Abstract
The aims of this study on human papilloma virus (HPV) 6/11/16/18 infection among females in Chengdu were to provide more targeted strategies for the prevention and treatment of cervical cancer and genital warts. In this study, the infection status of 20 genotypes was analysed by gene chip technology. The prevalence rates of HPV-6, -11, -16, and -18 infection among 180,276 cases were 0.94%, 0.57%, 3.22%, and 1.28%, respectively. The prevalence of HPV 6/11/16/18 showed a bimodal U-shaped curve with age; the first and second peak occurred among females < 20 and ≥ 60 years old, respectively. As the multiplicity of infections involving HPV6/11/16/18 increases, the infection rate decreases. The ratios of HPV16 single infection showed a yearly increase. The top five genotypes with HPV-16, -18, -6, and -11 in coinfection were HPV52/58/53/51/33, HPV 52/16/53/58/51, HPV52/16/58/51/53 and HPV16/52/58/59/18, respectively, HPV16/18/6/11 were mainly coinfected with HR-HPV. In sum, among the five vaccines available, nonavalent vaccine is more suitable for Chengdu females. For young females prioritizing vaccination is essential in the current context, while HPV screening remains an effective approach for older females. Additionally, in patients with genital warts, it is necessary to assess the presence of high-risk HPV infection and manage it appropriately in patients with genital warts.
Similar content being viewed by others
Introduction
Human papillomaviruses (HPVs) are non-enveloped, double-stranded circular DNA viruses of 8 kb in size that causes epithelial hyperplasia of the skin and is primarily transmitted through sexual contact or close contact and causes cervical cancer and other diseases (genital cancer, anal cancer, oropharyngeal cancers, precancers and genital warts)1. HPVs belong to the Papovaviridae family, and humans are the sole host. HPVs are classified into five diverse genera based on tissue tropism and subsequent host pathogenesis, which are Alphapapillomaviruses (alpha), Betapapillomaviruses (beta), Gammapapillomaviruses (Gamma), Mupapillomaviruses (mu) and Nupapillomaviruses (nu)2. HPV genomes are further classified into intratypic variants according to the diversity of their genome sequence3,4. The HPVs that infect the mucosal epithelium are classified into the genus Alphapapillomaviruses (alpha-HPVs)5. To date, 231 types of HPV have been identified, on the basis of the genomic sequence of L16, with more than 40 types infecting the genital area1. Based on their carcinogenic potential, mucosal alpha-PVs are designated as high-risk and low-risk (HR-HPV and LR-HPV). Currently, at least 14 HPV genotypes have been described as high-risk (HPV16, 18, 31, 33, 35, 39, 45,51, 52, 56, 58, 59, 66, and 68)7, while LR-HPV includes HPV6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81, and CP61088. Based on HPV activities, HPV genome is classified into three regions: the long control region (LCR) or the non-coding upstream regulatory region (URR), the late (L) region, and the early (E) region4,5among them, three oncogenes, E5, E6, and E7, modulate the transformation process, two regulatory proteins, E1 and E2, modulate transcription and replication, and two structural proteins, L1 and L2, compose the viral icosahedral capsid. Viral capsids have evolved to fulfil numerous roles that are critical to the establishment of viral infection9. HPVs infect host basal layer of the epithelium via micro-wounds10, and then in order to initiate viral infection, the L1 capsid protein binds to herparin sulfate proteoglycans (HSPGs) or interact with laminin-332 on the extracellular matrix Viral and capsids have evolved to fulfil numerous roles that are critical to the establishment of viral infection11. 60% of HPV infection in one year will be cleared spontaneously, and the great majority of infected women (more than 90%) resolve the infection spontaneously in two years12,13, while only a very small number of cases progress into cervical intraepithelial neoplasia (CIN), precancerous lesions and cancer. One of the reasons HPV causes cancer is that they have established molecular mechanisms to prevent cell cycle arrest and apoptosis14. Viral E6 and E7 oncoproteins inactivate the tumor suppressor proteins p53 and pRB, respectively, and are involved in uncontrolled cell proliferation and deliver their genetic material into the nucleus of the target cell15,16, Another cause of cancer is through the integration of HPV DNA into the host genome. When the E6 and E7 genes of the viral DNA remain intact and integrated into the host chromosome17,18, which causes additional chromosomal damages and genome destabilization in the infected cells, whereas the E6 and E7 oncogenes are extensively expressed. Approximately 98.7% of cervical cancers are attributable to HPV19. HR-HPV genotypes 16 and 18 are responsible for approximately 71% of cervical cancer cases20.
Cervical cancer is the second most common cancer in women worldwide and the third most common cause of cancer-related death worldwide. In 2020, an estimated 604127 new cases of cervical cancer and 341831 deaths worldwide were attributed to HPV infection21. Nearly 70% of the global burden of cervical cancer occurs in less developed countries. As the most populous developing country in the world, China bears a serious burden of cervical cancer. According to the latest statistics from the ICO/IARC HPV Information Centre in 2021, China experiences approximately 109,700 new cases and 59,100 deaths related to cervical cancer annually. The newly diagnosed cervical cancer cases in China constitute approximately 18.2% of the global incidence, while the mortality figures account for approximately 17.2% of global deaths attributed to cervical cancer22.
LR-HPV primarily causes genital warts and recurrent respiratory papillomatosis1, of which anogenital warts are more common. Ninety percent of genital warts are caused by noncarcinogenic LR-HPV genotypes 6 or/and 11, with HPV6 being the most common type of the disease, but HPV11 accounting for approximately a quarter of cases. In addition to anogenital warts, HPV types 6 and 11 are also associated with conjunctival, nasal, oral, and laryngeal warts1. Globally, genital warts are estimated to affect approximately 1% of the population23. The global incidence of genital warts in females (including both new and recurrent cases) is between 76 and 191 per 100,000 population (with a median of 120.5 per 100,000)24. Although genital warts are benign, they cause discomfort and affect psychological well-being. Even when addressed with physical therapy, they persist due to HPV infection and may recur upon external stimulation, which poses considerable challenges for patients and treatment.
The most effective approach for controlling cervical cancer and genital warts is prioritizing HPV vaccination. Currently, the US Food and Drug Administration (FDA) has approved three prophylactic HPV vaccines, and they are respectively Gardasil®4, a quadrivalent vaccine (6, 11, 16, 18) available in 2006, Cervarix™, a bivalent vaccine (16,18) available in 2007, and Gardasil®9, a nonavalent vaccine (6, 11, 16, 18, 31, 33, 45, 52, and 58) available in 201414. As of now, the China Food and Drug Administration (CFDA) has approved a total of five vaccines, including the three mentioned above (imported bivalent, quadrivalent, and nonavalent vaccines), which were respectively marketed in mainland China in 2016, 2017, and 2018. Subsequently, in 2019 and 2022, the CFDA granted approval for two locally produced bivalent vaccines to be marketed in mainland China, namely Cecolin produced by Xiamen Wantai Canghai Biotechnology Co., Ltd. and "Wozehui" by Yunnan Walvax Biotechnology Co., Ltd. HPV vaccines have shown 98% to 100.0% protective efficacy in clinical trials against HPV type-related diseases, offering effective protection for both vaccinated and unvaccinated females25,26,27. Due to various factors, the HPV vaccine was introduced to Chinese females a decade after their global counterparts, and owing to economic constraints, the vaccine is only promoted as a national secondary vaccine. Nationally, financial constraints have resulted in less than 0.05 percent coverage of the HPV vaccine among eligible women aged 9–45 years, with women aged 9–14 years accounting for less than 5 percent of the vaccinated population28. Alongside vaccination, HPV DNA and cytologicals creening is crucial for alleviating the burden of cervical cancer and other HPV-related diseases. The World Health Organization's "Global Strategy to Accelerate the Elimination of Cervical Cancer" aims to have 70% of women undergo regular HPV screening by 2030, with HPV DNA testing as the preferred method for cervical cancer screening29,30. China conducts limited large-scale screening for female HPV DNA, with most of it taking place in hospitals. Given the high incidence of cervical cancer and related diseases in Chinese women, coupled with low HPV vaccination rates and limited HPV-DNA testing, obtaining prevalence data on HPV infection in Chinese women is vital and timely. Although infection with genotypes other than HPV6/11/16/18 can also cause genital warts and cervical cancer in women, HPV6/11/16/18 infection is the primary cause, so in-depth analysis of these four genotypes is absolutely necessary. Currently, there is a lack of large-scale research data on HPV6/11/16/18 in Chengdu or even the entire nation. The data for this study were derived from a national tertiary-grade hospital in southwest China, comprising a central institution and three branch hospitals, covering virtually the entire Chengdu urban and suburban area. This study has provided a comprehensive analysis of the situation of HPV6/11/16/18 in different age groups, infection modes, and years.
Materials and methods
Subjects
Patients came from Chengdu Women's and Children's Central Hospital (CWCCH) for HPV screening motivated by diverse reasons, including patient requests, diagnostic needs prescribed by doctors, and opportunistic screening by doctors. Specific reasons included health examinations, abnormal vaginal bleeding, lower genital tract inflammation, genital warts, infertility, unknown lower abdominal pain, gynaecological tumours, and urethritis. Inclusion criteria consisted of a history of sexual activity, absence from menstruation and pregnancy, while exclusion criteria included women with no sex, menstruating and pregnant women, and women who had undergone uterine surgery. For cases that were reviewed, we only considered the results of the first screening. A total of 6254 cases were excluded from the study. Finally, a total of 180,276 participants were included in this retrospective study conducted at CWCCH between January 2015 and December 2021. The mean age was 38.8 ± 9.1 years (ranging from 12 to 91 years). categorized into age groups of < 20, 20–29, 30–39, 40–49, 50–59, and ≥ 60. This study was approved by the Medical Ethics Committee of Chengdu Women’s and Children’s Central Hospital, and all methods were performed in accordance with the relevant guidelines and regulations.
Ethics statement
This research received approval and a waiver for participant informed consent from the Medical Ethics Committee of Chengdu Women's and Children's Central Hospital due to the retrospective nature of the study, where patient identities were deliberately anonymized, rendering individual informed consent unnecessary.
Specimen collection
A gynaecologist wipes cervical secretions with a cotton swab before sampling, places the cervical brush head on the cervix and rotates the brush head clockwise 5 times to obtain a sufficient amount of cervical epithelial cells. Then, the cervical brush head is placed into the sample tube marked with the patient's name, the cap is tightened, and the sample is quickly sent for examination.
DNA extraction and HPV genotyping
DNA extraction and HPV genotyping were performed using an HPV genotyping test kit (Shenzhen GL Bio-Tech Co., Ltd.). The kit detected 20 types of HPV: HPV6, 11, 42, 43, 16, 18, 31,33, 35, 39, 45, 51, 52, 53.56, 58, 59, 66, 68, and 73. The process involved three steps: HPV-DNA extraction, PCR amplification and HPV-DNA hybridization. The Haema9600 (Zhuhai XZ Bio-Tech Co., Ltd.) was used for gene amplification. The amplification parameters were set as follows: ①50 °C for 2 min; ②95 °C for 10 min; ③40 cycles were performed at 95 °C for 30 s, 52 °C for 45 s and 65 °C for 30 s; and ④ 65 °C for 5 min. GL-HB-9600 (Shenzhen GL Bio-Tech Co., Ltd.) was used for DNA hybridization. After color rendering, positive detection results were indicated by clear blue dots. To ensure the reliability of each HPV test result, six probe arrays (including 3 positive anchor points, 1 negative anchor point, and 2 internal control points) were used as the internal control (IC), and HPV16 was added as a positive control. The testing doctor strictly follows the HPV interpretation rules to issue the patient's HPV test results. This study completely ruled out the impact of reproductive pathogens (BV, TV, VVC, NG, CT, etc.) other than HPV on the HPV detection results. Specific HPV extraction solution was added during the DNA extraction stage to ensure specificity. During the DNA amplification stage, HPV primers and probes were added to the reaction mixture to ensure the specific amplification of HPV fragments. The probes on the gene chip were specifically designed for HPV genotypes, ensuring no cross-reactivity between different genotypes during the DNA hybridization process.
Statistical analysis
Microsoft's Excel 2021 was used to process and analyse the data, the chi-square test was used to compare the sample rate between the groups, SPSS 26 (SPSS Inc., Chicago, IL, USA) was used to calculate chi-square data and P values, and two-sided P values of less than 0.05 were considered statistically significant.
Results
Overall prevalence of HPV infection
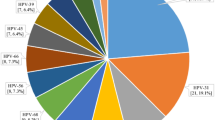
The total infection rate of in 180,276 cases was 21.97% (39,615 positive cases). the 16 high-risk types were HPV-52, -16, -58, -53, -51, -68, -56, -18, -59, -33, -39, -66, -31, -35, -45 and -73 in order. The prevalence of the 20 genotypes is shown in Fig. 1, in which HPV16 and HPV18 ranked second and eighth, respectively, with infection rates of 3.22% (5801 positive cases) and 1.28% (2310 positive cases). The four low risk infection rates were HPV-42, -43, -6 and -11 in order. HPV6 and HPV11 ranked third and fourth, with infection rates of 0.94% (1699 positive cases) and 0.57% (1019 positive cases), respectively (Fig. 1, Table 1).
Age-specific prevalence of HPV6/11/16/18 infection
The 30–39 years group made up the largest proportion of screened participants, accounting for 38.54% of the total population, yet the < 20 and ≥ 60 age groups made up fewer proportions, accounting for 0.44% and 14.4%, respectively (Table 1).
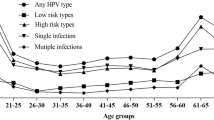
The infection curves of HPV-6, -11, -16 and -18 showed a bimodal U-shaped distribution with age, as did the coinfective genotypes of -16 and -18 (Tables 1, 2, 3 and Figs. 2, 3). The first peak occurred in females < 20 years old (at this age group, the prevalence of HPV-6, -11, -16 and -18 were 8.28% and 4.52%, 7.53% and 4.89%, respectively), sharply declining after the first peak and maintaining a plateau during middle age (40–49 years), followed by a slight increase with age, reaching a small second peak in the ≥ 60 age group (at this age group, the prevalence of HPV-6, -11, -16 and -18 were 1.23% and 1.27%, 7.35% and 1.96%, respectively) (Table 1). The prevalence of HPV6/11/18 infections, HPV-6 and -11 coinfection, HPV-16 and -18 coinfection, and HPV-6, -11, -16 and -18 coinfection with age was significantly different (P < 0.05) (Table 1).
Type-specific prevalence of HPV6/11/16/18 infection
The single infection ratios of HPV-6, -11, -16 and -18 were 48.56%, 57.41%, 60.71% and 56.23%, respectively. The double infection ratios were 28.37%, 27.48%, 25.44% and 26.15%, respectively. The triple infection ratios were 14.24%, 9.13%, 9.03% and 9.96%, respectively (Table 1). Single and multiple infections are almost equal, and double infections account for approximately half of single infections. With the increase in the patterns of multiple infections, the prevalence of multiple infections decreased gradually. This trend was observed almost every year and in almost every age group from 2015 to 2021 (Tables 1, 2 and Figs. 2, 4, 5). From 2015 to 2021, the ratios of single HPV16 infections showed a yearly increasing trend (Table 2 and Figs. 2, 4).
In the four genotypes of HPV6/11/16/18, whether HR-HPV or LR-HPV, they all present a variety of coinfection forms with other 19 genotypes, and are mainly coinfected with HR-HPV. The top ten genotypes of multiple infections with HPV-6, -11, -16, and -18 were HPV-52, -16, -58, -53, -51, -18, -59, -56, -6, and -43 (Table 4, Fig. 5). The top five genotypes with HPV-6 multiple infections were HPV-52, -16, -58, -51, and -53 in order. The top five genotypes with HPV11 multiple infection were HPV-16, -52, -58, -59, and -18 in order. The top five genotypes with HPV16 multiple infection were HPV-52, -58, -53, -51, and -33 in order. The top five genotypes with HPV18 multiple infections were HPV-52, -16, -53, -58, and -51 in order (Table 4, Fig. 5).
Year-specific prevalence of HPV6/11/16/18 infection
From 2015 to 2021, HPV16 consistently exhibited the highest infection rate (Table 1). With the exception of the five age distribution curves of HPV-6, -11, and -18 in 2015 and HPV-18 in 2018 and 2021 (the second peak was not pronounced), the age distribution curves of HPV-6, -11, -16, and -18 from 2015 to 2021 displayed a U-shaped . From 2015 to 2020, the annual infection rates of HPV6 significantly differed with age (P < 0.05). The infection rates of HPV11 were significantly different with age in 2015, 2016, 2018, and 2019 (P < 0.05). The age-related infection rates of HPV16 were significantly different in 2015, 2017, and 2021 (P < 0.05), and the age-related infection rates of HPV18 showed significant differences in 2015 and 2016 (P < 0.05) (Table 3, Fig. 3).
Discussion
To date, vaccination and HPV screening for women constitute two major strategies in the prevention of cervical cancer and genital warts. The focus of HPV vaccine research lies within the L region31 and the currently available HPV vaccines are designed using a combination of multiple subtypes of L1 virus-like particles (L1-VLPs), and the L1 spontaneously formed VLPs are highly immunogenic and produce high titers of neutralizing antibodies to prevent HPV infection14,32. VLPs mimic the organization and conformation of authentic native viruses but lack the viral genome, inducing an immune response without causing disease33. In contrast, attenuated or inactivated viruses may pose risks of incomplete attenuation or reversion to virulence, raising safety concerns. As a result, current HPV vaccines are safer than attenuated or inactivated viruses. A decade of HPV vaccination practice has demonstrated that HPV vaccine can reduce HPV infection rates, genital warts, low-grade and high-grade cervical intraepithelial neoplasia (CIN), and CIN2+27, moreover it has a strong group effect or indirect protection against unvaccinated females26. In terms of HPV screening, HPV DNA has a high sensitivity and negative predictive value (fewer high-grade lesions in the subsequent third year of screening) and is an effective screening method34.
In this study, the prevalence rates of HPV-16 and -18 infections in Chengdu were 3.22% and 1.28%, respectively. These rates were significantly higher than those of the less developed regions of Guangxi35 (2.7% and 1.11%, respectively) and the more developed regions of Shanghai36 (2.34% and 1.0%, respectively) but lower than those of the economically comparable Tianjin37 (5.36% and 1.57%, respectively). The prevalence rates of HPV-6 and -11 infections in Chengdu (0.94% and 0.57%, respectively) were lower than those in Shanghai36 (1.29% and 0.81%, respectively) and Guangxi35 (1.31% and 0.82%, respectively) but higher than those in Tianjin37 (0.30% and 0.51%, respectively). Disparities in HPV infection rates across different regions may be influenced by factors such as local economic conditions, awareness of prevention, lifestyle habits, and HPV detection methods. However, regardless, given the crucial roles of HPV 16/18 and HPV 6/11 in cervical cancer and genital warts, the aforementioned findings underscore the imperative need to enhance HPV vaccine coverage in the Chengdu region.
In terms of infection patterns, the ratios of HPV-6, -11, -16 and -18 single infection were 48.56%, 57.41%, 60.71% and 56.23%, respectively, which indicates that single infection is the primary mode of HPV infection, followed by double infection. As the multiplicity of coinfection increased, the prevalence of multiple infections gradually decreased. The occurrence of multiple infections is associated with having multiple sexual partners. The potential impact of multiple infections on cervical cancer risk remains debatable38,39. In this study, the top five genotypes in 180,276 cases were HPV-52,-16,-58,-53,-51, and the top five genotypes with HPV16 and HPV18 coinfection were HPV52, -58, -53, -51, -33 and HPV52, -16, -53, -58, -51, respectively, while the top five genotypes with HPV6 and HPV11 coinfection were HPV-52, -16, -58, -51, -53 and HPV-16, -52, -58, -59, -18, respectively. Thus it can be seen that HPV-52 and -58 are the top two genotypes among females in Chengdu. However, currently, among the five available HPV vaccines in the China mainland, only the nonavalent vaccine covers these two genotypes, so the nonavalent vaccine is more suitable for Chengdu females. These findings of this large-scale epidemiological investigation based on HPV genotyping screening hold significance for the prevention and control of diseases such as cervical cancer and genital warts in Chengdu. Additionally, they provide valuable insights for the future design of HPV vaccines.
HPV16, as a highly carcinogenic genotype, was found to have the highest infection rates in HPV6/11/16/18. The single infection positivity rate of HPV16 showed a continuous increase yearly, and studies40 have indicated that the proportion of high-grade cervical lesions among HPV16 single infections is the highest. In response to the increasing trend of HPV16 single infections, a stronger push for vaccines covering HPV 16 is warranted. While the infection rate of HPV18 is lower than that of HPV16, its coinfections with HPV52, -58, -53, -51, and -33 genotypes in multiple infections probably enhance its carcinogenic potential.
In this study, the coinfection of HPV6 and HPV11 was very low (42 cases), the single infection of HPV6 and HPV11 accounted for the majority (48.56% and 57.41%, respectively), and most of them had multiple coinfections with HR-HPV. In cases of multiple infections involving HPV 6, the most frequently cooccurring genotype is HPV-52, -16, -58, -51, and -53, whereas for HPV 11, the most commonly cooccurring genotype is HPV-16, -52, 58, -59, and -18. It can be seen that low-risk HPV6 and HPV11 are the most common co-infected LR-HPV with HR-HPV. Studies41 have pointed out that the most common HPV genotypes in genital warts in China are HPV-6, HPV-11 and HPV-16. In this study, HPV16 ranked second in HPV6 multiple infections (172 coinfections), and HPV16 ranked first in HPV11 multiple infections (97 coinfections), which also explains why HPV16 is the most frequent HR-HPV in genital warts. Moreover, multiple infections of HPV6/11 with HR-HPV may be the cause of genital warts appearing in LSIL, HSIL and tumour progression42; therefore, in patients with genital warts, it is necessary to test whether there is an HR-HPV infection and to address it appropriately.
In this study, the prevalence of HPV6/11/16/18 was bimodal with age, with young women (< 20 years old) having the highest prevalence, old women (≥ 60 years old) having higher prevalence, and middle-aged women (20–49 years old) having a modest decline in prevalence. In fact, HPV infection is bimodal with age and is very common in many regions, and almost all young women have the first peak, with the second peak occurring at different ages35,36,37,43,44. The first peak of HPV infection rates in young women can be up to 80% in some populations45, with most women suffering from transient infections that clear rapidly46. The highest infection rate among young women (< 20 years old) may be related to less mature immune protection and/or multiple sexual partners in this age bracket47 but also to the fact that HPV screening is performed only if the patient has a sexual history and associated symptoms (e.g., genital warts) or if the patient requests it, which results in a relatively high detection rate. In the face of the highest HPV6/11/16/18 infection rate among young girls, it is urgent to strengthen HPV vaccination among women who have not had sex before in Chengdu. After this first peak, the prevalence of infection gradually declined to a plateau in middle-aged women (20–49 years old), which is probably due to increased autoimmune function and stable sexual partners. For the second minor peak of infection, the higher rate of infection in old women (≥ 50 years old) may be due to immunosenescence, changes in sexual behaviour and sexual partner (both for male and female), and reappearance of past (latent) infections44,48,49,50,51. Some scholars52 point out that the geographic difference in this second peak may be partially explained by indirect indicators of menopausal hormonal patterns, such as body mass index (BMI) and ethnicity. Since young women (age ≤ 20 years) are HPV-susceptible because of high HPV infection rates, vaccination of women before sexual debut is the most cost-effective strategy to prevent cervical cancer. Data53,54 showed that the earlier young women were inoculated with the HPV vaccine, the higher the antibody titre and the better the protective effect of the vaccine. Studies55 showed that almost everywhere, most men and women started having sex in their late teens (ages 15–19), and most began having sex at 15. The crude median sexual debut age for the youngest age group was 17 years in China56. Considering Chinese cultural traditions and social factors such as nine-year compulsory education, Chinese scholars recommend that junior middle school girls (aged 13 to 15) to be the first group to be vaccinated. In mainland China, there is an ongoing initiative to gradually introduce free HPV vaccination services or provide subsidized HPV vaccine inoculation. Currently, 19 provinces, municipalities, or autonomous regions across China offer free HPV vaccine administration. As of now, locations within Sichuan Province where free HPV vaccination can be arranged for those junior middle school girls (aged 13 to 15) include the entire city of Chengdu, as well as Wenchuan County in Aba and the city of Barkam, the latter two being economically disadvantaged areas. Similarly, HPV vaccination is also necessary in women from less than the age of 20 to 45, and even in women already infected with HPV, data54 show that the immune response to the HPV vaccine appears to prevent reinfection or reactivation of disease with vaccine HPV type. Moreover, HPV 16 and 18 vaccines were well tolerated and highly efficacious against HPV 16 and 18-associated high-grade genital lesions and persistent infection57.
For the second minor peak of HPV infection, HPV and cytological screening is especially important in middle-aged and older women, as screening not only reduces the burden of precancerous lesions and related persistent HPV infections, but removal of lesions may have a direct antigen-presenting effect that could protect against subsequent HPV infections58. Given that HPV 16 or 18 infections have the highest risk for CIN 3 and occult cervical cancer, 2019 ASCCP59 specifically emphasized that additional evaluation (e.g., colposcopy with biopsy) is necessary even when cytology results are negative if HPV 16 or 18 testing is positive and that additional laboratory testing of the same sample is not feasible, the patient should proceed directly to colposcopy.
In sum, vaccination is of extreme importance in the current situation. The WHO global strategy to accelerate the elimination of cervical cancer as a public health problem highlights 90% vaccination coverage for girls younger than 15 years old by 2030 as one of the three targets60. For middle-aged and older women, regular HPV screening is a better strategy to prevent cervical cancer and precancerous lesions.
The study possesses several limitations. First, it lacked cell or pathological data from positive cases, rendering comparative statistics unfeasible. Second, female vaccination rates were not taken into account, making it impossible to calculate the specific impact of the vaccine on HPV infection rates. Third, the study's subjects comprised solely patients from hospitals within a 6-year timeframe, which does not represent the typical population of Chengdu. Therefore, these findings are exploratory in nature and merit further investigation. Fourth, only four genotypes were involved in this study, obviously, some other HR-HPVs and LR-HPVs are involved in malignant disorders, especially cervical cancer and other genital cancers, However, due to the need for detailed and in-depth study of the four genes, few other genotypes are taken in account, which need to be further presented in future studies.
Conclusions
In conclusion, the infection rate of HPV6/11/16/18 was high in Chengdu, with HPV16 exhibiting the highest infection rate and HPV16 single infections showing an annual increase. The prevalence of HPV6/11/16/18 infection in young women (< 20 years) was highest and in old women (≥ 60 years) was higher; therefore, prioritizing vaccination for young females is essential in the current context, while HPV screening remains an effective approach for older females. Among the available HPV vaccines in Chengdu, the nonavalent vaccine appears to be relatively most suitable for Chengdu females. Additionally, in patients with genital warts, it is necessary to assess the presence of HR-HPV infection and manage it appropriately.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author J.Y.Z upon reasonable request.
References
Human Papillomavirus (HPV). Centers for Disease Control and Prevention. https://www.cdc.gov/hpv/parents/about-hpv.html (2023).
Bernard, H.-U. et al. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 401, 70–79. https://doi.org/10.1016/j.virol.2010.02.002 (2010).
Bernard, H.-U. et al. Genome variation of human papillomavirus types: Phylogenetic and medical implications. Int J Cancer. 118, 1071–1076. https://doi.org/10.1002/ijc.21655 (2006).
Stanley, M. A., Pett, M. R. & Coleman, N. HPV: from infection to cancer. Biochem Soc T. 35, 1456–1460. https://doi.org/10.1042/BST0351456 (2007).
Bletsa, G. et al. Genetic variability of the HPV16 early genes and LCR. Present and future perspectives. Expert Rev. Mol. Med. 23, 1–13. https://doi.org/10.1017/erm.2021.18 (2021).
World Health Organization. Human papillomavirus vaccines: WHO position paper (2022 update). Wkly Epidemio. Rec. 97, 645–672. https://www.who.int/publications/i/item/who-wer9750-645-672 (2022).
World Health Organization. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention, 2nd edn. World Health Organization. https://www.who.int/publications/i/item/9789240040434 (2021).
Muñoz, N. et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. New Engl. J. Med. 348, 518–527. https://doi.org/10.1056/NEJMoa021641 (2003).
Horvath, C. A. J. et al. Mechanisms of cell entry by human papillomaviruses: An overview. Virol. J. 7, 11. https://doi.org/10.1186/1743-422X-7-11 (2010).
Schiller, J. T., Day, P. M. & Kines, R. C. Current understanding of the mechanism of HPV infection. Gynecol. Oncol. 118(1 Suppl), S12–S17. https://doi.org/10.1016/j.ygyno.2010.04.004 (2010).
Raff, A. B. et al. The evolving field of human papillomavirus receptor research: A review of binding and entry. J. Virol. 87, 6062–6072. https://doi.org/10.1128/JVI.00330-13 (2013).
Elfgren, K. et al. A population-based five-year follow-up study of cervical human papillomavirus infection. Am. J. Obstet. Gynecol. 183, 561–567. https://doi.org/10.1067/mob.2000.106749 (2000).
de Sanjose, S. et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 11, 1048–1056. https://doi.org/10.1016/S1470-2045(10)70230-8 (2010).
Tsakogiannis, D. et al. Mutation profile of HPV16 L1 and L2 genes in different geographic areas. Viruses. 15, 141. https://doi.org/10.3390/v15010141 (2022).
Martinez-Zapien, D. et al. Structure of the E6/E6AP/p53 complex required for HPV-mediated degradation of p53. Nature 529, 541–545. https://doi.org/10.1038/nature16481 (2016).
Aarthy, M. et al. E7 oncoprotein of human papillomavirus: Structural dynamics and inhibitor screening study. Gene 658, 159–177. https://doi.org/10.1016/j.gene.2018.03.026 (2018).
Tsakogiannis, D. et al. Determination of human papillomavirus 16 physical status through E1/E6 and E2/E6 ratio analysis. J. Med. Microbiol. 63, 1716–1723. https://doi.org/10.1099/jmm.0.076810-0 (2014).
Tsakogiannis, D. et al. Sites of disruption within E1 and E2 genes of HPV16 and association with cervical dysplasia. J. Med. Virol. 87, 1973–1980. https://doi.org/10.1002/jmv.24256 (2015).
Wang, X. L., Huang, X. M. & Zhang, Y. Z. Involvement of human papillomaviruses in cervical cancer. Front. Microbiol. 9, 2896. https://doi.org/10.3389/fmicb.2018.02896 (2018).
Moscicki, A.-B. et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet 364, 1678–1683. https://doi.org/10.1016/S0140-6736(04)17354-6 (2004).
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Human Papillomavirus and Related Diseases Report, China. HPV Information Centre https://hpvcentre.net/statistics/reports/CHN.pdf?t=1685267737262 (2023).
Gall, S. A. Female genital warts: Global trends and treatments. Infect. Dis. Obstet. Gynecol. 9, 149–154. https://doi.org/10.1155/S1064744901000278 (2001).
de Martel, C., Plummer, M., Vignat, J. & Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 141, 664–670. https://doi.org/10.1002/ijc.30716 (2017).
Kjaer, S. K. et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (Types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev. Res. 2, 868–878. https://doi.org/10.1158/1940-6207.CAPR-09-0031 (2009).
Rosenblum, H. G. et al. Human papillomavirus vaccine impact and effectiveness through 12 years after vaccine introduction in the United States, 2003 to 2018. Ann. Intern. Med. 175, 918–926. https://doi.org/10.7326/M21-3798 (2022).
Steben, M. et al. A review of the impact and effectiveness of the quadrivalent human papillomavirus vaccine: 10 Years of clinical experience in Canada. J. Obstet. Gynaecol. Can. 40, 1635–1645. https://doi.org/10.1016/j.jogc.2018.05.024 (2018).
Feng, X. J., Hou, H. L., Yu, Q. & Wang, J. S. Market analysis and counter measure of cervical cancer vaccine in China. China Biotechnol. 40, 96–101. https://doi.org/10.13523/j.cb.2006054 (2020).
Global strategy to accelerate the elimination of cervical cancer as a public health problem. World Health Organization. https://www.who.int/publications/i/item/9789240014107 (2020).
New recommendations for screening and treatment to prevent cervical. World Health Organization. https://www.who.int/news/item/06-07-2021-new-recommendations-for-screening-and-treatment-to-prevent-cervical-cancer (2021).
Balaji, D., Kalarani, I. B., Mohammed, V. & Veerabathiran, R. Potential role of human papillomavirus proteins associated with the development of cancer. VirusDisease 33, 322–333. https://doi.org/10.1007/s13337-022-00786-8 (2022).
Mo, Y. et al. Prophylactic and therapeutic HPV vaccines: current scenario and perspectives. Front. Cell Infect. Microbiol. 12, 909223. https://doi.org/10.3389/fcimb.2022.909223 (2022).
Wang, J. W. & Roden, R. B. S. Virus-like particles for the prevention of human papillomavirus-associated malignancies. Expert Rev. Vaccines. 12, 129–141. https://doi.org/10.1586/erv.12.151 (2013).
Rebolj, M. et al. Primary cervical screening with high risk human papillomavirus testing: observational study. BMJ (Clinical Research ed.). 364, l240. https://doi.org/10.1136/bmj.l240 (2019).
Wei, L., Ma, L., Qin, L. & Huang, Z. The prevalence and genotype distribution of human papillomavirus among women in Guangxi, southern China. Infect. Agent Cancer. 17, 19. https://doi.org/10.1186/s13027-022-00431-5 (2022).
Li, X. et al. Prevalence of cervicovaginal human papillomavirus infection and genotype distribution in Shanghai, China. Virol. J. 19, 146. https://doi.org/10.1186/s12985-022-01879-y (2022).
Chen, X. et al. Prevalence and genotype distribution of cervical human papillomavirus (HPV) among women in urban Tianjin, China. J. Med. Virol. 87, 1966–1972. https://doi.org/10.1002/jmv.24248 (2015).
Sandri, M. T. et al. Typing of human papillomavirus in women with cervical lesions: Prevalece and distribution of different genotypes. J. Med. Virol. 81, 271–277. https://doi.org/10.1002/jmv.21382 (2009).
Spinillo, A. et al. Multiple human papillomavirus infection and high grade cervical intraepithelial neoplasia among women with cytological diagnosis of atypical squamous cells of undetermined significance or low grade squamous intraepithelial lesions. Gynecol. Oncol. 113, 115–119. https://doi.org/10.1016/j.ygyno.2008.12.037 (2009).
Bruno, M. T., Scalia, G., Cassaro, N. & Boemi, S. Multiple HPV16 infection with two strains: A possible marker of neoplastic progression. BMC Cancer 20, 444. https://doi.org/10.1186/s12885-020-06946-7 (2020).
Chang, L. et al. Distribution of genital wart human papillomavirus genotypes in China: A multi-center study. J. Med. Virol. 85, 1765–1774. https://doi.org/10.1002/jmv.23646 (2013).
Clavero, O. et al. Squamous intraepithelial lesions of the anal squamocolumnar junction: Histopathological classification and HPV genotyping. Papillomavirus Res. 3, 11–17. https://doi.org/10.1016/j.pvr.2016.12.001 (2017).
Castle, P. E. et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J. Infect. Dis. 191, 1808–1816. https://doi.org/10.1086/428779 (2005).
Bruni, L. et al. Cervical human papillomavirus prevalence in 5 continents: Meta-analysis of 1 million women with normal cytological findings. J. Infect. Dis. 202, 1789–1799. https://doi.org/10.1086/657321 (2010).
Moscicki, A. B. et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA 285, 2995–3002. https://doi.org/10.1001/jama.285.23.2995 (2001).
Stanley, M. A. Epithelial cell responses to infection with human papillomavirus. Clin. Microbiol. Rev. 25, 215–222. https://doi.org/10.1128/CMR.05028-11 (2012).
Tota, J. E., Chevarie-Davis, M., Richardson, L. A., DeVries, M. & Franco, E. L. Epidemiology and burden of HPV infection and related diseases: Implications for prevention strategies. Prev. Med. 53, S12-21. https://doi.org/10.1016/j.ypmed.2011.08.017 (2011).
Li, B., Wang, H., Yang, D. & Ma, J. Prevalence and distribution of cervical human papillomavirus genotypes in women with cytological results from Sichuan province, China. J. Med. Virol. 91, 139–145. https://doi.org/10.1002/jmv.25255 (2019).
González, P. et al. Behavioral/lifestyle and immunologic factors associated with HPV infection among women older than 45 years. Cancer Epidemiol. Biomark. Prev. 19, 3044–3054. https://doi.org/10.1158/1055-9965.EPI-10-0645 (2010).
Franceschi, S. et al. Variations in the age specific curves of human papillomavirus prevalence in women worldwide. Int. J. Cancer. 119, 2677–2684. https://doi.org/10.1002/ijc.22241 (2006).
Smith, J. S. et al. Age-specific prevalence of infection with human papillomavirus in females: A global review. J. Adolesc. Health 43, S5-25. https://doi.org/10.1016/j.jadohealth.2008.07.009 (2008).
Althoff, K. N. et al. Correlates of cervicovaginal human papillomavirus detection in perimenopausal women. J. Womens Health (Larchmt). 18, 1341–1346. https://doi.org/10.1089/jwh.2008.1223 (2009).
Spinner, C. et al. Human papillomavirus vaccine effectiveness and herd protection in young women. Pediatrics. 143, e20181902. https://doi.org/10.1542/peds.2018-1902 (2019).
Olsson, S.-E. et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum. Vaccin. 5, 696–704. https://doi.org/10.4161/hv.5.10.9515 (2009).
Wellings, K. et al. Sexual behaviour in context: a global perspective. Lancet. 368, 1706–1728. https://doi.org/10.1016/S0140-6736(06)69479-8 (2006).
Zhao, F. H. et al. A multi-center survey of age of sexual debut and sexual behavior in Chinese women: Suggestions for optimal age of human papillomavirus vaccination in China. Cancer Epidemiol. 36, 384–390. https://doi.org/10.1016/j.canep.2012.01.009 (2012).
Zhao, F. H. et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced Human Papillomavirus (16 and 18) L1 virus-like-particle vaccine: end-of-study analysis of a phase 3, double-blind, randomised, controlled trial. Lancet Infect. Dis. 22, 1756–1768. https://doi.org/10.1016/S1473-3099(22)00435-2 (2022).
Passmore, J. A., Morroni, C., Shapiro, S., Williamson, A. L. & Hoffman, M. Papanicolaou smears and cervical inflammatory cytokine responses. J. Inflamm. (Lond). 4, 8. https://doi.org/10.1186/1476-9255-4-8 (2007).
Perkins, R. B. et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J. Low Genit. Tract. Dis. 24, 102–131. https://doi.org/10.1097/LGT.0000000000000525 (2020).
World Health Assembly adopts global strategy to accelerate cervical cancer elimination. World Health Organization https://www.who.int/news/item/19-08-2020-world-health-assembly-adopts-global-strategy-to-accelerate-cervical-cancer-elimination (2020).
Funding
This work was supported by the "13th Five-Year Plan" Science and Technology Major Project (2017ZX10103010-006).
Author information
Authors and Affiliations
Contributions
X.Q.W. participated in the collection of patient specimens, the design of the study, the discussion of the results, and the revision of the manuscript. J.Y.Z. contributed to data collection, statistics, figures, tables and analysis, and wrote and revised the manuscript. Y.W. M, Q.L.D. and X.L.Y. participated in patient diagnosis, specimen collection and specimen transportation. X.M.W. was responsible for HPV testing operations.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wei, X., Zhang, J., Mei, Y. et al. Prevalence and genotype distribution of HPV6/11/16/18 infections among 180,276 outpatient females from a Women’s and Children’s Central Hospital, 2015–2021, Chengdu, China. Sci Rep 13, 22249 (2023). https://doi.org/10.1038/s41598-023-48222-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48222-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.