
Bello, A. K. et al. Global kidney health atlas (GKHA): Design and methods. Kidney Int. Suppl. 7(2), 145–153 (2017).
Jha, V. et al. Chronic kidney disease: Global dimension and perspectives. Lancet 382(9888), 260–272 (2013).
Bikbov, B. et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 395(10225), 709–733 (2020).
Weiner, D. E. Causes and consequences of chronic kidney disease: Implications for managed health care. J. Manag. Care Pharm. 13(3 Suppl A), 1–9 (2007).
Romagnani, P. et al. Chronic kidney disease. Nat. Rev. Dis. Primers 3(1), 1–24 (2017).
Shen, Y. et al. Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: A systematic review and meta-analysis. Endocrine 55(1), 66–76 (2017).
Luyckx, V. A. et al. Reducing major risk factors for chronic kidney disease. Kidney Int. Suppl. 7(2), 71–87 (2017).
Jiang, L. et al. Metabolic syndrome and chronic kidney disease in a rural Chinese population. Clin. Chim. Acta 412(21–22), 1983–1988 (2011).
Huang, X. et al. Serum fatty acid patterns, insulin sensitivity and the metabolic syndrome in individuals with chronic kidney disease. J. Intern. Med. 275(1), 71–83 (2014).
Jung, C. H. et al. The risk of chronic kidney disease in a metabolically healthy obese population. Kidney Int. 88(4), 843–850 (2015).
Foster, M. C. et al. Overweight, obesity, and the development of stage 3 CKD: The Framingham Heart Study. Am. J. Kidney Dis. 52(1), 39–48 (2008).
Chang, Y. et al. Metabolically healthy obesity and development of chronic kidney disease: A cohort study. Ann. Intern. Med. 164(5), 305–312 (2016).
Hanks, L. J. et al. Metabolic subtypes and risk of mortality in normal weight, overweight, and obese individuals with CKD. Clin. J. Am. Soc. Nephrol. 8(12), 2064–2071 (2013).
Panwar, B. et al. Obesity, metabolic health, and the risk of end-stage renal disease. Kidney Int. 87(6), 1216–1222 (2015).
Chen, H.-Y. et al. Metabolic abnormalities, but not obesity per se, associated with chronic kidney disease in a Taiwanese population. Nutr. Metab. Cardiovasc. Dis. 30(3), 418–425. https://doi.org/10.1016/j.numecd.2019.09.029 (2020).
Hashimoto, Y. et al. Metabolically healthy obesity and risk of incident CKD. Clin. J. Am. Soc. Nephrol. 10(4), 578–583 (2015).
Mottaghi, A., Mirmiran, P., Delshad, H. & Azizi, F. Effect of different obesity phenotypes on incidence of chronic kidney disease in Tehranian adults. J. Am. Coll. Nutr. 35(7), 587–596 (2016).
Alizadeh, S. et al. Metabolic phenotypes of obese, overweight, and normal weight individuals and risk of chronic kidney disease: A systematic review and meta-analysis. Arch. Endocrinol. Metab. 63(4), 427–437. https://doi.org/10.20945/2359-3997000000149 (2019).
Zhang, J., Jiang, H. & Chen, J. Combined effect of body mass index and metabolic status on the risk of prevalent and incident chronic kidney disease: A systematic review and meta-analysis. Oncotarget 8(22), 35619–35629. https://doi.org/10.18632/oncotarget.10915 (2017).
Kanbay, M. et al. The risk for chronic kidney disease in metabolically healthy obese patients: A systematic review and meta-analysis. Eur. J. Clin. Investig. 53(1), e13878. https://doi.org/10.1111/eci.13878 (2023).
Verdecchia, P., Reboldi, G. & Angeli, F. The 2020 International Society of Hypertension global hypertension practice guidelines-key messages and clinical considerations. Eur. J. Intern. Med. 82, 1–6 (2020).
Moodley, N., Hariparshad, S., Peer, F. & Gounden, V. Evaluation of the CKD-EPI creatinine based glomerular filtration rate estimating equation in Black African and Indian adults in KwaZulu-Natal, South Africa. Clin. Biochem. 59, 43–49 (2018).
Consultation, W. E. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet (London, England). 363(9403), 157–163 (2004).
Grundy, S. M. et al. Clinical management of metabolic syndrome: Report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation 109(4), 551–556 (2004).
Huang, P. L. A comprehensive definition for metabolic syndrome. Dis. Model Mech. 2(5–6), 231–237. https://doi.org/10.1242/dmm.001180 (2009).
Jubber, I. et al. Non-visible haematuria for the detection of bladder, upper tract, and kidney cancer: An updated systematic review and meta-analysis. Eur. Urol. 77(5), 583–598 (2020).
Florindo, A. A. & Latorre, M. R. D. O. Validation and reliability of the Baecke questionnaire for the evaluation of habitual physical activity in adult men. Rev. Bras. Med. Esporte 9, 129–135 (2003).
Wang, Y., Chen, X., Song, Y., Caballero, B. & Cheskin, L. Association between obesity and kidney disease: A systematic review and meta-analysis. Kidney Int. 73(1), 19–33 (2008).
Webster, A. C., Nagler, E. V., Morton, R. L. & Masson, P. Chronic kidney disease. Lancet 389(10075), 1238–1252 (2017).
Wahba, I. M. & Mak, R. H. Obesity and obesity-initiated metabolic syndrome: Mechanistic links to chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2(3), 550–562 (2007).
He, Y. et al. Association between body mass index and mildly decreased estimated glomerular filtration rate in Chinese adults with early chronic kidney disease. J. Renal Nutr. 26(6), 367–372. https://doi.org/10.1053/j.jrn.2016.04.006 (2016).
Gelber, R. P. et al. Association between body mass index and CKD in apparently healthy men. Am. J. Kidney Dis. 46(5), 871–880. https://doi.org/10.1053/j.ajkd.2005.08.015 (2005).
Zhang, J., Jiang, H. & Chen, J. Combined effect of body mass index and metabolic status on the risk of prevalent and incident chronic kidney disease: A systematic review and meta-analysis. Oncotarget 8(22), 35619 (2017).
Cao, X., Zhou, J., Yuan, H., Wu, L. & Chen, Z. Chronic kidney disease among overweight and obesity with and without metabolic syndrome in an urban Chinese cohort. BMC Nephrol. 16(1), 1–9 (2015).
Moeinzadeh, Fh., Rouhani, M. H., Mortazavi, M. & Sattari, M. Prediction of chronic kidney disease in Isfahan with extracting association rules using data mining techniques. Tehran Univ. Med. J. 79(6), 459–467 (2021).
Chen, S. et al. Association between metabolically unhealthy overweight/obesity and chronic kidney disease: The role of inflammation. Diabetes Metab. 40(6), 423–430 (2014).
Brochu, M. et al. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women?. J. Clin. Endocrinol. Metab. 86(3), 1020–1025 (2001).
Koster, A. et al. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity 18(12), 2354–2361 (2010).
Stefan, N. et al. Identification and characterization of metabolically benign obesity in humans. Arch. Intern. Med. 168(15), 1609–1616. https://doi.org/10.1001/archinte.168.15.1609 (2008).
Ruan, X. Z., Varghese, Z. & Moorhead, J. F. An update on the lipid nephrotoxicity hypothesis. Nat. Rev. Nephrol. 5(12), 713–721 (2009).
Wu, Y. et al. Obesity-related glomerulopathy: Insights from gene expression profiles of the glomeruli derived from renal biopsy samples. Endocrinology 147(1), 44–50. https://doi.org/10.1210/en.2005-0641 (2006).
Phillips, C. M. & Perry, I. J. Does inflammation determine metabolic health status in obese and nonobese adults?. J. Clin. Endocrinol. Metab. 98(10), E1610–E1619 (2013).
Sarafidis, P. A. & Ruilope, L. M. Insulin resistance, hyperinsulinemia, and renal injury: Mechanisms and implications. Am. J. Nephrol. 26(3), 232–244 (2006).
Goldstein, B. J. & Scalia, R. Adiponectin: A novel adipokine linking adipocytes and vascular function. J. Clin. Endocrinol. Metab. 89(6), 2563–2568 (2004).
Ahl, S. et al. Adiponectin levels differentiate metabolically healthy vs unhealthy among obese and nonobese white individuals. J. Clin. Endocrinol. Metab. 100(11), 4172–4180 (2015).
Kim, S. et al. Metabolically healthy and unhealthy obesity phenotypes and risk of renal stone: A cohort study. Int. J. Obes. 43(4), 852–861 (2019).
Ronti, T., Lupattelli, G. & Mannarino, E. The endocrine function of adipose tissue: An update. Clin. Endocrinol. 64(4), 355–365 (2006).
Khan, S. R. Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl. Androl. Urol. 3(3), 256 (2014).
Aggarwal, K. P., Narula, S., Kakkar, M. & Tandon, C. Nephrolithiasis: Molecular mechanism of renal stone formation and the critical role played by modulators. BioMed Res. Int. 2013, 292953 (2013).
Wollin, D. A., Skolarikos, A. & Preminger, G. M. Obesity and metabolic stone disease. Curr. Opin. Urol. 27(5), 422–427 (2017).
Acknowledgements
We would like to thank the Isfahan’s Hazrat Abolfazl Health and Medical Charity association members for their support in this project. Also, the authors would like to thank all the study participants.
Funding
This study was financially supported by Isfahan University of Medical Sciences, Isfahan, Iran (grant number 196086). The funding sources were not involved in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
F.M., M.H.R., S.S., S.V., M.M., C.C., and F.S. contributed in conception, design, data collection, statistical analyses, data interpretation and manuscript drafting. All authors approved the final manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moeinzadeh, F., Rouhani, M.H., Seirafian, S. et al. Metabolic health status and renal disorders: a cross-sectional study. Sci Rep 13, 20794 (2023). https://doi.org/10.1038/s41598-023-48333-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48333-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.