Abstract
There is mounting evidence that malnutrition and systemic inflammation status are involved in the prognosis of various cancers. In this study, we aimed to evaluate the prognostic value of the pretreatment fibrinogen-albumin ratio index (FARI) in nasopharyngeal carcinoma (NPC) patients receiving definite radiotherapy. NPC patients who received definite radiotherapy between January 2013 and December 2019 were included. A receiver operating characteristic (ROC) curve was used to determine the optimal cutoff value. The clinicopathological characteristics of the patients were compared via the Chi-square test. Survival curves were analyzed by the Kaplan‒Meier method. The prognostic factors were evaluated by univariate and multivariate analyses via Cox hazards regression analysis. A total of 225 patients were enrolled, and the median follow-up time was 48.5 months. High FARI was correlated with worse ECOG score (p = 0.003), higher EBV-DNA titer (p = 0.047), and more advanced clinical stage (p < 0.001). In the multivariable analysis, FARI independently predicted OS (HR 2.399, 95% CI 1.294–4.450, P < 0.001), PFS (HR 2.085, 95% CI 1.200–3.625, P = 0.009), and DMFS (HR 2.527, 95% CI 1.288–4.958, P < 0.001). The current findings suggest that a high pretreatment FARI is an independent predictor of OS, PFS and DMFS in NPC patients undergoing definite radiotherapy.
Similar content being viewed by others
Introduction
Nasopharyngeal carcinoma (NPC) is the most common head and neck malignancy in southern China and southeast Asia1. Radiotherapy is the cornerstone of treatment due to the special anatomical site and radiation sensitivity of the tumor. With the development of radiotherapy and medical treatments, the prognosis of NPC patients, especially locally advanced NPC patients, has significantly improved2. However, approximately 10% of patients will still suffer from relapse, and 20% will suffer from metastasis3. Thus, exploring effective and economical biomarkers to predict the prognosis of NPC patients and facilitate patient stratification and treatment modification is urgently needed.
Promotion of inflammation and deregulation of metabolism are widely accepted as a hallmark of cancer4. Increasing evidence has shown that the inflammatory and nutritional status of the host is closely related to treatment sensitivity and prognosis. Albumin is the classical biomarker for nutritional status, and previous studies have shown that it is a prognostic factor in various malignancies, including hepatocellular carcinoma, esophageal carcinoma and non-small cell lung cancer5,6,7. Fibrinogen is an important coagulation factor in the blood. However, increasing evidence has shown that fibrinogen also acts as an inflammatory mediator that is involved in systemic inflammation, cancer cell adhesion, and cancer progression8,9. Basic studies have shown that fibrinogen could affect innate immunity by inhibiting the function of macrophages and NK cells. The correlation between plasma fibrinogen level and prognosis has been reported in several kinds of cancers10,11,12. FARI is an index that reflects measures of both albumin and fibrinogen. It has been reported that FARI is an independent prognostic factor in several kinds of cancers, including head and neck squamous cell carcinoma13, gastric cancer14, cholangiocarcinoma15, and pancreatic ductal adenocarcinoma16. However, the prognostic role of FARI in NPC patients has not yet been reported.
In the present study, we retrospectively analyzed the prognostic value of FARI, as well as other nutritional and inflammation indices, in a cohort of NPC patients.
Methods and materials
Patients
The data from patients diagnosed with NPC who underwent definitive radiotherapy, with or without chemotherapy, at the Second Xiangya Hospital, Central South University from January 2013 to December 2019 were retrospectively analyzed. The exclusion criteria were as follows: (1) patients with incomplete clinical-pathological data; (2) patients with missing laboratory test results; (3) patients with incomplete follow-up data; (4) patients with a history of chronic inflammatory diseases such as inflammatory bowel disease; and (5) patients with recent acute infectious diseases. The research was carried out in line with the Declaration of Helsinki and approved by the Ethics Committee of the Second Xiangya Hospital of Central South University, and informed consent was waived.
Data collection
The demographic and clinical pathological data and laboratory results were obtained from the hospital medical records system. Data regarding patients’ age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS) scores, T stage, N stage, clinical stage, treatment modality, height, body weight, neutrophil, lymphocyte, platelet, monocyte, serum albumin, plasma fibrinogen, and EBV-DNA were collected. The plasma fibrinogen was tested via the Clauss method, and the serum albumin was tested via the bromocresol green method. The method of quality control is to use two levels of quality control products for machine testing, and the error should be within the range set by the laboratory. The FARI was calculated as follows: FARI = fibrinogen/albumin.
RT procedure
All patients were treated with intensity-modulated radiation therapy (IMRT). The gross tumor volume and clinical tumor volume were defined according to the guidelines17. The prescribed dose was 70 Gy/2.12 Gy/33 F for PGTV, 60 Gy/1.82 Gy/33 F for PTV1, and 54 Gy/1.64 Gy/33 F for PTV2. Radiotherapy was delivered once daily 5 days per week. Concurrent chemotherapy (cisplatin or nedaplatin) and targeted therapy (nimotuzumab) were administered according to the stage and tolerability of the patients.
Follow-up
Patients were followed up by telephone review or inpatient and outpatient medical records. The last follow-up date was April 30, 2023. Overall survival (OS) was calculated from the date of diagnosis to the date of death from any cause or to the last date of follow-up. Progression-free survival (PFS) was defined between the date of diagnosis and the date of disease progression or death.
Statistical analysis
SPSS statistical software (version 22.0; SPSS Inc., Chicago, IL, USA) was used for data analysis. Receiver operating characteristic (ROC) curves were used to calculate the cutoff value for FARI. The Chi-square test was used to analyze the relationship between FARI and clinicopathological features. The Kaplan–Meier method was used to calculate survival curves. Multivariate analysis was based on the Cox regression model. A two-sided p value < 0.05 was considered statistically significant.
Ethics statement
This research was carried out in line with the Declaration of Helsinki and approved by the Ethics Committee of the Second Xiangya Hospital of Central South University. The Ethics Committee of the Second Xiangya Hospital of Central South University waived the requirement of written informed consent.
Results
Patient characteristics
The characteristics of 225 enrolled NPC patients are presented in Table 1. The median age was 49 years (range: 15–71 years). One hundred and sixty-two out of 225 (72.0%) were male. One hundred thirteen patients (59.1%) had an ECOG score of 0. The mean body mass index (BMI) was 23.56 ± 3.38 kg/m2, with 8.4% of patients being underweight. Sixty-one patients (27.1%) had a positive EBV-DNA test, which was defined as more than 400 copies/ml, and 81 patients (36.0%) had a negative EBV-DNA test. However, there were 83 patients (36.9%) whose EBV-DNA status was unknown. Thirty-four patients (15.1%) had stage II tumors, 132 patients (58.7%) had stage III tumors, and 59 patients (26.2%) had stage IVa tumors according to the AJCC 8th edition staging system. Among all the patients, 71 (31.6%) received concurrent chemoradiotherapy (CCRT), and the rest of the patients received radiotherapy or radiotherapy concurrent with other drugs (nimotuzumab, etc.). With a median follow-up time of 48.5 months (range: 5.7–117.3 months), 19 (8.4%) patients suffered from recurrence, 46 (20.4%) patients had experienced metastasis, and 53 (23.6%) patients died.
Cutoff value of FARI and the association with clinicopathological characteristics
By setting overall survival status as the endpoint, ROC analysis was used to determine the cutoff value of FARI, and the optimal cutoff value of FARI was 0.0761 (Fig. 1). All patients were categorized into a high FARI group and a low FARI group according to the cutoff value. The association between FARI and the clinicopathological characteristics of the patients is shown in Table 2. High FARI was associated with worse ECOG score (p = 0.003), higher EBV-DNA titer (p = 0.047), and more advanced T stage and clinical stage (p < 0.001). FARI status was not associated with age, sex, BMI, N stage or treatment modality (all p > 0.05).
Prognostic value of FARI
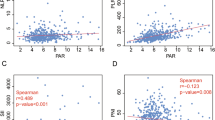
Kaplan‒Meier analysis showed that FARI (p < 0.001), clinical stage (p = 0.010), T stage (p = 0.034), N stage (p = 0.022), and EBV-DNA (p = 0.025) were associated with patient OS. (Table 3, Fig. 2A). FARI (p < 0.001), clinical stage (p = 0.003), T stage (p = 0.012), N stage (p = 0.015), ECOG (p = 0.030), and EBV-DNA (p = 0.025) were associated with patient PFS (Table 3, Fig. 2B). Only clinical stage (p = 0.021).
Kaplan–Meier survival curves of NPC patients (A–D). (A) Kaplan–Meier curves for OS according to FARI; (B) Kaplan–Meier curves for PFS according to FARI; (C) Kaplan–Meier curves for LRRFS according to FARI; (D) Kaplan–Meier curves for DMFS according to FARI; OS overall survival, PFS progression free survival, LRRFS local–regional relapse-free survival, DMFS distant metastasis-free survival.
and N stage (p = 0.022) were associated with patient LRRFS. (Table 3, Fig. 2C). FARI (p < 0.001), clinical stage (p = 0.012), T stage (p = 0.018), ECOG (p = 0.015), treatment modality (p = 0.045), and EBV-DNA (p = 0.006) were associated with patient DMFS (Table 3, Fig. 2D).
The variables that were found to be significantly correlated with patient prognosis, including clinical stage, ECOG, treatment modality, and FARI, were incorporated into the multivariate analysis. Cox regression analysis showed that clinical stage (HR 1.567, 95% CI 0.998–2.461, P = 0.051) and FARI (HR 2.399, 95% CI 1.294–4.450, P < 0.001) were independent prognostic factors of OS. Clinical stage (HR 1.607, 95% CI 1.050–2.461, P = 0.029) and FARI (HR 2.085, 95% CI 1.200–3.625, P = 0.009) were independent prognostic factors of PFS. Only clinical stage (HR 2.938, 95% CI 1.342–6.430, P = 0.005) was an independent prognostic factor of LRRFS. Clinical stage (HR 1.629, 95% CI 1.005–2.641, P = 0.048), treatment modality (HR 0.426, 95% CI 0.204–0.889, P = 0.023), and FARI (HR 2.527, 95% CI 1.288–4.958, P < 0.001) were independent prognostic factors of DMFS (Table 4).
Discussion
In the present study, we evaluated the prognostic importance of FARI in a cohort of 225 NPC patients. The results showed that high FARI was related to unfavorable clinical characteristics and outcomes. Furthermore, FARI was an independent prognostic factor of OS, PFS, and DMFS. To the best of our knowledge, this is the first report on the prognostic role of FARI in NPC patients. This result indicated that FARI may be a promising blood-based prognostic index for NPC patients.
Increasing evidence has shown that the systemic inflammatory response18,19,20 and malnutrition21,22,23 play a critical role in the development and progression of various malignancies. Fibrinogen is a classical coagulation-related protein; however, an increasing number of studies have proven that it is also a marker of systemic inflammation and is involved in the progression of cancer via multiple mechanisms. Fibrinogen could impair macrophage migration and prevent fibrinogen-leukocyte interactions by mutating the leukocyte integrin binding motif on fibrinogen, which harms the antitumor immunity of the host24. Fibrinogen could also block the ability of NK cells to clear tumor cells, facilitating evasion of immune surveillance and metastasis25. Fibrinogen could promote epithelial-mesenchymal transition (EMT) via the AKT-mTOR pathway8. Preclinical research has proven that suppression of fibrinogen by miRNA could decrease the metastatic potential of tumor cells in mouse lung cancer models26. Thus, decreasing fibrinogen might be a potential strategy to improve outcomes in cancer patients by minimizing metastasis.
Malnutrition is a common complication of cancer that is related to poorer quality of life, more treatment interruption, and worse prognosis27. Cancer-related symptoms, such as dysphagia and bowel obstruction, as well as cancer-induced excess catabolism and inflammation, could significantly affect nutritional status28. Albumin (ALB) is a typical repetitiveness of nutritional status that is synthesized by the liver and suppressed by malnutrition and systemic inflammation29. In clinical studies,
ALB30,31 and ALB-related indices, including the CRP-albumin ratio32 and prognostic nutritional index33, have been proven to be independent prognostic factors in various cancers, including NPC.
FARI is a comparatively new marker of nutrition-inflammation status. Several studies have reported the prognostic value of FARI in many cancers, including esophageal squamous cell carcinoma34, hepatocellular carcinoma35, pancreatic neuroendocrine neoplasms36, and gastric cancer37. Although the cutoff value of FARI varies in different studies, all of the studies showed that elevated FARI is an adverse factor in cancer patients. This result indicated that fibrinogen and albumin have consistent effects in different cancer patients. Our group previously also reported that FARI is an independent prognostic factor of OS in lung adenocarcinoma patients38 and head and neck squamous cell carcinoma patients13. In the present study, we found that FARI is an independent prognostic factor of OS, PFS, and DMFS, but not LRRFS, in NPC patients. This result provides further support for the idea that the immune-inflammation status mainly affects the prognosis of cancer patients by influencing metastasis, not local relapse, which is in accordance with a previous report on mechanical and clinical research.
There are several limitations in this study. First, this is a single-center retrospective study with a long time span that may inherently carry some bias. Second, EBV-DNA has widely been accepted as a prognostic factor in Chinese patients. Some research has indicated that EBV-DNA > 4000 copies/ml predicts a worse prognosis in NPC patients39. However, the testing procedure of EBV-DNA has not been standardized thus far, which makes the results from different centers incomparable. In our center, the cutoff value of EBV-DNA is 400 ml/copy. In the present study, only 63.1% of the patients had an EBV-DNA test result. We found in univariate analysis that EBV-DNA was a prognostic factor with a cutoff value of 400 copies/ml. However, due to the limited sample size, it was not included in the multivariate analysis. Third, due to the limited sample size, no internal or external validation cohort was set to confirm the results. Therefore, more studies are still needed to further verify the clinical importance of FARI in NPC patients.
Conclusions
In summary, a high FARI level is related to poorer prognosis, and FARI is an independent factor of OS, PFS and DMFS in NPC patients. It is an effective and economical marker that may facilitate prognosis stratification and indicate a new perspective in intervention strategies to improve the clinical outcomes of NPC patients.
Data availability
The raw data supporting the conclusions of this article will be made available by the corresponding author (email address: houtao@csu.edu.cn), without undue reservation.
References
Chen, Y. P. et al. Nasopharyngeal carcinoma. Lancet 394(10192), 64–80 (2019).
Li, J. Y., Chen, Y. P., Li, Y. Q., Liu, N. & Ma, J. Chemotherapeutic and targeted agents can modulate the tumor microenvironment and increase the efficacy of immune checkpoint blockades. Mol. Cancer 20(1), 27 (2021).
Zhang, Y. et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N. Engl. J. Med. 381(12), 1124–1135 (2019).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: The next generation. Cell 144(5), 646–674 (2011).
Jeng, L. B., Chan, W. L. & Teng, C. F. Prognostic significance of serum albumin level and albumin-based mono- and combination biomarkers in patients with hepatocellular carcinoma. Cancers (Basel) 15(4), 1 (2023).
Ji, S. et al. A combined immune prognostic index in esophageal squamous cell carcinoma patients treated with anti-PD-1 therapy. Ther. Adv. Med. Oncol. 15, 17588359231174868 (2023).
Zhang, C. L. et al. Research progress and value of albumin-related inflammatory markers in the prognosis of non-small cell lung cancer: A review of clinical evidence. Ann. Med. 55(1), 1294–1307 (2023).
Zhang, F. et al. Fibrinogen promotes malignant biological tumor behavior involving epithelial-mesenchymal transition via the p-AKT/p-mTOR pathway in esophageal squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 143(12), 2413–2424 (2017).
Angelidakis, E. et al. Impact of fibrinogen, fibrin thrombi, and thrombin on cancer cell extravasation using in vitro microvascular networks. Adv. Healthc. Mater. https://doi.org/10.1002/adhm.202202984e2202984 (2023).
Yunpeng, P. et al. Establishment and validation of a nomogram based on coagulation parameters to predict the prognosis of pancreatic cancer. BMC Cancer 23(1), 548 (2023).
Xu, J. et al. Preoperative alpha fetoprotein, total bilirubin, fibrinogen, albumin, and lymphocytes predict postoperative survival in hepatocellular carcinoma. Cancer Med. https://doi.org/10.1002/cam4.6030 (2023).
Wang, Y. et al. Plasma fibrinogen acts as a predictive factor for pathological complete response to neoadjuvant chemotherapy in breast cancer: A retrospective study of 1004 Chinese breast cancer patients. BMC Cancer 21(1), 542 (2021).
Wang, S. et al. High fibrinogen-albumin ratio index (FARI) predicts poor survival in head and neck squamous cell carcinoma patients treated with surgical resection. Eur. Arch. Otorhinolaryngol. 279(9), 4541–4548 (2022).
Shen, Y. et al. The prognostic value of FAR and a novel FAR-CA125 score in resectable gastric signet ring cell carcinoma patients. J. Cancer Res. Clin. Oncol. https://doi.org/10.1007/s00432-023-04870-4 (2023).
Liu, H., Qiu, G., Hu, F. & Wu, H. Fibrinogen/albumin ratio index is an independent predictor of recurrence-free survival in patients with intrahepatic cholangiocarcinoma following surgical resection. World J. Surg. Oncol. 19(1), 218 (2021).
Tomita, K. et al. Prognostic significance of plasma fibrinogen/serum albumin ratio in the postoperative outcome of pancreatic ductal adenocarcinoma. Anticancer Res. 40(12), 7017–7023 (2020).
Li, W. F. et al. Locoregional extension patterns of nasopharyngeal carcinoma and suggestions for clinical target volume delineation. Chin. J. Cancer 31(12), 579–587 (2012).
Cai, H., Chen, Y., Zhang, Q., Liu, Y. & Jia, H. High preoperative CEA and systemic inflammation response index (C-SIRI) predict unfavorable survival of resectable colorectal cancer. World J. Surg. Oncol. 21(1), 178 (2023).
Yi, J., Xue, J., Yang, L., Xia, L. & He, W. Predictive value of prognostic nutritional and systemic immune-inflammation indices for patients with microsatellite instability-high metastatic colorectal cancer receiving immunotherapy. Front. Nutr. 10, 1094189 (2023).
Wang, P. et al. Systemic inflammation influences the prognosis of patients with radically resected non-small cell lung cancer and correlates with the immunosuppressive microenvironment. Int. J. Cancer 153(4), 826–842 (2023).
Muscaritoli, M. et al. The impact of nutritional status at first medical oncology visit on clinical outcomes: The NUTRIONCO study. Cancers (Basel) 15(12), 1 (2023).
Morton, M. et al. Malnutrition, sarcopenia, and cancer cachexia in gynecologic cancer. Gynecol. Oncol. 175, 142–155 (2023).
Sandini, M. et al. Independent effect of fat-to-muscle mass ratio at bioimpedance analysis on long-term survival in patients receiving surgery for pancreatic cancer. Front. Nutr. 10, 1118616 (2023).
Silva, L. M. et al. Plasmin-mediated fibrinolysis enables macrophage migration in a murine model of inflammation. Blood 134(3), 291–303 (2019).
Palumbo, J. S. et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 105(1), 178–185 (2005).
Juang, L. J. et al. Suppression of fibrin(ogen)-driven pathologies in disease models through controlled knockdown by lipid nanoparticle delivery of siRNA. Blood 139(9), 1302–1311 (2022).
Aredes, M. A., Garcez, M. R. & Chaves, G. V. Influence of chemoradiotherapy on nutritional status, functional capacity, quality of life and toxicity of treatment for patients with cervical cancer. Nutr. Diet. 75(3), 263–270 (2018).
Baracos, V. E., Martin, L., Korc, M., Guttridge, D. C. & Fearon, K. C. H. Cancer-associated cachexia. Nat. Rev. Dis. Primers 4, 17105 (2018).
Baracos, V. E. Cancer-associated malnutrition. Eur. J. Clin. Nutr. 72(9), 1255–1259 (2018).
Danan, D. et al. Prognostic value of albumin in patients with head and neck cancer. Laryngoscope 126(7), 1567–1571 (2016).
Yang, H. et al. Prognostic role of pre-treatment serum albumin in patients with nasopharyngeal carcinoma: A meta-analysis and systematic review. Clin. Otolaryngol. 45(2), 167–176 (2020).
Keinanen, A., Uittamo, J., Marinescu-Gava, M., Kainulainen, S. & Snall, J. Preoperative C-reactive protein to albumin ratio and oral health in oral squamous cell carcinoma patients. BMC Oral Health 21(1), 132 (2021).
Jiang, Y. M., Huang, S. T., Pan, X. B., Ma, J. L. & Zhu, X. D. The prognostic nutritional index represents a novel inflammation-nutrition-based prognostic factor for nasopharyngeal carcinoma. Front. Nutr. 10, 1036572 (2023).
Tan, Z. et al. A novel blood tool of cancer prognosis in esophageal squamous cell carcinoma: The Fibrinogen/Albumin Ratio. J. Cancer 8(6), 1025–1029 (2017).
Xu, Q. et al. A novel inflammation-based prognostic score: The fibrinogen/albumin ratio predicts prognoses of patients after curative resection for hepatocellular carcinoma. J. Immunol. Res. 2018, 4925498 (2018).
Deng, S. et al. Fibrinogen/albumin ratio as a promising marker for predicting survival in pancreatic neuroendocrine neoplasms. Cancer Manag. Res. 13, 107–115 (2021).
Lin, G. T. et al. Fibrinogen-albumin ratio as a new promising preoperative biochemical marker for predicting oncological outcomes in gastric cancer: A multi-institutional study. Ann. Surg. Oncol. 28(12), 7063–7073 (2021).
Zhao, X. et al. High fibrinogen-albumin ratio index predicts poor prognosis for lung adenocarcinoma patients undergoing epidermal growth factor receptor-tyrosine kinase inhibitor treatments. Medicine (Baltimore) 99(46), e23150 (2020).
Yip, P. L., Lee, A. W. M. & Chua, M. L. K. Adjuvant chemotherapy in nasopharyngeal carcinoma. Lancet Oncol. https://doi.org/10.1016/S1470-2045(23)00266-8 (2023).
Funding
This research is sponsored by the Clinical Medical Technology Innovation Guidance Project of Hunan Province (2021SK53515), the Natural Science Foundation of Hunan Province (2021JJ40882, 2022JJ40682), and the National Natural Science Foundation of China (81802476).
Author information
Authors and Affiliations
Contributions
C.D.—conceptualization, methodology, and writing-original draft; S.J.-Z. and J.L.—data collection and methodology; Z.C.—data collection and software; Y.H.-F. and Y.C.-X.—data curation and writing-review and editing; X.L.-L. and C.H.-H.—investigation; T.H.—resources, software, conceptualization, writing-review and editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deng, C., Zhang, S., Ling, J. et al. Prognostic value of the fibrinogen albumin ratio index (FARI) in nasopharyngeal carcinoma patients undergoing radiotherapy. Sci Rep 13, 20630 (2023). https://doi.org/10.1038/s41598-023-48029-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48029-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.