Abstract
Immuno-oncology (IO) combination therapy is utilized as a first-line systemic treatment for advanced renal cell carcinoma. However, evidence supporting the use of cabozantinib after IO combination therapy is lacking. We retrospectively analyzed patients who received second-line cabozantinib after IO combination therapy using the Japanese Urological Oncology Group (JUOG) database. In total, 254 patients were enrolled in the JUOG global study, and 118 patients who received second-line cabozantinib comprised the study cohort. The objective response rate, disease control rate, second-line cabozantinib progression-free survival (PFS), and overall survival from second-line for overall were 32%, 75%, 10.5 months, and not reached, respectively, for first-line IO-IO therapy were 37%, 77%, 11.1 months, and not reached, respectively, versus 24%, 71%, 8.3 months, and not reached, respectively, for first-line IO-tyrosine kinase inhibitor therapy. In univariate and multivariate analyses, discontinuation of first-line treatment because of progressive disease and liver metastasis were independent risk factors for PFS. All-grade adverse events occurred in 72% of patients, and grade 3 or higher adverse events occurred in 28% of patients. Second line-cabozantinib after first-line IO combination therapy for advanced renal cell carcinoma was expected to be effective after either IO-IO or IO-TKI treatment and feasible in real-world practice.
Similar content being viewed by others
Introduction
The estimated number of new cases of kidney cancer globally was 431,288 in 2020, and the disease was linked to 179,368 deaths1. Currently, immuno-oncology (IO) combination therapy is commonly used as a systemic treatment for advanced renal cell carcinoma (RCC)2,3,4,5,6. The emergence of this strategy has resulted in changes in both first- and second-line therapy. A promising phase 3 trial assessing second-line therapy after IO combination therapy is underway, but the results have not yet been published7. The tyrosine kinase inhibitor (TKI) cabozantinib is frequently used in clinical trials and clinical practice in the first-line setting both as monotherapy and in combination with IO therapy. In fact, the phase 3 METEOR trial compared cabozantinib with everolimus in patients previously treated with TKI monotherapy8. However, there are little data assessing the use of this treatment after IO combination therapy despite its frequent clinical use. It is important to recognize the therapeutic outcomes of cabozantinib after IO combination therapy in real-world settings. Some new second-line treatments including combination regimens could be approved in the future. Such treatments might not be suitable for all patients, especially those at higher risk of adverse events (AEs). There is a need to confirm the efficacy and safety of cabozantinib after IO combination therapy, which has not yet been verified on a large scale, using large-scale real-world clinical data. It is also necessary to clarify the outcomes of sequential treatments after cabozantinib. In this study, we assessed the use of cabozantinib in the second-line setting after IO combination therapy using large-scale study data from the Japanese Urological Oncology Group (JUOG) study data. This study is novel in that it examines the therapeutic outcomes, evaluation of progression risk factor and outcomes of sequential treatments of cabozantinib after IO combination in a real clinical setting on a large scale.
Methods
Thirty-five university hospitals and cancer centers participated in this large-scale retrospective study in Japan. Patients with advanced renal cancer who started second-line therapy after the approval of nivolumab plus ipilimumab in 2018 were eligible. We retrospectively analyzed data for patients who received second-line cabozantinib therapy after IO combination therapy. This study was approved by the institutional review board of Hamamatsu University School of Medicine (No. 22-008). The institutional review board of Hamamatsu University School of Medicine approved that this research was properly conducted in an opt-out format. All methods were performed in accordance with the relevant guidelines and regulations by including a statement.
IO combination therapy includes IO-IO and IO-TKI. In this study, IO-IO is nivolumab + ipilimumab and IO-TKI is avelumab or pembrolizumab + axitinib. Second-line cabozantinib progression was defined as progression after administration of cabozantinib. Death after administration of cabozantinib was defined as an OS event with reference to past similar reports9.
The following patient data were retrospectively collected from hospital records: age, sex, smoking status, Eastern Cooperative Oncology group performance status (ECOG PS), primary tumor diameter, first-line combination therapy regimen, nephrectomy, histology, presence of sarcomatoid change, IMDC risk in the first and second lines, Best overall response (BOR) in the first and second lines, second-line cabozantinib progression free survival (PFS) and overall survival (OS) from second-line, reason for discontinuing first-line IO combination therapy, duration between first-line combination therapy and second-line cabozantinib therapy, metastatic organ during second-line cabozantinib therapy, lactate dehydrogenase levels, albumin levels, estimated glomerular filtration rate (eGFR), C-reactive protein levels at the time of cabozantinib initiation and third-line PFS and overall survival OS from third-line. All AEs that occurred during cabozantinib administration were collected.
All statistical analyses were performed using JMP Pro 16.0.1 (SAS Institute, Cary, NC, USA). A two-sided P-value of < 0.05 denoted statistical significance. Intergroup comparisons were performed using the Mann–Whitney U test and χ2 test. Risk factors related to progression were analyzed by the Cox proportional hazards model and Kaplan–Meier method. Variables significant at P < 0.05 in univariate analysis were included in multivariate analysis.
Results
In total, 254 patients were enrolled in the JUOG global study, of whom 118 were included in this analysis. In the first-line setting, 73 patients received IO-IO therapy, and 45 patients received IO-TKI therapy. IO-IO consisted of nivolumab plus ipilimumab in all cases. Ten patients in the IO-TKI group received avelumab plus axitinib, and 35 received pembrolizumab plus axitinib.
Patients’ background
Table 1 presents the patients’ background data. The median patient age was 67 years. The median duration of observation from second-line cabozantinib initiation was 10.5 months. In total, 60% of patients underwent nephrectomy, and 76% had clear cell histology. The IMDC risk at the start of first-line therapy was favorable, intermediate, and poor in 13%, 54%, and 31% of patients, respectively. The objective response rate (ORR) in the first-line setting was 33%. Meanwhile, 77% of patients discontinued first-line treatment because of disease progression, whereas 23% discontinued treatment because of AEs. The period between first-line combination therapy and second-line cabozantinib therapy was 6.7 months in the entire population, 7.8 months in the IO-IO group, and 6.4 months in the IO-TKI group. There were no differences in background data between the IO-IO and IO-TKI groups.
Efficacy of second-line cabozantinib
Table 2 presents the BOR, PFS, and OS by first-line therapy. Overall objective response rate and disease control rate (DCR) were 32% and 75%. The IO-TKI group had a slightly lower ORR than the IO-IO group (37% vs. 24%). The DCR was 77% in the IO-IO group, versus 71% in the IO-TKI group. The BOR was progressive disease (PD) in 18% of patients in the IO-IO group and 22% of patients in the IO-TKI group.
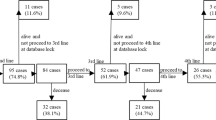
Figure 1 presents PFS for second-line cabozantinib treatment. PFS in the entire cohort was 10.5 months (95% confidence interval [CI] = 8.5–15.4). PFS in the IO-IO group was 11.1 months (95% CI = 9.1–16.9), compared with 8.3 months (95% CI = 5.2–not evaluable [NE]) in the IO-TKI group. Figure 2 presents OS for second-line cabozantinib treatment. OS for the total population was not reached (NR) (95% CI = 15.3–NE, 6-month OS rate was 84%, 12-month OS rate was 71%). OS was NR in both the IO-IO (95% CI = 15.1–NE, 6-month OS rate was 86%, 12-month OS rate was 74%) and IO-TKI groups (95% CI = 12.1–NE, 6-month OS rate was 82%, 12-month OS rate was 68%).
Progression-free survival for second-line cabozantinib after first-line immuno-oncology (IO) combination therapy. Rates were estimated using the Kaplan–Meier method. (a) All patients. (b) In patients who received first-line IO-IO therapy. (c) Patients who received first-line IO-tyrosine kinase inhibitor therapy. NE not evaluable.
Overall survival for second-line cabozantinib after first line immuno-oncology (IO) combination therapy. Rates were estimated using the Kaplan–Meier method. (a) All patients. (b) In patients who received first-line IO-IO therapy. (c) Patients who received first-line IO-tyrosine kinase inhibitor therapy. NR not reached, NE not evaluable.
We examined the risk factors for PFS in all 118 patients (Table 3). According to univariate analysis, the reason for first-line treatment discontinuation (PD vs. AE: hazard ratio [HR] = 2.84, P = 0.0284), ECOG PS (0 vs. 1–2: HR = 0.35, P = 0.0157), and liver metastasis (present vs. absent: HR = 6.43, P = 0.0003) were risk factors for PFS. According to multivariate analysis, the reason for first-line treatment discontinuation and liver metastasis were independent risk factors for PFS. PFS according to these risk factors is presented in Fig. 3. Supplementary Fig. S1 presents PFS by reason for discontinuing each first-line regimen. In the IO-IO group, PFS tended to be worse for patients who discontinued treatment because of PD than for those who discontinued because of AEs.
Progression-free survival for second-line cabozantinib after first-line immuno-oncology combination therapy based on independent risk factors. Rates were estimated using the Kaplan–Meier method. (a) Reason for first-line treatment discontinuation. (b) Presence of liver metastasis. AE adverse event, PD progressive disease, HR hazard ratio, CI confidence interval.
Safety of second-line cabozantinib
AEs of any grade occurred in 72% of patients, whereas grade 3 or higher AEs occurred in 28% of patients (Table 4). Hypertension and hand–foot syndrome were the most frequent AEs, followed by liver dysfunction, fatigue, and diarrhea. There were no Grade 5 AEs. Select AEs are presented in Supplementary Table S1.
Efficacy of third-line treatment after cabozantinib
Finally, we analyzed PFS and OS in the third-line setting after second-line cabozantinib treatment in 49 patients (Fig. 4). The third-line therapy was axitinib in 19 patients, nivolumab in 12 patients, pazopanib in 6 patients, sunitinib in 5 patients, everolimus in 3 patients, temsirolimus in 1 patient, and others in 3 patients. In this group, PFS was 3.9 months (95% CI = 1.8–6.6), whereas OS was 7.9 months (95% CI = 5.0–NE).
Institutional review board statement
This study was approved by the Institutional Review Board of Hamamatsu Medical University Hospital (IRB No. 22-008).
Informed consent
Informed consent was obtained by the opt-out method in the study. The requirement for patient consent was waived because this was a retrospective review of medical practice covered by health insurance.
Discussion
In this study, we analyzed the actual clinical situation of cabozantinib as a second-line treatment after IO combination therapy using large-scale retrospective data from JUOG. Although this study included a large cohort and the treatment strategies slightly differed at each facility, it is meaningful to investigate the actual situation of second-line treatment after IO combination treatment in Japan in recent years. The purpose of this study was to clarify the utility of second-line cabozantinib treatment after IO combination therapy. The ORR for second-line cabozantinib after IO combination therapy was 32%, PFS was 10.5 months, and OS was NR (6-month OS rate was 84%, 12-month OS rate was 71%). The factors significantly associated with efficacy were first-line treatment discontinuation because of PD and liver metastasis. None of the AEs significantly exceeded those previously reported. Sequential treatment remains important for metastatic RCC treatment. Third-line treatment after cabozantinib administration was associated with PFS and OS of 3.9 and 7.9 months, respectively, indicating that efficacy can be expected after cabozantinib treatment.
Based on the results of the phase 3 METEOR trial8, we are using cabozantinib in clinical practice. Compared with everolimus, cabozantinib provided clear benefits for the primary endpoint of PFS and secondary endpoint of OS. The AEs of this drug were also manageable. However, in METEOR trial, approximately 5% of patient received IO before cabozantinib. It is unlikely that this situation reflects the efficacy of cabozantinib after IO administration.
The recently reported phase 3 CONTACT-03 trial evaluated the efficacy of cabozantinib in patients previously treated with IO10. This trial compared the efficacy of cabozantinib alone and in combination with atezolizumab. First, it should be noted that the cabozantinib monotherapy group had median PFS of 10.8 months, in line with the current study results. The results support the efficacy of cabozantinib as sequential treatment in the IO era. Median OS was NR. The results of this phase 3 trial failed to demonstrate the efficacy of add-on atezolizumab. The BREAKPOINT trial, a phase 2 study in 31 cases has been reported. Median PFS was 8.3 months, OS was 13.8 months and ORR was 37.9%. Grade 3–4 adverse events occurred in 47%. Although this study involved a relatively small number of cases, it is a very important result when considering the theme of this paper9.
The After-IO trial was conducted as a follow-up study of a phase 3 trial of patients treated with nivolumab11. Axitinib and other TKIs have produced high response rates. However, information on cabozantinib was lacking in this study because of the timing of its approval. A report from the Italian Meet Uro 7 group provided real-world results for cabozantinib after IO monotherapy12. According to this study, cabozantinib was linked to longer PFS than everolimus and other TKIs. In addition, cabozantinib is the most frequently selected drug in clinical practice. Thus, the results of the Italian study do not reflect the efficacy of cabozantinib in clinical practice after IO combination therapy, which is currently the mainstream strategy.
The reason for first-line treatment discontinuation was an independent predictor of PFS in patients treated with cabozantinib. In patients who discontinued first-line therapy because of AEs, median PFS was not reached in the IO-IO or IO-TKI group. We believe that cabozantinib administration in a state of relatively high drug sensitivity led to good results. The time from first-line treatment to cabozantinib administration was not a significant predictor of PFS. In this regard, some patients are expected to be highly sensitive to drug therapy in general. Conversely, second-line treatment is not effective in some patients with rapid tumor growth and a short period between first- and second-line treatment. We anticipate that the efficacy of cabozantinib will be limited in some patients with accelerated tumor growth after PD. Median PFS did not reach 10 months in patients who discontinued first-line treatment because of PD, highlighting the need for new systemic drugs.
Analyses have been conducted by metastatic organ in patients with RCC. Metastasis to the liver, bone, and brain is associated with poor prognoses. Xue et al. also found that liver metastases carried the worst prognosis13. In our study, the efficacy of cabozantinib in patients with bone and brain metastases was relatively satisfactory. Cabozantinib has inhibitory effects on MET and AXL, and it is expected to be effective against bone metastasis14. The METEOR trial also recorded a high response rate in patients with bone metastasis8. Cabozantinib exhibited considerable intracranial activity and an acceptable safety profile in patients with RCC and brain metastases15. In this study, we were unable to examine the details of local treatment of the brain. In addition, few cases of brain metastasis have been investigated, and further analysis is required. A certain effect has been demonstrated, as indicated by the results of this study. It is suggested that efficacy can be expected even after IO combination treatment in these metastatic organs. Meanwhile, the efficacy in patients with liver metastasis was limited. Liver metastases from RCC are reported to carry a poorer prognosis than liver metastases from other cancer types treated with IO16. Several reports discussed poor prognosis associated with liver metastases. James et al. found that the presence of liver metastasis significantly reduced tumor-specific immunity in an antigen-specific, PD-1-dependent manner. This process was associated with the coordinated activation of regulatory T cells and modulation of intratumoral CD11b+ monocytes17. The presence of liver metastasis was correlated with fewer CD8+ T cells at the invasive margin in distant tumors18. We expect new systemic treatments in the future for patients with liver metastasis.
The evaluation of AEs is expected to differ between retrospective studies and clinical trials. In addition, this study was based on multi-institutional data, and there are disparities in the awareness of AEs among institutions. The rate of all-grade AEs was somewhat low compared with the findings for cabozantinib in clinical trials8. This finding should not be interpreted as a low incidence of AEs in the Japanese population but rather as a limitation of information collection. However, the incidence of grade 3 or higher AEs was 28%, in line with prior findings8. These data serve as an index for Japanese data after IO combination treatment. No grade 5 AEs were observed in this population. It is considered that cabozantinib can be used safely in this population after molecular targeted therapy. A report found that Japanese patients are relatively prone to liver dysfunction19, and the present data recorded Grade 3 or higher liver dysfunction in six patients, which should be noted.
Expectations for sequential therapy might be lower than that in the previous age of molecular targeted drugs, but in real-world practice, sequential systemic therapy is often used to treat metastatic RCC. When performing sequential treatment, it is desirable to avoid the situation in which subsequent therapeutic effects are not anticipated. Cabozantinib is a relatively effective TKI, and the effects of subsequent systemic treatment after cabozantinib were analyzed in this study. Although PFS and OS were not substantially extended, a certain effect can be expected in patients who started third-line treatment. Luigi et al. summarized systemic treatment after cabozantinib in 56 patients. Median OS after cabozantinib was 7.7 months, while median TTF after cabozantinib was 2.8 months. However, only three of the participants in this study used IO combination as first-line treatment20. There are no large-scale reports of the use of cabozantinib after IO combination followed by systemic treatment. Of course, some patients receive best supportive care after cabozantinib administration, suggesting that AE management is possible.
Several combination treatments have been reported as second-line treatments after IO combination in recent years. One study verified the effect of adding atezolizumab to cabozantinib10. Unfortunately, no benefit was observed. Another phase 2 single-arm trial examined combination therapy with cabozantinib and belzutifan, in which PFS was 13.8 months21. Although a simple comparison cannot be made, the addition of belzutifan could be promising given the PFS of approximately 10 months in our study and the aforementioned CONTACT03 trial10. The phase 3 LITESPARK011 trial comparing cabozantinib with the combination of lenvatinib and belzutifan is currently underway7. The result of this study should be watched closely. It is hoped that the approval of these promising treatments will lead to improved prognoses in patients who discontinued IO combination therapy because of PD and patients with liver metastases, who had poor prognoses in this study.
This research had some limitations. First, this was a retrospective study. Treatment selection was left to the discretion of each facility, leading to varied treatments. In addition, this research used information from facilities such as university hospitals and cancer centers, and there is a possibility that the protocols of these situations slightly deviate from those used in hospitals throughout Japan. At the time of enrollment in this study, no patients in whom lenvatinib plus pembrolizumab was discontinued and cabozantinib was administered were included. In actual clinical practice, this strategy is frequently employed. New research is expected to clarify this issue in the future. We were not able to verify the effects of cabozantinib at different starting doses in this study. This is an issue that should be verified in future research. Phase II CaboPoint trial is now on-going22. It is hoped that the results will become clearer once the results of this trial are published.
In this study, we analyzed the real-world data of cabozantinib in Japan after IO combination therapy using the JUOG database. A relatively high response rate was obtained even after IO combination therapy, and AE management was possible. The results of this relatively large-scale study clarified the usefulness of cabozantinib and identified factors associated with poor efficacy, namely first-line treatment discontinuation because of PD and liver metastasis.
Conclusions
To the best of our knowledge, this is the first study to evaluate the efficacy and safety of second-line cabozantinib therapy after first-line IO combination treatment for advanced RCC using a Japanese multi-institutional database. ORR was 32%, DCR was 75%, median PFS was 10.5 months, and median OS was NR. No adverse events were reported after IO combination therapy.
Data availability
The data presented in this study are available on request from the corresponding author.
References
Bukavina, L. et al. Epidemiology of renal cell carcinoma: 2022 update. Eur. Urol. 82, 529–542 (2022).
Albiges, L. et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: Extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 5(6), e001079 (2020).
Choueiri, T. K. et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 384(9), 829–841 (2021).
Haanen, J. et al. Extended follow-up from JAVELIN Renal 101: Subgroup analysis of avelumab plus axitinib versus sunitinib by the International Metastatic Renal Cell Carcinoma Database Consortium risk group in patients with advanced renal cell carcinoma. ESMO Open 8(3), 101210 (2023).
Motzer, R. et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N. Engl. J. Med. 384(14), 1289–1300 (2021).
Plimack, E. R. et al. Pembrolizumab plus axitinib versus sunitinib as first-line treatment of advanced renal cell carcinoma: 43-month follow-up of the phase 3 KEYNOTE-426 study. Eur. Urol. 84(5), 449–454 (2023).
Motzer, R. J. et al. LITESPARK-011: Belzutifan plus lenvatinib vs cabozantinib in advanced renal cell carcinoma after anti-PD-1/PD-L1 therapy. Future Oncol. 19(2), 113–121 (2023).
Choueiri, T. K. et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 17(7), 917–927 (2016).
Procopio, G. et al. A multicenter phase 2 single arm study of cabozantinib in patients with advanced or unresectable renal cell carcinoma pre-treated with one immune-checkpoint inhibitor: The BREAKPOINT trial (Meet-Uro trial 03). Tumori 109(1), 129–137 (2023).
Pal, S. K. et al. Atezolizumab plus cabozantinib versus cabozantinib monotherapy for patients with renal cell carcinoma after progression with previous immune checkpoint inhibitor treatment (CONTACT-03): A multicentre, randomised, open-label, phase 3 trial. Lancet 402(10397), 185–195 (2023).
Tomita, Y. et al. Erratum to: Subgroup analysis of the AFTER I-O study: A retrospective study on the efficacy and safety of subsequent molecular targeted therapy after immune-oncology therapy in Japanese patients with metastatic renal cell carcinoma. Jpn. J. Clin. Oncol. 51(12), 1770 (2021).
Santini, D. et al. Clinical outcomes of metastatic renal carcinoma following disease progression to programmed death (PD)-1 or PD-L1 inhibitors (IO): A Meet-URO Group Real World Study (Meet-Uro 7). Am. J. Clin. Oncol. 44(3), 121–125 (2021).
Xue, J. et al. Patterns of distant metastases in patients with clear cell renal cell carcinoma—A population-based analysis. Cancer Med. 10(1), 173–187 (2021).
Grüllich, C. Cabozantinib: Multi-kinase inhibitor of MET, AXL, RET, and VEGFR2. Recent Results Cancer Res. 211, 67–75 (2018).
Hirsch, L. et al. Clinical activity and safety of cabozantinib for brain metastases in patients with renal cell carcinoma. JAMA Oncol. 7(12), 1815–1823 (2021).
Tian, B. W. et al. Effect of liver metastasis on the efficacy of immune checkpoint inhibitors in cancer patients: A systemic review and meta-analysis. Clin. Exp. Metastasis 40(4), 255–287 (2023).
Lee, J. C. et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci. Immunol. 5(52), eaba0759 (2020).
Tumeh, P. C. et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol. Res. 5(5), 417–424 (2017).
Tomita, Y. et al. Cabozantinib in advanced renal cell carcinoma: A phase II, open-label, single-arm study of Japanese patients. Int. J. Urol. 27(11), 952–959 (2020).
Cerbone, L. et al. Activity of systemic treatments after cabozantinib failure in advanced metastatic renal cell carcinoma. Clin. Genitourin. Cancer 20(1), 80–87 (2022).
Choueiri, T. K. et al. Belzutifan plus cabozantinib for patients with advanced clear cell renal cell carcinoma previously treated with immunotherapy: An open-label, single-arm, phase 2 study. Lancet Oncol. 24(5), 553–562 (2023).
Albiges, L. et al. CaboPoint: A phase II study of cabozantinib as second-line treatment in patients with metastatic renal cell carcinoma. Future Oncol. 18(8), 915–926 (2022).
Acknowledgements
We thank the JUOG members. We additionally thank Joe Barber Jr., PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
The present study was supported by a Grant-in-Aid for Scientific Research (C) (22K09494 to T.S.).
Author information
Authors and Affiliations
Contributions
T.S. contributed to data collection and analysis, table and figure preparation, reference collection, and manuscript writing. Y.M. and H.M. contributed to data collection and analysis and supervised all activities. H.S., T.O., N.H., S.H., K.N., K.U., T.K., M.T., H.T., Y.K., T.K., T.K., K.F., M.M., and T.K. contributed to data collection. H.K and T.I. contributed to the supervision of all activities. The first draft of the manuscript was prepared by T.S. All authors have read and approved the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Tomokazu Sazuka has received honoraria from Takeda and BMS. Takahiro Osawa has received honoraria from Takeda and ONO. Takahiro Kimura is a paid consultant/advisor of Astellas, AstraZeneca, Bayer, Janssen, and Sanofi. Masayuki Takahashi has received honoraria from MSD, Eizai, Merck and Pfizer. Kazutoshi Fujita has received honoraria from ONO, BMS, MSD, Pfizer and Takeda. Hiroshi Kitamura has received honoraria from BMS and MSD, Research expenses from BMS and MSD. The funders had no role in the design of the study; data collection, analysis, and interpretation; manuscript writing; or the decision to publish the results.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sazuka, T., Matsushita, Y., Sato, H. et al. Efficacy and safety of second-line cabozantinib after immuno-oncology combination therapy for advanced renal cell carcinoma: Japanese multicenter retrospective study. Sci Rep 13, 20629 (2023). https://doi.org/10.1038/s41598-023-48087-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48087-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.