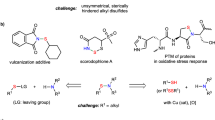
Abstract
A metal-free dehydrative thioetherification method has been reported, enabling the conversion of various alcohols and thiols into thioethers. By employing triflic acid as a catalyst or utilizing a recyclable NAFION® superacid catalyst, these methods significantly improve the efficiency and practicality of sulfide preparation.
Similar content being viewed by others
Introduction
Organosulfur compounds have significant industrial importance, ranging from polymer production to their use as agrochemicals and pharmacologically active compounds1,2,3. Among the S-containing derivatives with such broad applications, thioethers, which contain a C–S–C bonds in their structure, are certainly included. At first glance, their resemblance to ethers is highly deceptive. This is because they exhibit completely different chemical and biological properties, primarily due to the divalent sulfur center's greater polarizability, surpassing that of oxygen in ethers1. This, in turn, drives chemists to constantly seek new methods for synthesizing this group of chemical compounds4,5,6,7,8,9,10,11,12,13,14,15.
Thioethers can be readily obtained by several methods (Fig. 1), but the main ones are those based on nucleophilic substitution or addition reactions (hydrothiolation16). The classical synthesis relies on the utilization of easily removable groups like halides17 or carboxylates18. However, these methods have a significant drawback as they produce substantial amounts of salt waste and pose challenges concerning process efficiency and chemoselectivity. On the other hand, we come across the aforementioned hydrothiolation. Here, it becomes paramount to ensure strict control over the process's selectivity, as it can result in the formation of various regioisomers16,19,20. So far, many methods have been developed primarily based on the use of free radicals21, transition metals22,23,24, and Lewis acids25,26,27,28. Taking into consideration the pros and cons discussed earlier and giving due importance to green chemistry aspects (e.g., the generation of non-toxic byproducts, the use of cost-effective and commercially available catalysts, the substitution of noble metal complexes, etc.), an ideal approach for synthesizing this compound involves a nucleophilic addition to alcohols, wherein the sole byproduct generated is water. In the existing literature, the procedures commonly describe the use of catalysts primarily composed of transition metal compounds29,30,31,32,33, particularly employing sub-stoichiometric amounts of their triflates34,35,36,37. From a synthetic perspective, the presence of residual metal impurities can create additional obstacles for industrial and pharmaceutical applications. Consequently, there is a strong need and desire to develop gentle and selective methods for constructing thioethers under metal-free conditions.
Building upon these studies and considering our previous successes in using metal triflates in catalysis27,38,39, we decided to thoroughly investigate their application in thioetherification. Initially, we hypothesized that simple HOTf40,41 should serve as an equally effective catalyst for this process, thereby excluding the need for d-block metals. This allowed us to develop a highly efficient method for thioether synthesis. Furthermore, this work addresses a significant practicality concern by presenting a heterogeneous alternative42,43 in the form of commercially available Nafion44,45. Additionally, the aspect of hidden Brønsted acid catalysis is discussed in the context of metal triflates application46,47,48,49.
Methods
General information
All reactions were carried out in the ambient atmosphere. Solvents used for all experiments were purchased from Honeyweel or Sigma Aldrich (Merck), and used as received. Triflic acid was purchased from ABCR GmBH. Metal triflates and NAFION® (in the form of pellets) were purchased from Sigma Aldrich (Merck). Commercially available thiols and alcohols were purchased from Sigma Aldrich (Merck), Angene or Ambeed, and used as received. The progress of reactions (conversion of thiols) was monitored by GC chromatography using Bruker Scion 460-GC and Agilent 5977B GC/MSD with Agilent 8860 GC System. The structures of products were determined by NMR spectroscopy, IR spectroscopy, and MS spectrometry. The 1H NMR (400 or 600 MHz), and 13C NMR (101 or 151 MHz) spectra were recorded on Bruker Avance III HD NanoBay spectrometer, using chloroform-d (CDCl3) as the solvent. Deuterated solvents were purchased from Sigma Aldrich (Merck) (CDCl3 99.8 atom% D) and used as received. The enantiomeric purity was determined by HPLC analysis (Daicel Chiralcel OD-H). FT- IR spectra were taken on a Nicolet™ iS50 FTIR Spectrometer. In the case of IR spectroscopy in real-time, the measurements were made using a ReactIR 15 Mettler Tolledo spectrophotometer, equipped with a 9-reflection probe with a diamond window of 9.5 mm AgX DiComp Mettler Tolledo and an MCT detector cooled with nitrogen.
General synthetic procedures
All procedures can be also found in Supporting Information. Here, we present representative procedures.
The synthesis of compounds 3a–3p and 3aa–3af
To a 10 mL vial equipped with a magnetic stirring bar, alcohol (1, 1 mmol), thiol (2, 1 mmol), nitromethane (1 mL), and HOTf (0.01 mmol) were added under an ambient atmosphere. Subsequently, the reaction mixture was stirred at 80 °C for 2 h. After the reaction was completed, in order to neutralize HOTf the potassium carbonate (0.01 mmol) was added. After this time, the solvent was evaporated under reduced pressure. Next, the crude products were separated via extraction (diethyl ether-water), to give corresponding products 3a–3p, and 3aa–3af. The pure products were identified by 1H NMR, 13C NMR, IR, and MS spectrometry.
The synthesis of compounds 3q and 3r
To a 10 mL vial equipped with a magnetic stirring bar, alcohol (1, 2 mmol), thiol (2, 1 mmol), nitromethane (1 mL), and HOTf (0.02 mmol) were added under an ambient atmosphere. Subsequently, the reaction mixture was stirred at 80 °C for 2 h. After the reaction was completed, in order to neutralize HOTf the potassium carbonate (0.02 mmol) was added. After this time, the solvent was evaporated under reduced pressure. Next, the crude products were separated via extraction (diethyl ether-water), to give corresponding products 3q–3r. The pure products were identified by 1H NMR, 13C NMR, IR, and MS spectrometry.
The synthesis of compounds 3ag-3am, and 3ao-3ap
To a 10 mL vial equipped with a magnetic stirring bar, alcohol (1, 1 mmol), thiol (2, 1 mmol), nitromethane (1 mL), and HOTf (0.05 mmol) were added under an ambient atmosphere. Subsequently, the reaction mixture was stirred at 80 °C for a definite time (2–6 h). After the reaction was completed, in order to neutralize HOTf the potassium carbonate (0.01 mmol) was added. After this time, the solvent was evaporated under reduced pressure. Next, the crude products were separated via extraction (diethyl ether-water), to give corresponding products 3ag-3am, and 3ao-3ap. The pure products were identified by 1H NMR, 13C NMR, IR, and MS spectrometry.
Results and discussion
The optimization studies, presented in Table 1, involved an in-depth investigation of a dehydrative coupling reaction between thiophenol (2a) and tert-amyl alcohol (1a). Significantly, all experiments were carried out utilizing new vials and magnetic stirrers. This meticulous approach holds immense significance in eradicating any potential impact stemming from trace amounts of other transition metal impurities50,51. Since the substrates are not air-sensitive, we performed all reactions under an ambient atmosphere. Importantly, comprehensive details related to optimization studies (especially the use of metal triflates as the catalysts) can be found in the Supporting Information file (Table S1).
Initially, we tested the activity and selectivity of simple HOTf in a dehydrative coupling reaction (Table 1). The optimal attempt was conducted in CH3NO2 as a solvent (80 °C, 2 h), in the presence of 1 mol% of HOTf. As a result, we obtained the desired product 3a in 90% yield (entry 1). Attempt to decrease the loading of the catalyst gave a bit inferior conversion (entry 2). The control reaction revealed that the process does not proceed without the catalyst (entry 3). Instead, it was found that the reaction works under solvent-free conditions. However, in such a case we observed the formation of three different thioethers (entry 4). Finally, several solvents including H2O, 2-MeTHF, and CH3CN were utilized (entries 9–11). We demonstrated that nitromethane was the solvent of choice (other mediums were less efficient and provided noticeably lower chemoselectivity). The above-mentioned processes can also proceed in the presence of commercially available metal triflates (for details please see Table 1, entry 7 and 8, as well as SI, Table S1).
Having the optimized conditions at our disposal, we conducted tests using various thiols to demonstrate the broad applicability of our protocol (Fig. 2, top). The reaction conditions proved to be effective for a diverse set of thiophenol derivatives (3a–3j). As an initial example 3a, benzenethiols bearing electron-donating groups were readily alkylated (3b–3e), including difunctional 4-hydroxythiophenol 3d (88% yield). Gratifyingly, halogenated thiophenols were readily adopted in this protocol (3f–3h, 82–84% yield), as were electron-deficient ones bearing trifluoromethyl and trifluoromethoxy functionalities (3i–3j, 87% yield). Motivated by these findings, we subsequently explored the utilization of heterocyclic thiols, which serve as biorelevant frameworks. Each of them yielded the desired products with moderate efficiencies (3k–3l, 70–73% yields). Next, we were pleased to find that HS-terminated carboxylic acids and esters were also successfully alkylated under standard conditions leading to products 3m–3n in very good yields (85–90%).
Subsequently, we tested our methodology on variously substituted aliphatic mercaptans. Simple 1-octanethiol, as well as chloro-substituted benzylthiol reacted well, providing 3o and 3p in good yields (80–85%). Next, we sought to obtain alkylated bis(thioethers). Using our catalytic system, two dialkylated symmetrical variants were afforded (3q and 3r, in 83% and 94% yields).
Following the high efficiency and chemoselectivity of the transformation, we proceeded to advance the exploration of additional applications for our catalytic system. Encouragingly, this strategy also enabled the dehydrative coupling of other alcohols with benzenethiol. As shown in Fig. 2 (bottom), another tertiary alcohol such as 3-ethyl-3-pentanol (1b), 1-adamantanol (1c), 1-methylcyclopentanol (1d), and 4-hydroxy-4-methyl-2-pentanone (1e) were exclusively converted into valuable sulfides (3aa–3ad). In the last example, the ketone group remains untouched, which is particularly noteworthy (3ad). When the reaction is catalyzed by Lewis acids, it results in the formation of a dithioacetal. Furthermore, commercial secondary alcohols also participated effectively in this reaction (3ae–3an, 64–99% yield), while in the case of 2-cyclohexen-1-ol (1f) preserving the ene-functionality untouched. Regarding the more complex compound, we tested enantiomerically pure (-)-borneol, which is used, among other things, in pharmaceuticals and fragrances. The reaction required a longer time (48 h), but led only to the expected endo-diastereoisomer (3an, 64% yield). Finally, two primary (benzylic and allylic) alcohols were also readily adopted in this protocol (3ao–3ap, 84–89% yield).
Lastly, the reaction on a larger scale demonstrated the robustness and preparative scale utility of the process, under procedurally convenient conditions (Fig. 3).
The concept of Brønsted acid catalysis mentioned above motivated us to explore a heterogeneous system using Nafion®, a perfluorinated copolymer with sulfonic acid groups. This particular copolymer has proven useful in dehydration and esterification reactions52,53,54. In our experiment, we initially selected benzenethiol (1 mmol) and tert-amyl alcohol (1.5 mmol) as the coupling partners. We conducted the reaction using approximately 0.22 g of Nafion® NR50 pellets (approx. 5 pellets) (Fig. 4, top). To obtain a detailed understanding of the heterogeneous catalyst, we became intrigued to further probe its reusable nature (Fig. 4, bottom). We were hence delighted to observe that Nafion was successfully reused without a significant loss of performance over 10 cycles.
Next, to get some mechanistic insights into Brønsted acid catalysis, we carried out some of preliminary experiments (Fig. 5). As a first investigation, we conducted a radical clock experiment. It gives the desired product containing an untouched cyclopropyl ring (3z), thereby implying that radical pathways were likely, not operative (Fig. 5a). Therefore, the most probable mechanism involves the SN1-type nucleophilic substitution. With the formation of a planar sp2 hybridized carbocation, racemization occurs.
Confirmation of this reaction pathway is provided by using an R-enantiomer of 1-phenylethan-1-ol. As a result, a racemic mixture was observed (Fig. 5b). Next, we monitored the dehydration reaction using reactIR (Fig. 5c). The kinetic plots obtained for the coupling of tert-amyl alcohol (1a) to thiophenol (2a) confirmed a rapid disappearance of the distinguishing bands at 880 and 940 cm−1 (Fig. 5c). Thus, a mechanism is presented in Fig. 5d. Finally, we proceeded to find an answer to the question of whether this reaction can occur using both Brønsted and Lewis catalysis. In this case, the hydrolysis of metal triflates is known to occur with the formation of triflic acid. Therefore, we conducted a test reaction using indium(III) triflate as the catalyst. However, we carried it out in the presence of 2,6-di-tert-butylpyridine, which does not coordinate to Lewis acids but readily undergoes protonation. Despite the use of pyridine, the reaction proceeded unchanged in terms of efficiency and selectivity, suggesting that both Brønsted and Lewis acids can catalyze this reaction (Fig. 5e).
Conclusions
In summary, we have successfully developed an innovative metal-free approach for thioesterification of alcohols utilizing thiols. Unlike previous methods that heavily relied on transition metals or metal triflates, our new method offers distinct advantages. Through our studies, we have demonstrated that a simple triflic acid serves as a highly efficient and selective catalyst. Moreover, we have addressed practicality concerns by exploring the option of heterogeneous catalysis using superacidic Nafion. This exciting development allows for the catalyst's repeated usage without significant loss in process efficiency. Furthermore, our research has provided valuable insights into the reaction mechanism. In addition, we conducted a comparison between Lewis acid and Brønsted acid catalysis, revealing that both approaches exhibit equally remarkable effectiveness. These significant advancements open up exciting possibilities for sustainable and green thioesterification processes, with reduced environmental impact and enhanced applicability in industrial and pharmaceutical contexts.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Geiger, V. J., Oechsner, R. M., Gehrtz, P. H. & Fleischer, I. Recent metal-catalyzed methods for thioether synthesis. Synthesis 54, 5139–5167 (2022).
Beletskaya, I. P. & Ananikov, V. P. Transition-metal-catalyzed C–S, C–Se, and C–Te bond formations via cross-coupling and atom-economic addition reactions. Achievements and challenges. Chem. Rev. 122, 16110–16293 (2022).
Han, M. İ & Küçükgüzel, ŞG. Thioethers: An overview. Curr. Drug Targets 23, 170–219 (2022).
Robertson, F. & Wu, J. Convenient synthesis of allylic thioethers from phosphorothioate esters and alcohols. Org. Lett. 12, 2668–2671 (2010).
Jaimes-Romano, E. et al. C–S couplings catalyzed by Ni(II) complexes of the type [(NHC)Ni(Cp)(Br)]. J. Catal. 426, 247–256 (2023).
Pramanik, M., Choudhuri, K., Mathuri, A. & Mal, P. Dithioacetalization or thioetherification of benzyl alcohols using 9-mesityl-10-methylacridinium perchlorate photocatalyst. Chem. Commun. 56, 10211–10214 (2020).
Liu, C. & Szostak, M. Forging C−S bonds through decarbonylation: New perspectives for the synthesis of privileged aryl sulfides. ChemCatChem 13, 4878–4881 (2021).
Liu, C. & Szostak, M. Decarbonylative sulfide synthesis from carboxylic acids and thioesters via cross-over C–S activation and acyl capture. Org. Chem. Front. 8, 4805–4813 (2021).
Dong, B., Shen, J. & Xie, L.-G. Recent developments on 1,2-difunctionalization and hydrofunctionalization of unactivated alkenes and alkynes involving C–S bond formation. Org. Chem. Front. 10, 1322–1345 (2023).
Dinda, T. K., Kabir, S. R. & Mal, P. Stereoselective synthesis of Z-styryl sulfides from nucleophilic addition of arylacetylenes and benzyl thiols. J. Org. Chem. 88, 10070–10085 (2023).
Yang, X. et al. Synthesis of α-aryl sulfides by deaminative coupling of α-amino compounds with thiophenols. Org. Biomol. Chem. 21, 3794–3799 (2023).
Liu, W. et al. Synthesis of trifluoromethylated thioethers via Ni-catalyzed reductive C–S coupling. Org. Chem. Front. 10, 2943–2948 (2023).
Ji, H. et al. Predominant intermolecular decarbonylative thioetherification of carboxylic acids using nickel precatalysts. Org. Chem. Front. 10, 4275–4281 (2023).
Khan, J., Tyagi, A., Ghosh, D. & Hazra, C. K. HFIP-assisted reductive C–S, C–N, and C–X coupling of carbonyl compounds: A combined computational and experimental mechanistic study. Org. Chem. Front. 10, 1275–1282 (2023).
Cuffaro, D. et al. Ionic liquid-promoted green synthesis of biologically relevant diaryl thioethers. Green Chem. Lett. Rev. 23, 295–302 (2020).
Castarlenas, R., Giuseppe, A. D., Pérez-Torrente, J. J. & Oro, L. A. The emergence of transition-metal-mediated hydrothiolation of unsaturated carbon-carbon bonds: A mechanistic outlook. Angew. Chem. Int. Ed. 52, 211–222 (2013).
Nguyen, K. N., Duus, F. & Luu, T. X. T. Benign and efficient preparation of thioethers by solvent-free S-alkylation of thiols with alkyl halides catalyzed by potassium fluoride on alumina. J. Sulfur Chem. 37, 349–360 (2016).
Venkatesham, K., Rao, C. B., Dokuburra, C. B., Bunce, R. A. & Venkateswarlu, Y. Direct synthesis of thioethers from carboxylates and thiols catalyzed by FeCl3. J. Org. Chem. 80, 11611–11617 (2015).
Savolainen, M. A. & Wu, J. Markovnikov-selective hydrothiolation of styrenes: Application to the synthesis of stereodefined trisubstituted olefins. Org. Lett. 15, 3802–3804 (2013).
Dondoni, A. & Marra, A. Metal-catalyzed and metal-free alkyne hydrothiolation: Synthetic aspects and application trends. Eur. J. Org. Chem. 2014, 3955–3969 (2014).
Pakuła, D. et al. Sulfur-containing silsesquioxane derivatives obtained by the thiol-ene reaction: Synthesis and thermal degradation. ChemPlusChem 87, e202200099 (2022).
Ren, S. et al. E-selective hydrothiolation of terminal arylallenes with arylthiols catalyzed by Ni(PMe3)4. Appl. Organomet. Chem. 34, e5291 (2020).
Jawale, D. V. et al. Catalytic hydrothiolation of alkenes and alkynes using bimetallic RuRh nanoparticles on carbon nanotubes. Green Chem. 24, 1231–1237 (2022).
Capurso, M., Radivoy, G., Nador, F. & Dorn, V. Hydrothiolation of alkynes with thiol–catechol derivatives catalysed by CuNPs/TiO2: Exploring the reaction mechanism by DFT calculations. RSC Adv. 13, 8025–8033 (2023).
Weïwer, M., Coulombel, L. & Duñach, E. Regioselective indium(III) trifluoromethanesulfonate-catalyzed hydrothiolation of non-activated olefins. Chem. Commun. https://doi.org/10.1039/B513946E (2006).
Kuciński, K., Pawluć, P., Marciniec, B. & Hreczycho, G. Highly selective hydrothiolation of unsaturated organosilicon compounds catalyzed by scandium(III) triflate. Chem. Eur. J. 21, 4940–4943 (2015).
Kuciński, K., Pawluć, P. & Hreczycho, G. Scandium (III) triflate-catalyzed anti-Markovnikov hydrothiolation of functionalized olefins. Adv. Synth. Catal. 357, 3936–3942 (2015).
Mosaferi, E., Ripsman, D. & Stephan, D. W. The air-stable carbocation salt [(MeOC6H4)CPh2][BF4] in Lewis acid catalyzed hydrothiolation of alkenes. Chem. Commun. 52, 8291–8293 (2016).
Corma, A., Navas, J., Rõdenas, T. & Sabater, M. J. One-pot palladium-catalyzed borrowing hydrogen synthesis of thioethers. Chem. Eur. J. 19, 17464–17471 (2013).
Hikawa, H., Machino, Y., Toyomoto, M., Kikkawa, S. & Azumaya, I. Cationic palladium(II)-catalyzed dehydrative nucleophilic substitutions of benzhydryl alcohols with electron-deficient benzenethiols in water. Org. Biomol. Chem. 14, 7038–7045 (2016).
Sorribes, I. & Corma, A. Nanolayered cobalt–molybdenum sulphides (Co–Mo–S) catalyse borrowing hydrogen C–S bond formation reactions of thiols or H2S with alcohols. Chem. Sci. 10, 3130–3142 (2019).
Singh, A., Gupta, S. & Khurana, J. M. Zinc chloride mediated nucleophilic substitution: Amination and thioetherification of alcohols at room temperature. Org. Prep. Proc. Int. 52, 110–119 (2020).
Nishio, T. et al. Direct nucleophilic substitution of alcohols using an immobilized oxovanadium catalyst. Eur. J. Org. Chem. 2021, 4417–4422 (2021).
Han, X. & Wu, J. Ga(OTf)3-catalyzed direct substitution of alcohols with sulfur nucleophiles. Org. Lett. 12, 5780–5782 (2010).
Kuciński, K. & Hreczycho, G. Highly efficient and chemoselective tertiary and secondary benzylation of thiols catalyzed by indium(III) triflate. Eur. J. Org. Chem. 2017, 5572–5581 (2017).
Xu, B. et al. Benzyl thioether formation merging copper catalysis. RSC Adv. 12, 692–697 (2021).
Xu, B. et al. Cu-catalyzed coupling of unactivated tertiary alkyl alcohols with thiols via C–O bond cleavage. Tetrahedron Lett. 89, 153604 (2022).
Kaźmierczak, J., Kuciński, K. & Hreczycho, G. Highly efficient catalytic route for the synthesis of functionalized silsesquioxanes. Inorg. Chem. 56, 9337–9342 (2017).
Kaźmierczak, J., Kuciński, K., Stachowiak, H. & Hreczycho, G. Introduction of boron functionalities into silsesquioxanes—novel independent methodologies. Chem. Eur. J. 24, 2509–2514 (2018).
Falconnet, A., Arndt, J.-D., Hashmi, A. S. K. & Schaub, T. Triflic-acid-catalyzed friedel-crafts reaction for the synthesis of diaryl sulfones. Eur. J. Org. Chem. 2022, e202200477 (2022).
To, T. A., Mai, B. K. & Nguyen, T. V. Toward homogeneous Brønsted-acid-catalyzed intramolecular carbonyl-olefin metathesis reactions. Org. Lett. 24, 7237–7241 (2022).
Manabe, K., Iimura, S., Sun, X.-M. & Kobayashi, S. Dehydration reactions in water. Brønsted acid−surfactant-combined catalyst for ester, ether, thioether, and dithioacetal formation in water. J. Am. Chem. Soc. 124, 11971–11978 (2002).
de Souza, P. M., de Sousa, L. A., Noronha, F. B. & Wojcieszak, R. Dehydration of levoglucosan to levoglucosenone over solid acid catalysts. Tuning the product distribution by changing the acid properties of the catalysts. Mol. Catal. 529, 112564 (2022).
Kaźmierczak, J. & Hreczycho, G. Nafion as effective and selective heterogeneous catalytic system in O-metalation of silanols and POSS silanols. J. Catal. 367, 95–103 (2018).
Kuciński, K. & Hreczycho, G. S-acetylation of thiols mediated by triflic acid: A novel route to thioesters. Org. Process Res. Dev. 22, 489–493 (2018).
Taylor, J. G., Adrio, L. A. & Hii, K. K. Hydroamination reactions by metal triflates: Brønsted acid vs. metal catalysis?. Dalton Trans. 39, 1171–1175 (2010).
Dang, T. T., Boeck, F. & Hintermann, L. Hidden Brønsted acid catalysis: Pathways of accidental or deliberate generation of triflic acid from metal triflates. J. Org. Chem. 76, 9353–9361 (2011).
Šolić, I., Lin, H. X. & Bates, R. W. Testing the veracity of claims of Lewis acid catalysis. Tetrahedron Lett. 59, 4434–4436 (2018).
Šolic, I., Seankongsuk, P., Loh, J. K., Vilaivan, T. & Bates, R. W. Scandium as a pre-catalyst for the deoxygenative allylation of benzylic alcohols. Org. Biomol. Chem. 16, 119–123 (2018).
Pentsak, E. O., Eremin, D. B., Gordeev, E. G. & Ananikov, V. P. Phantom reactivity in organic and catalytic reactions as a consequence of microscale destruction and contamination-trapping effects of magnetic stir bars. ACS Catal. 9, 3070–3081 (2019).
Sau, S., Pramanik, M., Bal, A. & Mal, P. Reported catalytic hydrofunctionalizations that proceed in the absence of catalysts: The importance of control experiments. Chem. Rec. 22, e202100208 (2022).
López, D. E., Goodwin, J. G. & Bruce, D. A. Transesterification of triacetin with methanol on Nafion® acid resins. J. Catal. 245, 381–391 (2007).
Kidwai, M., Chauhan, R. & Bhatnagar, S. Nafion-H®: A versatile catalyst for organic synthesis. Curr. Org. Chem. 19, 72–98 (2015).
Kim, S. et al. Self-catalyzed esterification of 2-hydroxyisobutyric acid: A simple method to produce methyl 2-hydroxyisobutyrate. Mol. Catal. 532, 112721 (2022).
Acknowledgements
This work was supported by a National Science Centre (Poland) Grant UMO-2021/43/D/ST4/00132 (K.K.). L. A. and A. L. thanks the University of the Basque Country UPV/EHU (UFIQOSYC11/22), Basque Government (EJ grant IT-1583-22), and Ministerio de Ciencia e Innovación, Agencia Estatal de Investigación (grants PID2019-109633GB-C21/AEI/10.13039/501100011033 and PID2022-137153NB-C21/AEI/10.13039/501100011033) for financial support. We owe a very special thanks to Dr. Rafał Januszewski (AMU Poznań) for his invaluable assistance involving FT-IR analyses.
Author information
Authors and Affiliations
Contributions
Methodology, K.K.; Synthesis of Products—K.K., M.M., K.L., L.A.; Formal analysis, K.K., M.M., L.A., A.L.; writing—original draft preparation, K.K. and A.L.; visualization, K.K.; supervision, K.K., A.L.; funding acquisition, K.K., A.L. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Markwitz, M., Labrzycki, K., Azcune, L. et al. Access to thioethers from thiols and alcohols via homogeneous and heterogeneous catalysis. Sci Rep 13, 20624 (2023). https://doi.org/10.1038/s41598-023-47938-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47938-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.