Abstract
Pathogens affect wild bird populations worldwide, contributing to their decline. Considering the scarce health data regarding the endangered Pyrenean Capercaillie (Tetrao urogallus aquitanicus), we molecularly surveyed selected pathogens (Newcastle disease virus, Avian influenza virus, Chlamydia psittaci, avian pathogenic Escherichia coli, Campylobacter jejuni, and Salmonella spp.) in 30 Pyrenean Capercaillie feces collected in the field (Catalonia, northeastern Spain). Additionally, swab and tissue samples from eight wild Pyrenean Capercaillies of Catalonia and Andorra were molecularly tested for herpesvirus and hemosporidians (Plasmodium spp., Haemoproteus spp., and Leucocytozoon spp.). All fecal samples were negative for the pathogens tested. Nevertheless, we detected a novel herpesvirus in 50% (4/8) of the Pyrenean Capercaillies, and hemosporidian DNA in 62.5% (5/8) of the tissue samples (i.e., Haemoproteus sp. [4 of 8] and/or Leucocytozoon sp. [3 of 8]). To our knowledge, this is the first detection of herpesvirus and hemosporidians infections in Pyrenean Capercaillies. The putative novel herpesvirus belongs to the genus Iltovirus. The presence of hemosporidian parasites in this mountain bird species is of concern, and could be related to the marked increase in the average temperature in the Pyrenees as a consequence of climate change. Our findings are fundamental to improve the conservation plans for the endangered Pyrenean Capercaillie population.
Similar content being viewed by others
Introduction
The Pyrenean Capercaillie (Tetrao urogallus aquitanicus) is a very elusive endangered subspecies of Western Capercaillie (T. urogallus, order Galliformes) that sustains local endemism in the Pyrenees and inhabits narrow elevation ranges1. This mountain bird is highly sensitive to environmental changes and stress2. Several factors are considered threats to Pyrenean Capercaillies, including climate change, habitat fragmentation, increased extensive livestock and wild ungulate populations, and disturbances resulting from off-track practices2. Additionally, increased temperatures may promote their exposure to novel pathogens that were not originally present and/or that normally belong to other altitudinal ranges3. Responses to these environmental stressors may lead to increased stress hormone levels, immunosuppression and increased susceptibility to environmental changes and/or diseases4,5.
Pathogens may contribute to wild bird population decline due to mortality and decreased reproductive success6,7. Furthermore, some wild bird infectious agents have zoonotic potential and/or relevance in poultry7,8. To this date, there are no reports of pathogens affecting Pyrenean capercaillie, and their descriptions in other Wester Capercaillie subspecies are very scarce, limited to infections by Usutu virus, Plasmodium relictum, Capillaria sp., and Eimeria spp. in a Western Capercaillie (T. u. crassirostris) kept in captivity in Switzerland9, Haemoproteus sp. in two Western Capercaillies from Austria10, and Escherichia coli, Clostridium perfringens, Enterococcus spp. or Aspergillus fumigatus in Cantabrian Capercaillie (T. u. cantabricus) from a breeding center in Spain11.
The aim of this study was to generate basic health data and investigate potential factors contributing to the current decline of the Pyrenean Capercaillie population. Thus, we molecularly surveyed the presence of Avian influenza virus, Newcastle virus, Chlamydia psittaci, avian pathogenic E. coli (APEC), Campylobacter jejuni and Salmonella spp. in Pyrenean Capercaillie fecal samples, and tested swab and tissue samples for herpesviruses and hemosporidians (Plasmodium, Haemoproteus and Leucocytozoon).
Materials and methods
Study species
The Pyrenean Capercaillie is a subalpine bird, distributed year-round along the Pyrenean range, southwestern Europe1. This subspecies was recently reclassified as “Endangered” by the Spanish List of Wildlife Species under Special Protection Regime12.
Study area
The study was performed in the Catalonian Pyrenees (Val d’Aran, Pallars Sobirà, Pallars Jussà, Alt Urgell, Cerdanya, Berguedà, and Ripollès counties, Spain), and Andorra (Fig. 1), from 1571 to 2302 m above sea level (a.s.l.). These territories are placed in the axial Pyrenees (Val d’Aran, Pallars Sobirà, Cerdanya, Andorra), Pre-Pyrenees (Pallars Jussà, Berguedà) or in the Pre-Pyrenees, with some areas in the Pyrenees (Alt Urgell, Ripollès).
Location of the Pyrenean Capercaillies (Tetrao urogallus aquitanicus) sampled for the study and results of the herpesvirus and Leucocytozoon sp. and Haemoproteus sp. analyses. The figure was created with ArcGIS Pro v3.1 (https://www.esri.es/es-es/arcgis/productos/arcgis-pro/introduccion).
Samples
Fresh fecal samples
Fresh fecal samples (n = 30) were collected in the field during the spring, summer and fall of 2020 and 2021, from areas within the species’ distribution range in the Catalonian Pyrenees (Val d’Aran [n = 3], Pallars Sobirà [n = 13], Pallars Jussà [n = 1], Alt Urgell [n = 7], Cerdanya [n = 1], Berguedà [n = 2], and Ripollès [n = 3] counties). The characteristic morphological features of capercaillie feces facilitated their identification (Supplementary Fig. 1). Fecal samples were collected with latex gloves and sterilized lingual depressors, stored without media in autoclaved microtubes, and kept frozen at − 20/− 80 °C until processed.
Tissue and swab samples
We collected tissue samples (blood, central nervous system, lung, liver, spleen, and kidney) from eight adult male Pyrenean Capercaillies (Table 1). Six of these animals were sampled (blood, oropharyngeal and/or cloacal swab samples) during Pyrenean Capercaillie spring captures for tagging in Catalonia (Spain), and two additional cases were found in the field in Catalonia and Andorra (Fig. 1, Table 1). Specifically, blood samples (5 to 7 ml) from five individuals were taken from the brachial vein using 10 ml syringes (23-gauge, 25 mm needles), during sedation procedures for GPS placement. Blood samples were collected in EDTA and biochemistry tubes, kept refrigerated, and used to perform a complete hematology and biochemical study (data not shown), while a volume of 0.5 ml of whole blood was kept frozen (− 20 ºC) for molecular studies. Oropharyngeal (n = 5) and cloacal (n = 5) swab samples were taken during captures. Additionally, tissue samples aside from blood (i.e., central nervous system, lung, liver, kidney, spleen, and kidney) were taken during the necropsy of five individuals (22038–22040, 22163, 23001, Table 1). Necropsies were conducted following a standard protocol13.
Histopathology
Selected tissue samples were placed in 4% neutral buffered formalin, routinely processed, sectioned at 3–4 μm, stained with hematoxylin and eosin, and examined under light microscopy. Sections included brain, trachea, thyroid and parathyroid glands, lungs, heart, liver, kidney, spleen, skeletal muscle, adrenal gland, pancreas, testis, esophageal-proventricular junction, and any gross lesion detected during necropsy. In all cases, intestinal samples were in advanced stages of autolysis, which prevented histopathological examination.
Blood smear
Blood smears were performed immediately after sampling and stained with May-Grunwald-Giemsa. In cases where postmortem samples were collected, blood was sampled from the heart. Three blood smears were performed in each of these animals, and stained with a wright stain (Sigma-Aldrich Inc., St. Louis, USA) in an automatic stainer Hematek 2000 (Siemens Healthcare Diagnostic Inc., Tarrrytown, NY, USA),
Molecular methods
Fecal samples
Fecal samples (n = 30) were thawed at room temperature, and aliquots of 0.2 mg were processed for simultaneous RNA and DNA extraction by pressure filtration (QuickGene DNA tissue kit S, Fujifilm), following the manufacturer’s instructions, and added RNA carrier supplementation during lysis. Real-time PCR (rt-PCR) protocols were used to detect Salmonella spp. and C. jejuni14. E. coli was tested by a conventional PCR followed by an octuplex PCR for avian pathogenicity factors15. Avian influenza virus and Newcastle virus RNA, and Ch. psittaci DNA were detected using rt-PCR protocols, respectively based on TaqMan probe16,17, and on high resolution melting (HRM) analysis18.
Blood and tissue samples
Solid tissue (n = 19) samples were mechanically homogenized using 20 mg of solid tissue with 300 µl PBS, 200 µl lysis buffer and ceramic beads placed in 2 ml tubes. Cloacal (n = 5) and oropharyngeal (n = 5) swab samples were mixed with 300 µl of PBS and 200 µl of lysis buffer, vortexed for 1 min, mixed with 25 µl proteinase K and 5.9 µl of RNA carrier, and vortexed for 1 min. Blood samples (n = 5, 200 µl each) were directly extracted. Total DNA and RNA from these samples were extracted using the PureLink Viral RNA/DNA Mini Kit (Invitrogen, Waltham, Massachusetts, USA), following the manufacturer’s instructions. The extractions of blood, solid tissues, oropharyngeal and/or cloacal samples of eight animals were tested against herpesviruses using a nested broad-spectrum PCR that amplifies a fragment (215–315 bp) of DNA polymerase (DPOL) gene19. The extracted samples (excluding swabs) from the eight animals were also analyzed for Plasmodium spp., Haemoproteus spp., and Leucocytozoon spp. with a nested PCR that partially amplifies the mitochondrial cytochrome b gene of Apicomplexan hemoparasites20. The internal PCR has a specific pair of primers to amplify an approximately 480 bp fragment of Plasmodium spp. and Haemoproteus spp., and another one for Leucocytozoon spp. (also around 480 bp)20.
Amplicons of the expected size were purified with Exo-SAP IT (Thermo Fisher Scientific) and directly sequenced in both directions. The consensus sequences were constructed using the ClustalW alignment of forward and reverse sequences in MEGA 7.021 and Geneious®. The obtained consensus sequences, without primers, were compared to the closest ones from GenBank/EMBL/DDBJ by BLAST search, and the percentage of nucleotide and amino acid (aa) similarity was calculated based on p-distance using MEGA 7.0 software, as described by Ewbank et al.22. Herpesvirus and hemosporidian maximum likelihood phylograms were constructed with MEGA 7.0 and RaXM 1.523, data set was resampled 1000 times for bootstrap values, and the evolutionary model was selected with ProtTest (v3.4.2). To construct the hemosporidians phylograms, previously identified representative lineages were obtained from MalAvi database.
Ethics approval
The Pyrenean Capercaillie captures included in this article were carried out on public lands, in strict compliance with the European (Directives 92/43/CEE and 147/2009/CE), Spanish (Act 42/2007 and Act 12/1985), and Catalan (Decree 148/1992, of 9 June) regulations regarding photographic, scientific, and sports activities that may affect wildlife species and Legislative Decree 2/2008, of April 15, approving the Revised Text of the Animal Protection Act). Exceptional permits for trapping, sedation, sampling, movement, and equipping the target species with transmitters -protected under the Spanish law- were obtained from the competent authorities (Capercaillie capture and sedation permits number: SF/005/2020, SF/002/2020, SF/036/2020, SF/0089/2021, SF/0029/2022–Department of Environment and Housing and Alt Pirineu Natural Park; Government of Catalonia). The protocols for trapping, movement, sedation, tagging and sampling the Pyrenean Capercaillies were consistent with the best practices and technical and scientific recommendations related to animal welfare of European (Directives 92/43/CEE and 147/2009/CE), Spanish (Act 42/2007 and Act 12/1985), and Catalan (Decree 148/1992, of 9 June) regulations and were approved by Government of Catalonia. Ethic committee code for Pyrenean Capercaillies captures and sedation was “2/2020/DG”, granted the Climatic Action, Food and Rural Agenda of the Government of Catalonia.
ARRIVE guidelines
Not applicable, given that these guidelines are for reporting in vivo experiments.
Results
Anatomopathological findings
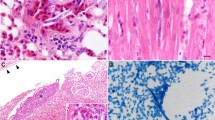
Necropsies were performed in five Pyrenean Capercaillies (cases 22038–22040, 22163, 23001), and histopathology was conducted in four of these birds. The main gross and histopathologic findings are described in Supplementary Table 1. Of note, none of the examined animals presented lesions consistent with those associated with herpesvirus, Plasmodium, Haemoproteus or Leucocytozoon (see below). Specifically, exo-erythrocytic development of haemoproteids (meronts and megalomeronts) were not identified in any of the available tissue sections.
Blood smear
Blood smears were performed in five animals: 22036–22039, and 22041. Structures consistent with hemoparasites were identified under light microscopy in three animals (i.e., Haemoproteus-like structures in animals 22036, 22038, 22039, and Leucocytozoon-like structures in case 22039) (Fig. 2).
Molecular techniques
None of the tested fecal samples was PCR-positive to Avian influenza virus, Newcastle disease virus, Ch. psittaci, APEC, Salmonella spp. or C. jejuni.
Four out of eight animals were herpesvirus-positive (22037, 22038, 22039, 22163) (Table 1). These animals were positive in cloacal swab samples (n = 3), brain (n = 1), liver (n = 1) and kidney (n = 1). The same herpesvirus sequence type was obtained in all sequenced amplicons, and presented the highest nucleotide (79.9%) and aa (80.6%) identities with an alphaherpesvirus sequence retrieved from a Neotropical Cormorant (Phalacrocorax brasilianus) of Chile (KY769943) and with several Gallid alphaherpesvirus 1 sequences from chicken (Gallus gallus) (e.g., JX458824, MK895003, MF405079)—the latter also known as infectious laryngotracheitis virus. The obtained sequence clustered in the phylogram (bootstrap value of 86) with a herpesvirus sequence from a Neotropical Cormorant (KY769943) and with a reference sequence of Gallid alphaherpesvirus 1 (NC_006623) (Fig. 3A).
Maximum likelihood phylograms of the ClustalW alignment of: (A) The deduced amino acid herpesviral DNA polymerase sequences (i) found in Pyrenean Capercaillies (Tetrao urogallus aquitanicus) (blue dot), (ii) the closest sequences from GenBank database, and iii) other alphaherpesvirus sequences described in birds, with Mardivirus and Iltovirus genera accepted species. Human gammaherpesvirus 8 was selected as outgroup. The herpesvirus phylogram was based on the gamma distributed Le and Gascuel model with Invariant sites and Gamma distribution (LG + I + G). (B) The Haemoproteus cytochrome b sequence found in Pyrenean Capercaillies and other birds, with Leucocytozoon schoutedeni selected as outgroup; it was based on Gamma distribution (GTRGAMMA). (C) The cytochrome b sequence of Leucocytozoon sp. found in Pyrenean Capercaillies and other birds, with Haemoproteus belopolskyi selected as outgroup, and based on Gamma distribution (GTRGAMMAI). The reliability of all phylograms was tested by a 1000 bootstrap analysis; those bootstrap values lower than 70 were omitted.
Overall, Apicomplexa hemoparasites were identified in five out of eight (62.5%) Pyrenean Capercaillies: Haemoproteus sp. in four out of eight and Leucocytozoon spp. in three out of eight birds (Table 1). Of note, Haemoproteus-like structures were present in blood smears of three of the Haemoproteus-PCR-positive cases (220036, 22038, 22039), and Leucocytozoon-like structures in one of the Leucocytozoon-positive cases (22039), coinfected with Haemoproteus. None of the samples was PCR-positive to Plasmodium spp. The same Haemoproteus sequence type was present in all positive capercaillies, and had the highest nucleotide similarity (98.1%) with Haemoproteus sp. sequences of birds of Thailand (MK390808) and Papua New Guinea (MK061657, MK061654, AY714134), and the highest aa identity (100%) with Haemoproteus sequences from birds of Thailand (MK390808), South Korea (MW296829, MW296831, MW296830), and China (KT944102). Regarding Leucocytozoon spp., we obtained the same sequence type in three animals, more similar to the Leucocytozoon sp. sequence KU257627 (nucleotide and aa similarities of 97.1% and 95.6%, respectively) retrieved from a Spruce Grouse (Falcipennis canadensis) of Alaska, USA. The Haemoproteus phylogram showed that our Pyrenean Capercaillie sequence clustered together in well-supported clades with sequences of Western Capercaillie from Austria and with sequences of different species of raptors from Asia and Europe (Fig. 3B). The Leucocytozoon sp. sequence retrieved in Pyrenean Capercaillie clustered together, in well-supported clades, with Leucocytozoon sp. sequences obtained from different species within the family Phasianidae, from North America (Fig. 3C).
Representative herpesvirus, Haemoproteus sp. and Leucocytozoon sp. sequences obtained in this study were submitted to GenBank under accession numbers OR426924, OR354895 and OR354896, respectively.
Discussion
To our knowledge, this is the first report of herpesvirus, Haemoproteus sp., and Leucocytozoon in Pyrenean Capercaillies. The detection of a novel herpesvirus sequence type in four animals from different counties and in a new host species (i.e., Pyrenean Capercaillie) supports its classification as a novel alphaherpesvirus species, tentatively named Tetrao urogallus alphaherpesvirus 1. Based on the phylogram and identity analysis results, this virus belongs to genus Iltovirus, which includes other species, such as Gallid alphaherpesvirus 1. Gallid alphaherpesvirus 1 can cause infectious laryngotracheitis, a respiratory disease that affects chickens, pheasants, partridges, and peafowl24; however, the pathogenic potential of Tetrao urogallus alphaherpesvirus 1 remains unknown.
Avian malaria is caused by parasites of the genus Plasmodium, while avian malaria-like disease is caused by parasites of the genera Haemoproteus and Leucocytozoon; all of them comprised into the phylum Apicomplexa, order Haemosporidia25. In avian species, hemosporidian infections range from subclinical disease, anemia or slight plumage coloration change, to severe and even fatal disease26,27, potentially leading to severe declines in naïve wild bird populations28. Herein, we detected Haemoproteus in four Pyrenean Capercaillies. The genus Haemoproteus, a sister genus to malaria parasites (Plasmodium), parasitizes only birds and reptiles29,30. In birds, Haemoproteus species are considered cosmopolitan and are often prevalent29,31. Haemoproteus parasites are transmitted mainly by Culicoides biting midges (Ceratopogonidae), with only a few species vectored by louse flies (Hippoboscidae)29,32. This genus does not multiply in blood cells and presents predominantly light or moderate parasitemia29.
Leucocytozoon sp. was identified in three Pyrenean Capercaillies. This genus is transmitted by hematophagus black flies (Simuliidae)—with the exception of the species Leucocytozoon caulleryi, transmitted by biting midges (Culicoides)29. In the tribe Tetraonini, which comprises capercaillies, grouse and ptarmigan, the parasite Leucocytozoon lovati was detected in ptarmigan species inhabiting northern Norway and alpine areas of Japan33,34, while diverse Leucocytozoon haplotypes were identified in four grouse and three ptarmigan species of Alaska, USA35. This suggests that Leucocytozoon vectors are present in cold areas and at high altitudes similar to the Pyrenees.
Regarding the pathogenicity of the detected genera of hemoparasites, lesions associated with Leucocytozoon are more common than those of Haemoproteus36. High Leucocytozoon spp. parasitemia can cause emaciation, dehydration, convulsions, severe tissue damage, and mortality36,37. Additionally, a Leucocytozoon species—Leucocytozoon lovati—was associated with decreased reproductive success in the Black Grouse (Lyrurus tetrix)38, a galliform of the same family (Phasianidae) as the Pyrenean Capercaillie. The effects of Leucocytozoon sp. over the reproductive success of Pyrenean Capercaillies are still unknown. Although initially considered relatively benign to their hosts39, the Haemoproteus genus was recently associated with organ damage in naturally infected birds10,40. In our study, none of the Leucocytozoon- and/or Haemoproteus-positive animals analyzed by histopathology presented moderate or marked lesions associated with these parasites. These results are consistent with chronic parasitemia, a phase in which identifying persisting meronts in tissue samples can be difficult41. Nevertheless, potential costs to the immune-system or fitness of the animals should not be disregarded, especially in those individuals sustaining coinfection.
Although Leucocytozoon is better adapted to mountain conditions, the presence of insect-borne hemoparasites (Haemoproteus and Leucocytozoon) in the mountain galliforms analyzed herein was unexpected, particularly of Haemoproteus42. These animals were sampled in territories over 1570 m a.s.l., and one of the Haemoproteus-positive cases was sampled at 2255 m a.s.l. As explained above, these agents are mainly transmitted by the Culicoides biting midges and black flies, respectively29,32. The infection by the sister genus Plasmodium, transmitted by Culicidae and Ceratopogonidae43, was recently reported based on the hematology of a bearded vulture chick (Gypaetus barbatus) in the Pyrenees, also considered a mountain bird44. Culicoides biting midges are also important vectors of bluetongue virus, which causes an important disease of ungulates. In recent years, bluetongue virus has been detected in two of 89 Pyrenean chamois (Rupicapra pyrenaica pyrenaica) from the French Pyrenees, strongly suggesting the presence of the vector in the region45. It has been hypothesized that climate change may alter the geographic distribution of hemosporidians and increase parasite transmission (e.g., expanding vector ranges, promoting longer vector breeding seasons, and enhancing the development of avian haematozoa), consequently altering these parasites’ host range3,46. Worryingly, the average temperature in the Pyrenees has increased 1.2 °C from 1949 to 201047, 30% over the average world increase (0.85 °C). Thus, long-term studies are warranted to address the impact of climate change on Pyrenean capercaillies and other mountain species inhabiting the Pyrenees, a hot spot of biodiversity.
All the Pyrenean Capercaillie fecal samples were PCR-negative to Avian influenza virus, Newcastle disease virus, Ch. psittaci, APEC, Salmonella spp. and C. jejuni. To the authors’ knowledge, there are no previous reports of the above mentioned agents in capercaillies. A previous study surveyed highly pathogenic avian influenza virus in 145 capercaillies in Europe, between 2016 and 2017, where all of the individuals tested negative to that agent48. Additionally, Avian influenza virus was not detected in the 79 mountain galliforms tested in the Italian Alps49. The negative results for APEC, Salmonella spp. and C. jejuni were not completely unexpected, once these pathogens have been previously described in other wild free-ranging galliforms, although generally at low prevalences11,50,51,52, and not necessarily considered part of their normal microbiota51. In capercaillies, the presence of E. coli was confirmed in captive and free-ranging birds of Germany, with higher occurrence in the captive individuals50. Nevertheless, the authors did not further characterize the isolates, and thus, it is not possible to know if these animals presented APEC. The same difference in E. coli prevalence between captive and free-raging birds was observed in red-legged partridges (Alectoris rufa) by Díaz-Sánchez et al.51, whom suggested that the bacterium could not be part of the normal intestinal microbiota of that species. In addition, the unique diet of Pyrenean Capercaillies, one of the few bird species able to eat coniferous needles53 is likely associated with a distinct intestinal microbiota, as observed in other capercaillie subspecies in Germany53. To this date, there is only one report of Western Capercaillie microbiome, which identified marked differences between cecal samples of captive and free-ranging birds from Germany53. Interestingly, the phylum Proteobacteria, which includes species like E. coli, Campylobacter spp. and Salmonella spp., only represented 4% of the microbiome of the analyzed free-ranging capercaillies, but 46% of the microbiome of the captive ones53. This could also be the case for Pyrenean capercaillies. Recently, García-Rodríguez et al.11 reported several cases of colibacillosis (caused by E. coli) in captive Cantabrian Capercaillies (i.e., isolated E. coli infections in nine chicks and one adult, and a co-infection by E. coli, Clostridium perfringens, and Enterococcus gallinarum in one chick). The potential impact of this bacterium in free-ranging Pyrenean capercaillie chicks is unknown. Further studies are necessary to characterize the Pyrenean Capercaillie intestinal microbiome. Additionally, larger sample sizes will be required to confirm our negative results.
Herein, we report the first description of herpesvirus, Haemoproteus and Leucocytozoon in Pyrenean Capercaillies, based on detailed molecular data and light microscopy. None of the tested fecal samples were positive to Newcastle disease virus, Avian influenza virus, Ch. psittaci, APEC, C. jejuni, and Salmonella spp. Our findings contribute to the current scarce knowledge about infectious agents in this species. In light of the endangered status of the Pyrenean Capercaillie and the expected consequences of climate change for hemosporidian parasite dynamics and mountain bird health, further studies are necessary to clarify the presence of clinical signs and pathologic changes associated with herpesvirus, Haemoproteus and Leucocytozoon. Although sedation methods have been developed to reduce the mortality risk related to capture myopathy and invasive sampling during Pyrenean Capercaillies captures2, this species is very sensitive to stress related to handling. Therefore, non-invasive methods and collection of carcasses should be prioritized to study Pyrenean Capercaillie diseases. Additionally, the presence of hemoparasites in mountain environments inhabited by Pyrenean capercaillies could also be assessed by sampling other birds species inhabiting the same areas (e.g., chicks in nests), and vectors. The surveillance results of these infectious agents will provide valuable insights for the development of in situ and ex situ management strategies, crucial for the conservation of this threatened galliform species.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files]. The sequences obtained in the study were deposited in GenBank under accession numbers OR426924, OR354895 and OR354896.
References
Canut, J., Garcia-Ferré, D. & Afonso-Jordana, I. Pyrenean capercaillie (Tetrao urogallus). In Catalan Breeding Bird Atlas Distribution and Abundance 2015–2018 and Change since 1980 (eds Franch, M. et al.) 112–113 (Institut Català d’Ornitologia and Cossetània Editions, 2021).
Nicolás, F. O. et al. Sedation of wild Pyrenean Capercaillie (Tetrao urogallus aquitanicus) using intramuscular midazolam. Animals. 12, 1773 (2022).
Garamszegi, L. Z. Climate change increases the risk of malaria in birds. Glob. Chang. Biol. 17, 1751–1759 (2011).
Fowler, G. S. Behavioral and hormonal responses of Magellanic penguins (Speniscus magellanicus) to tourism and nest site visitation. Biol. Conserv. 90, 143–149 (1999).
Cyr, N. E. & Michael Romero, L. Chronic stress in free-living European starlings reduces corticosterone concentrations and reproductive success. Gen. Comp. Endocrinol. 151, 82–89 (2007).
Pennycott, T. W., Dagleish, M. P., Wood, A. M. & Garcia, C. Chlamydophila psittaci in wild birds in the UK. Vet. Rec. 164, 157–158 (2009).
Nemeth, N. M. et al. Bald eagle mortality and nest failure due to clade 2.3.4.4 highly pathogenic H5N1 influenza a virus. Sci. Rep. 13, 191 (2023).
Recht, J., Schuenemann, V. J. & Sánchez-Villagra, M. R. Host diversity and origin of zoonoses: The ancient and the new. Animals. 10, 1672 (2020).
Meister, S. L., Wyss, F., Wenker, C., Hoby, S. & Basso, W. U. Avian haemosporidian parasites in captive and free-ranging, wild birds from zoological institutions in Switzerland: Molecular characterization and clinical importance. Int. J. Parasitol. 20, 46–55 (2022).
Himmel, T. et al. Molecular probes for the identification of avian Haemoproteus and Leucocytozoon parasites in tissue sections by chromogenic in situ hybridization. Parasit. Vectors. 12, 282 (2019).
García-Rodríguez, A. et al. Mortality causes in captive Cantabrian capercaillie (Tetrao urogallus cantabricus) in Spain. Animals. 13, 1255 (2023).
Spanish Ministry for the Ecological Transition. Spanish Extinct Species List. (BOE 83, April 7th, 2023). https://www.boe.es/boe/dias/2023/04/07/pdfs/BOE-A-2023-8751.pdf (2023).
Wakamatsu-Utsuki, N. & Pollock, C. 2019. A guide to avian necropsy. LafeberVet Web site. https://lafeber.com/vet/a-guide-to-avian-necropsy/ (2019).
Wiemer, D. et al. Real-time multiplex PCR for simultaneous detection of Campylobacter jejuni, Salmonella, Shigella and Yersinia species in fecal samples. Int. J. Med. Microbiol. 301, 577–584 (2011).
Ewers, C., Janssen, T., Kießling, S., Philipp, H.-C. & Wieler, L. H. Rapid detection of virulence-associated genes in avian pathogenic Escherichia coli by multiplex polymerase chain reaction. Avian Dis. 49, 269–273 (2005).
Spackman, E. et al. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J. Clin. Microbiol. 40, 3256–3360 (2002).
Wise, M. G. et al. Development of a real-time reverse-transcription PCR for detection of Newcastle disease virus RNA in clinical samples. J. Clin. Microbiol. 42, 329–338 (2004).
Mitchell, S. L. et al. Genotyping of Chlamydophila psittaci by real-time PCR and high-resolution melt analysis. J. Clin. Microbiol. 47, 175–181 (2009).
VanDevanter, D. R. et al. Detection and analysis of diverse herpesviral species by consensus primer PCR. J. Clin. Microbiol. 34, 1666–1671 (1996).
Hellgren, O., Waldenström, J. & Bensch, S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 90, 797–802 (2004).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016).
Ewbank, A. C. et al. Herpesvirus, flavivirus, and coronavirus surveillance in magnificent frigatebirds (Fregata magnificens), Alcatrazes Archipelago, southeastern Brazil. J. Wildl. Dis. 59, 353–358 (2023).
Stamatakis, A., Hoover, P. & Rougemont, J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 57(5), 758–771 (2008).
WOHA (World Organisation for Animal Health). Chapter 10.3. Avian Infectious Laryngotracheitis. In OIE Terrestrial Manual 2021. Terrestrial Animal Health Code. https://www.woah.org/fileadmin/Home/fr/Health_standards/tahm/3.03.03_AVIAN_INF_LARYNGO.pdf (2021).
Atkinson, C.T. & Van Riper, C. Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon, and Haemoproteus. In Oxford Ornithology Series. Bird-Parasite Interact, vol. 2, 19–48 (1991).
Ots, I. & Hörak, P. Health impact of blood parasites in breeding great tits. Oecologia 116, 441–448 (1998).
del Cerro, S. et al. Carotenoid-based plumage colouration is associated with blood parasite richness and stress protein levels in blue tits (Cyanistes caeruleus). Oecologia. 162, 825–835 (2010).
LaPointe, D. A., Atkinson, C. T. & Samuel, M. D. Ecology and conservation biology of avian malaria. Ann. N. Y. Acad. Sci. 1249, 211–226 (2012).
Valkiūnas, G. Pathogenicity. In Avian Malaria Parasites and Other Haemosporidia 1st edn (ed. Valkiūnas, G.) 75–107 (CRC Press, Taylor & Francis Group, 2005).
Telford, S. R. Plasmodiid parasites. In The Hemoparasites of the Reptilia. Color Atlas and Text (ed. Telford, S. R., Jr.) 15–95 (CRC Press, Taylor & Francis Group, 2009).
Clark, N. J., Clegg, S. M. & Lima, M. R. A review of global diversity in avian haemosporidians (Plasmodium and Haemoproteus: Haemosporida): New insights from molecular data. Int. J. Parasitol. 44, 329–338 (2014).
Valkiūnas, G. & Atkinson, C. T. Introduction to life cycles, taxonomy, distribution and basic research techniques. In Avian Malaria and Related Parasites in the Tropics: Ecology, Evolution and Systematics (eds Santiago-Alarcon, D. & Marzal, A.) 45–80 (Springer, 2020).
Holmstad, P. R. & Skorping, A. Covariation of parasite intensities in willow ptarmigan, Lagopus lagopus L. Can. J. Zool. 76, 1581–1588 (1998).
Hagihara, M., Yamaguchi, T., Kitahara, M., Hirai, K. & Murata, K. Leucocytozoon lovati infections in wild rock ptarmigan (Lagopus mutus) in Japan. J. Wildl. Dis. 40, 804–807 (2004).
Smith, M. M., Van Hemert, C. & Merizon, R. Haemosporidian parasite infections in grouse and ptarmigan: Prevalence and genetic diversity of blood parasites in resident Alaskan birds. Int. J. Parasitol. Parasites Wildl. 5, 229–239 (2016).
Wernery, U. Infectious diseases. In Avian Medicine 2nd edn (ed. Samour, J.) 434–521 (Elsevier, 2016).
Bowman, D. D. Protista (chapter 3). In Georgis’ Parasitology for Veterinarians 11th edn (ed. Bowman, D. D.) 93–134 (Elsevier, 2021).
Höglund, J., Alatalo, R. V. & Lundberg, A. The effects of parasites on male ornaments and female choice in the lek-breeding black grouse (Tetrao tetrix). Behav. Ecol. Sociobiol 30, 71–76 (1992).
Bennett, G. F., Pierce, M. A. & Ashford, R. W. Avian haematozoa: Mortality and pathogenicity. J. Nat. Hist. 27, 993–1001 (1993).
Ortiz-Catedral, L. et al. Haemoproteus minutus is highly virulent for Australasian and South American parrots. Parasit. Vectors. 12, 40 (2019).
Valkiūnas, G. & Iezhova, T. A. Exo-erythrocytic development of avian malaria and related haemosporidian parasites. Malar. J. 16, 101 (2017).
Cuevas, E. et al. Latitudinal gradients of haemosporidian parasites: Prevalence, diversity and drivers of infection in the Thorn-tailed Rayadito (Aphrastura spinicauda). Int. J. Parasitol. Parasites Wildl. 11, 1–11 (2019).
Nourani, L., Zakeri, S. & Dinparast Djadid, N. Dynamics of prevalence and distribution pattern of avian Plasmodium species and its vectors in diverse zoogeographical areas—A review. Infect. Genet. Evol. 81, 104244 (2020).
Gil, J. A., Blanco, J. M., Höfle, U. & Alcántara, M. Estado sanitario de pollos de quebrantahuesos (Gypaetus barbatus) en el Pirineo central (Aragón-España). AODA. 2012–2014(8), 47–58 (2017).
Corbière, F. et al. Bluetongue virus serotype 1 in wild ruminants, France, 2008–10. J. Wildl. Dis. 48, 1047–1051 (2012).
Zamora-Vilchis, I., Williams, S. E. & Johnson, C. N. Environmental temperature affects prevalence of blood parasites of birds on an elevation gradient: Implications for disease in a warming climate. PLoS ONE. 7, e39208 (2012).
OPCC-CTP (Pyrenean Climate Change Observatory) Crops and mountain agro-pastoralism. In Climate Change in the Pyrenees: Impacts, Vulnerabilities and Adaptation Bases of Knowledge for the Future Climate Change Adaptation Strategy in the Pyrenees Vol. 8 (eds Terrádez, J. & Arauzo, I.) 87–100 (Collserola, 2018).
European Food Safety Authority & et al,. Avian influenza overview October 2016-August 2017. EFSA J. 15, e05018 (2017).
Delogu, M. et al. Virological investigation of avian influenza virus on postglacial species of phasianidae and tetraonidae in the Italian alps. ISRN Vet. Sci. 2013, 601732. https://doi.org/10.1155/2013/601732 (2013).
Schales, K., Gerlach, H. & Kösters, J. Investigations on the aerobic flora and Clostridium perfringens in fecal specimens from free-living and captive capercaillies (Tetrao urogallus L., 1758). Zentralbl Veterinarmed B. 40, 469–477 (1993).
Díaz-Sánchez, S., Mateo-Moriones, A., Casas, F. & Höfle, U. Prevalence of Escherichia coli, Salmonella sp. and Campylobacter sp. in the intestinal flora of farm-reared, restocked and wild red-legged partridges (Alectoris rufa): Is restocking using farm-reared birds a risk?. Eur. J. Wildl. Res. 58, 99–105 (2012).
Botti, V. et al. Salmonella spp. and antibiotic-resistant strains in wild mammals and birds in north-western Italy from 2002 to 2010. Vet. Ital. 49, 195–202 (2013).
Wienemann, T. et al. The bacterial microbiota in the ceca of Capercaillie (Tetrao urogallus) differs between wild and captive birds. Syst. Appl. Microbiol. 34, 542–551 (2011).
Acknowledgements
We thank the Conselh Generau d’Aran, the Departament of Climatic Action, Food and Rural Agenda of the Government of Catalonia, the Ministry for the Ecological Transition and the Demographic Challenge of the Government of Spain, the Departament de Medi Ambient i Sostenibilitat of Govern d’Andorra, Forestal Catalana, Paisatges Vius Foundation, Parc Natural de l’Alt Pirineu, Elena Vega, Carles Juan Sallés (Noah’s Path), Cos d’Agents Rurals and Centre de Ciència i Tecnologia Forestal de Catalunya for their administrative and technical support.
Funding
We thank the Association of Avian Veterinarians, Government of Catalonia, and Conselh Generau d’Aran for the financial support. CS and IS receive Juan de la Cierva incorporación (IJC2020–046019-I) and Juan de la Cierva formación (process number FJC2020–046311–I) fellowships, respectively, granted by Agencia Estatal de Investigación/Ministerio de Ciencia e Innovación, Spain.
Author information
Authors and Affiliations
Contributions
Perform experiments O.N.F, I.S., R.V., I.A., B.M.-M, F.E., C.S. Analyze experiments O.N.F, I.S., A.C.E., R.V., I.A., D.G.-F., B.M.-M, F.E., I.I., A-de la T., A.M and C.S. Design the study O.N.F., A.C.E., F.E., A.M and C.S. Wrote the paper O.N.F., I.S., A.C.E., and C.S. with the contribution of all coauthors. Supervise the study F.E., A.M., and C.S. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Francisco, O.N., Sacristán, I., Ewbank, A.C. et al. First detection of herpesvirus and hemosporidians in the endangered Pyrenean Capercaillie (Tetrao urogallus aquitanicus). Sci Rep 13, 21936 (2023). https://doi.org/10.1038/s41598-023-48123-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48123-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.