Abstract
IGT family genes play essential roles in shaping plant architecture. However, limited amount of information is available about IGT family genes in peanuts (Arachis hypogaea). In the current study, 13 AhIGT genes were identified and classified into three groups based on their phylogenetic relationship. Gene structure, conserved domain analyses indicated all AhIGTs were observed to share a similar exon–intron distribution pattern. AhIGTs within the same subfamily maintained a consistent motif composition. Chromosomal localization and synteny analyses showed that AhIGTs were unevenly localized on 9 chromosomes and that segmental duplication and purifying selection may have played important roles in the evolution of AhIGT genes. The analysis of conserved motifs, GO annotation, and transcript profile suggested that AhLAZY1-3 may play roles in gravity sensing and shaping peanut plant architecture. Transcript profile analysis suggested that AhTAC1 could potentially be involved gynophore (‘peg’) penetration into the soil. The cis-element analysis revealed that the light-responsive elements accounted for most of all cis-acting elements. Furthermore, qRT-PCR analysis showed that the expression of several AhIGT genes, like AhTAC1-2/4, was light-dependent, indicating that these genes may regulate plant architecture in response to light signals. This study may facilitate functional studies of the IGT genes in peanut.
Similar content being viewed by others
Introduction
Optimizing plant architecture has been demonstrated as one of the best methods to improve planting density, stress-tolerance and overall productivity of crops1. The IGT gene family, which is identified based on a conserved motif (GφL(A/T)IGT), has been observed to play a significant role in regulating gravitropism and shaping the structure of plants2. The IGT gene family comprises a set of genes (LAZY1-6) and TILLER ANGLE CONTROL 1 (TAC1). IGT genes have relatively low levels of sequence conservation across gene-family members. Nevertheless, 5 short conserved motifs, called I–V domains, have been identified in IGT family proteins, but not all were present in all members of the family3. Additionally, a unique intron–exon arrangement was also detected for IGT genes. This arrangement was characterized by a short exon encoding two amino acids and the last exon encoding a short peptide including ethylene-responsive amphiphilic repression (EAR) motif containing transcriptional repressors3,4. TAC1 lacks this motif (domain V) which is known to be essential for functions of LAZY1 and LAZY45. Domain I located at N terminus, and it is necessary for anchoring AtLAZY1 at the plasma membrane6. The IGT conserved domain II contains the family-specific GφL (A/T) IGT sequence. Any mutation in this directly resulted in the loss of function of LAZY14,6. Much less information about the domain III and IV is available7,8,9.
The lazy1 traits, initially described in the 1930s, exhibited a prostrate growth habit in rice and maize10,11. Over the decades, the LAZY1, which responsible for lazy traits12,13,14 have been identified. LAZY1 is a crucial regulator of negative gravitropism, and its loss leads to the formation of wide-angled branches in Arabidopsis thaliana15. LAZY4/DEEPER ROOTING 1 (DRO1) was identified as a key QTL controlling deep rooting in rice16. LAZY4 is involved in gravitropic responses via auxin signaling and promotes deep rooting in rice17. TAC1 was described as a key QTL that controlled the production of compact and erect tillers in rice18. TAC1 showed a similar expression pattern to LAZY119, which modulated plant branch growth angle, but had opposite functions19,20. Several studies have shown that light and gravity signals function concurrently to shape plant architecture21,22,23. A report showed that the expression of AtTAC1, which modulated plant architecture in response to photosynthetic signals, was light dependent24. The expression of AtIGT genes was also found to respond differentially to alterations in light signaling, and the loss of several LAZY and DRO genes in Arabidopsis resulted in a lack of branch angle reaction to light stimuli25. These studies have demonstrated that IGT family genes play a role in regulating plant architecture in response to both light and gravity stimuli.
The peanut (Arachis hypogaea L.) is one of the world’s most important economic oilseed crops. The peanut seeds are rich in vegetable oils, proteins, vitamins and minerals. Peanuts produce aerial flowers but subterranean fruits (pods). Embryo development remains arrested until the fertilized ovary is buried in the soil with the help of a specialized organ called the peg or gynophore. Positive gravitropism is the most typical features of pegs as it enables them to grow downwards and penetrate in the soil26. Then the height of flowers directly influences pegs to penetrate the soil and affect peanut yield. As a result, optimizing plant architecture improves planting density and increases pod number per plant in peanut. Although the critical importance of IGT genes involved in regulating gravitropism and shaping the structure of plants27,28,29, little is known about this gene family in A. hypogaea. This study is the first genome-wide study to identify the IGT genes in the genome of peanuts. To gain a better understanding of IGT genes in peanuts, their phylogenetic relationships, chromosome locations, synteny study, gene structures, conserved motifs, and cis-element were examined. Additionally, their expression profiles in numerous tissues and their response to light and dark signals were evaluated. The results may facilitate functional studies of the IGT genes in peanut.
Materials and methods
Identification and characterization of IGT genes in peanut
The protein and genomic sequences of A. hypogaea var. Shitoqi were downloaded from the Peanut Genome Resource (PGR) database (http://peanutgr.fafu.edu.cn/Download.php)30. The sequences of seven AtIGTs (protein sequences are listed in supplementary Table S1) were downloaded from the Arabidopsis Information Resource (TAIR) database (https://www.arabidopsis.org/). These sequences were then subjected to the blastp program to search for the peanut protein sequence database with an E-value ≤ 1.0e−6. Further, 7 AtIGT protein sequences were subjected to MAFFT version 7.0 software31 for multiple sequence alignment. The alignment results were used to build HMM profile using hmmbuild program32. Then, the HMM profile was used to screen protein sequences of peanuts on HMMER software32 with an E-value ≤ 1.0e−6. The amino acid sequences of candidate AhIGTs from blastp and HMMER search were aligned with those of the corresponding AtIGTs using DNAMAN version 5.0 software (Lynnon Biosoft, San Ramon, CA, USA) to confirm whether they were the orthologs of the target AtIGTs or not. The protein sequences of Arachis duranensis and Arachis ipaensis were obtained from the PeanutBase database (https://data.legumeinfo.org/Arachis/)33, and the IGT proteins in these two species were identified using the blastp method. The sequences of the confirmed proteins were submitted to the ExPASy tool34 to predict protein molecular weight (MW) and isoelectric point (PI). Subcellular localization of the proteins was predicted by WoLF PSORT35.
The structure of AhIGT genes was analyzed by the Gene Structure Display Server 2.036, and conserve protein motifs were predicted by the MEME software37 with a maximum number of motifs (10) and motif length ranging from 6 to 20 amino acids. The conserved motifs were visualized using the TBtools software38. The final figures prepared for gene structures and conserved motifs were refined using Adobe Illustrator software (Adobe Systems Incorporated, San Jose, CA, USA).
Chromosome location, phylogenetic tree, and synteny analysis of AhIGT genes
The chromosomal location of AhIGTs was obtained from the PGR database30. The distribution of AhIGTs was visualized using MapChart software39, and the final figure was refined with Adobe Illustrator software (Adobe Systems Incorporated, San Jose, CA, USA).
The full-length amino acid sequences of IGT proteins from A. thaliana, A. hypogaea, A. duranensis and A. ipaensis were submitted to ClustalW software40 to perform sequence alignment. Sequence alignment results were used as the basis to construct a phylogenetic tree by maximum likelihood method using MEGA 7.0 software41. The phylogenetic tree was visualized by the Evolview website42.
The synteny analysis of AhIGTs in A. hypogaea was performed with McScanX software and the results were plotted by Circos software43. The collinear relationships among IGT proteins in A. hypogaea, A. duranensis and A. ipaensis were determined using the Python version of McScanX44. The Ka/Ks values of IGT proteins were calculated using the KaKs calculator 2.0 program45.
Analysis of cis-regulatory element distribution in AhIGT gene promoters and GO annotation
The 1500-bp sequences occurring upstream of the start codon of AhIGTs were extracted from the PGR database30. The putative cis-elements of each promoter were analyzed using the PlantCARE database46, and the results were visualized using the Gene Structure Display Server 2.0 online tools36. The full-length amino acid sequences of AhIGTs were submited to the eggNOG website47. GO enrichment analysis was performed using the TBtools software38.
Expression profiles and qRT-PCR analysis of AhIGT genes
The tissue-specific expression profiles were obtained from the PGR database (http://peanutgr.fafu.edu.cn/Transcriptome.php)30. The fragment per kilobase per million reads (FPKM) value of 9 different tissues including cotyledon, root, stem, leaves, stem tip, gynophore, testa, pericarp, and embryo were downloaded, and the heatmap was drawn using Heatmapper48.
A peanut variety PX05 provided by the Crops Research Institute, Anhui Academy of Agricultural Science, Hefei, China, was used as the plant material and exposed to continuous light and dark conditions. The use of plant parts and seeds in the present study complies with relevant institutional, national, and international guidelines and legislation. The seedlings were grown in a growth chamber at 25–30 ℃ under 14:10 h light/dark conditions. The 5- or 6-week-old seedlings were transferred to chambers in continuous darkness for 48 h, and thereafter, leaves were sampled at 0, 0.5, 1, 2, 4, 8, 12, 24 and 48 h after the treatment. The plants were then transferred to chambers under continuous light, and leaves were sampled at 0.5, 1, 2, 4, 8, 12, 24 and 48 h after the treatment. We combined equal amounts of leaves from 5 plants as a biological replicate, and three biological replicates were considered in our experiment. Total RNA was extracted using the SDS-LiCl method which was described by Vennapusa49. We used the PrimeScript™ RT reagent Kit (TaKaRa, Japan) to synthesize cDNA according to developer instructions. qRT-PCR assays were performed using SYBR Premix Ex Taq™ (TaKaRa, Japan) with three technical replicates on a CFX Connect Real-Time PCR Detection System (Bio-Rad, USA). The conditions for the qRT-PCR reaction were set as follows: initial denaturation at 95 ℃ for 5 min, then 40 cycles of denaturation at 95 ℃ for 30 s, annealing at 60 ℃ for 1 min. The quantified data were calculated using the 2−ΔΔCt method. The Actin gene from peanut (A. hypogaea)50 was used as an internal control and all AhIGT primers are listed in Supplementary Table S2.
Results
Identification and characterization of IGT family genes in peanut
A total of 13 IGT genes unevenly distributed along 9 out of the 20 A. hypogaea chromosomes, were identified in peanut genome, with 7 genes located on AA subgenome and 6 located on BB subgenome. Chromosome A06 contained the maximum number of IGT genes (3); while chromosomes A02 and B02 each contained 2 IGT genes, and chromosomes A03, A09, B03, B04, B06, and B09 each contained 1 IGT gene (Table 1, Fig. 1). Most homologous IGT gene pairs that had strong collinear relationships were observed to be located on the homologous chromosomes between the AA and BB subgenomes. For example, the IGT genes AhTAC1-1 and AhLAZY5-1 present on chromosome A02 were found to have strong collinear relationships with the IGT genes AhTAC1-2 and AhLAZY5-2 present on chromosome B02. The lengths of AhIGT proteins varied from 241 (AhTAC1-2) to 417 (AhLAZY1-2) amino acids, whereas their predicted molecular weight ranged from 27.67 (AhTAC1-2) to 46.50 kDa (AhLAZY1-2), and the isoelectric points ranged from 5.03 (AhLAZY3-1) to 9.73 (AhLAZY5-2) (Table 1). After conducting in silico prediction of subcellular localization, AhTAC1-1 and AhTAC1-3 were shown to be located in the cytoplasm, while the others were located in the nuclear (Table 1). Additional information such as gene position and coding sequence lengths can be found in Table 1.
The physical map of AhIGT genes located on chromosomes; The diagram was drawn by the Mapchart software, and the physical location of AhIGTs was obtained from the Peanut Genome Resource (PGR) database (http://peanutgr.fafu.edu.cn/Download.php).
Phylogenetic relationships among IGT genes
To investigate the evolutionary relationships among IGT genes, a phylogenetic tree was constructed using the sequences of 33 IGT proteins from A. thaliana, A. hypogaea, A. duranensis, and A. ipaensis (protein sequences are shown in Table S1). As shown in Fig. 2, the tree was divided into three groups (LAZY-like, DRO-like, and TAC). The LAZY-like group included LAZY1, LAZY5, and LAZY6, while the DRO-like group consisted of LAZY2, LAZY3, and LAZY4, and the last group (TAC) only contained TAC1. Most AhIGTs within each group were paired with their orthologs in either A. ipaensis or A. duranensis. For example, in the TAC1 clade, AhTAC1-1 (A02) was clustered with its A. duranensis counterpart Adu.M7LVY (A02), while AhTAC1-3 (B02) was clustered with its ortholog Aip.HP7FW (B02) in A. ipaensis. Some AhIGTs like AhLAZY1-3 (B04) had corresponding orthologs both in A. ipaensis (Adu.TK7RU, A04) and A. duranensis, (Api.9A27H, B04). However, in the DRO-like subgroup, 3 pairs of genes, including AtLAZY2 and AtLAZY4, AhLAZY2/4-1 and AhLAZY2/4-2, and Aip.MR79R and Adu.JH1LG were clustered together. It was difficult to determine which proteins were orthologs of AtLAZY2 or AtLAZY4. AtLAZY6 formed a separate branch, with no ortholog found in A. hypogaea, A. duranensis, or A. ipaensis.
A phylogenetic tree of IGT family genes in A. thaliana, A. hypogaea, A. duranensis, and A. ipaensis constructed using the maximum likelihood method; Overall, 13 IGTs from A. hypogaea (red stars), 7 IGTs from A. thaliana (light blue triangles), 7 IGTs from A. ipaensis (orange boxes), and 6 IGTs from A. duranensis (light green boxes) were clustered into three major classes, denoted by different colors. The bootstrap is displayed in orange circles.
Gene structures and conserved motifs of IGT genes in peanut
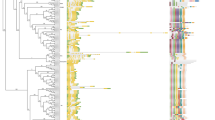
To have a better understanding of the structure of AhIGT genes, the exon–intron arrangements were generated (Fig. 3). The results revealed that most AhIGT genes shared similar structural patterns. For example, all 13 AhIGT genes consisted of two short exons followed by a long exon. In addition, a short exon was observed to appear at the end of AhLAZY1-3 and all members of the AhLAZY2/4, AhLAZY3, AhLAZY5 clades. Expect for LAZY1-1, which had 3 exons, all LAZY-like genes and DRO-like genes contained 5 exons, while all AhTAC1 clade members contained 4 exons.
The structures and conserved motifs of IGT genes in peanut; (a) A phylogenetic tree of IGT genes in peanuts was constructed by the MEGA7.0 software. (b) The structure of AhIGT genes; Blue boxes represent the 5’ or 3’ untranslated regions, while red boxes denote exons and black lines symbolize introns. (c) Conserved motifs of AhIGTs; Different colored boxes indicate different motifs.
Furthermore, the full-length amino acid sequences of all AhIGTs were examined to identify their conserved motifs (Fig. 3). As shown in Fig. 3, motif 1 contained the family-specific GφL (A/T) IGT sequence, which was identified in all AhIGT proteins except for the proteins of the AhLAZY5 clade. All AhIGTs contained motif 2 at the N terminus. Motif 3, which contained the EAR motif (Figure S1), was found in all members of AhLAZY3, AhLAZY2/4, and AhLAZY5 clades. However, in AhLAZY1 clade, only AhLAZY1-3 contained motif 3 with the EAR motif. Motif 4 was identified in the TAC1, LAZY2/4, and LAZY3 clades. Motifs 5 and 7 were specific to DRO-like proteins, while motifs 6 and 8 were only specific to the LAZY1 clade. Motif 9 was only detected in the TAC1 clade, whereas motif 10 was only detected in the LAZY5 clade.
Duplication and synteny analysis of IGT genes in peanut
Synteny analysis was performed to explore the evolutionary relationships among IGT genes in A. hypogaea (Fig. 4A). A total of 9 segmentally duplicated gene pairs were detected, and no tandem duplication was found. After calculating the Ka/Ks ratio, all duplicated IGT gene pairs in peanut were shown to have a Ka/Ks ratio less than 1 (Table 2), indicating that purifying selective pressure acted on evolutionary process of the AhIGT genes.
Duplications and synteny analysis of IGT genes in peanut; (a) Collinearity analysis of AhIGTs in A. hypogaea; Grey lines in the background represent all collinear blocks among different chromosomes, while red lines indicate segmentally duplicated gene pairs. (b) Collinearity analysis of AhIGTs in A. hypogaea, A. ipaensis and A. duranensis; All collinear blocks among A. hypogaea, A. ipaensis and A. duranensis are represented by grey lines in the background and syntenic IGT gene pairs are represented by red lines.
Further, the collinearity analysis of genes among A. hypogaea, A. ipaensis and A. duranensis was performed (Fig. 4B). A total of 10 AhIGTs in A. ipaensis and A. duranensis showed the correspondent relationships. Four AhIGTs in the AA subgenome exhibited a one-to-one homologous relationship with their orthologs in A. duranensis, and 6 AhIGTs in the BB subgenome had a one-to-one homology relationship with their orthologs in A. ipaensis. No IGT gene pair was found between the AA subgenome and the A. ipaensis genome or between the BB subgenome and the A. ipaensis genome. The results indicated that gene duplication played a crucial role in the evolution of the IGT gene family in the peanut genome. The Ka/Ks ratio was also determined for A. hypogaea and its two diploid ancestors (Table S3). All duplicated gene pairs had a Ka/Ks ratio < 1, indicating the role of strong purifying selective pressure in the evolutionary process of IGT genes in these species.
Cis-element analysis and functional annotation of IGT genes in peanut
The 1500-bp upstream sequences of AhIGT genes were extracted to perform the cis-element analysis. Apart from the general transcriptional regulators and elements with unknown functions, most cis-elements could group into three categories, which involved light-responsive elements, hormone-responsive elements, and transcription factor (TF)-binding elements (Fig. 5). Light-responsive elements included AE-box, Box4, G-box, GA-motif, GATA-motif, GT1-motif, TCT-motif, chs-CMA1a, chs-CMA2a and Sp1. Hormone-responsive elements included CGTCA-motif and TGACG-motif (Methyl jasmonate, MeJA), P-box (gibberellic acid, GA), TCA-element (salicylic acid, SA), ABRE (abscisic acid, ABA) and TGA-element (auxin). TF-binding elements, however, included Myb, MYB, Myc, MYC. Interestingly, the light-responsive elements accounted for the majority of all cis-acting elements, which indicated that AhIGT genes may play roles in the regulation of photoactivity.
GO annotation and GO enrichment analysis of AhIGTs were performed to gain an understanding of their functions (Table S4). Only three members of AhLAZY1 were annotated, which were found to be involved in the biological process (BP) and the cellular component (CC). In the biological process, AhLAZY1-3 was annotated in gene ontology (GO) terms of hormone transport and regulation of polar auxin transport, whereas AhLAZY1-1 and AhLAZY1-2 were annotated in gravitropism. In the cellular component, three annotated genes were located in the nucleus, which was consistent with the subcellular location prediction of these genes.
Expression patterns of AhIGT genes in various tissues
To explore the expression patterns of AhIGT genes in different tissues, we downloaded the transcriptome profiles of 13 AhIGT genes across 9 different tissues from the Peanut Genome Resource (PGR) database. As shown in Fig. 6 and Table S5, some AhIGT genes displayed relatively lower expression. For example, AhLAZY3-1, AhLAZY3-2, AhLAZY5-1, AhLAZY5-2, and AhAZY1-1 had FPKM values less than 1 in all 9 tissues. In addition, most AhIGT genes were also observed to have relatively lower FPKM values in cotyledon, root, testa, pericarp, and embryo. High expression of AhIGTs was usually detected in the stem, leaves, and stem tip. For example, AhLAZY1-3 had the highest FPKM values in the stem, while AhLAZY2/4-1 and AhLAZY2/4-2 had the highest FPKM values in the stem tip. These genes also exhibited relatively higher expression in leaves or the stem. The highest expression of AhTAC1s was detected in the gynophore, and their relatively higher expression was observed in the stem, leaves and some other tissues.
Expression patterns of AhIGT genes in continuous darkness and light
The cis-elements analysis showed that the light-responsive elements accounted for the majority of all cis-acting elements in the promoter region of AhIGT genes. Next, we explored the dynamic expression profiles of these genes under continuous dark and light conditions (Fig. 7). A total of 9 genes including AhLAZY1-1, AhLAZY1-3, AhLAZY3-1, four paralogs of AhTAC1 and two paralogs of AhLAZY5 were evaluated, out of which three (AhLAZY3-1 and two paralogs of AhLAZY5) exhibited no expression in leaves.
In continuous darkness, the expression of AhLAZY1-1 increased after 4 h of the treatment and peaked at 12 h, followed by a decrease thereafter and an increase after 24 h of darkness (Fig. 7A). Similarly, the expression of AhLAZY1-3 increased after 1 h, peaked at 4 h, and then declined, with a final increase after 12 h of dark treatment (Fig. 7B). The expressions of both AhTAC1-1 and AhTAC1-3 were variable (Fig. 7C, D). However, AhTAC1-2 and AhTAC1-4 showed different expression patterns. Although their expression showed a relatively lower level at 1 and 12 h, it started increasing in continuous darkness and peaked at 48 h (Fig. 7E, F).
In continuous light, the expressions of both AhLAZY1-1 and AhLAZY1-3 were variable (Fig. 7A, B). AhTAC1-1 and AhTAC1-3 showed similar expression patterns, i.e., their expression was first downregulated gradually and then upregulated in a stepwise manner in the late stage. In addition, both these two genes demonstrated sharply increased expression at 8 h (Fig. 7C, D). Similar expression patterns with little difference were also observed in AhTAC1-2 and AhTAC1-4, with a sharp decrease after 30 min, followed by a gradual decline after 24 h (Fig. 7E, F).
Discussion
The IGT family genes are known as key players in gravity sensing, which is critical to the optimization of crop architecture1. Currently, 4, 2, 27 and 4 members of the IGT gene family have been identified in rice27, maize1, rapeseeds28 and apple29. In this study, 13 AhIGT genes were identified, unevenly distributed on 9 chromosomes. The 13 AhIGT genes could be classified into three group, consistent with previous studies’ results1,25. WGD is recognized as a major driving force of plant evolution, resulting in gene duplication and subsequent silencing and removal of duplicate genes51. Phylogenetic, chromosomal locations and synteny analysis revealed strong collinear relationships among most IGT genes in A. hypogaea between the AA and BB subgenome. Additionally, most AhIGT genes in the AA and BB subgenomes showed corresponding relationship with those in A. duranensis and A. ipaensis, respectively. These indicated the crucial role of segmental duplications in the expansion of the IGT gene family. Although tetraploid peanut contains the same number of IGT genes in A. duranensis and A. ipaensis combined, the number of IGT homologous genes between their subgenome in A. hypogaea and its two wild progenitors was not equivalent. This suggests that the chromosome doubling and then sequence arrangement would cause the loss of IGT genes during the polyploidization process.
In Arabidopsis, 5 short conserved regions and a unique intro-exon arrangement were presented in IGT family genes1,52. These conserved regions and intro-exon arrangement are also identified in IGT family genes in rice27, rapeseeds28 and apple29. In this study, motif 1, 2 and 3 corresponded exactly to the conserved regions II, I and V, and a similar intro-exon arrangement was also presented in AhIGT family. The exon–intron arrangement could also explain the evolutionary history of the gene family53. It is wide accepted that homologous proteins with similar gene structures and conserved motifs across different species may possess identical or similar functions1,52,54. So, it is conjectured that AhIGTs might participated gravity sensing and shaping plant architecture in peanuts.
Using public databases30, the expression patterns of AhIGTs across 9 different tissues were obtained. Interestingly, the expression level of AhLAZY1-3 in the stem tip and stem was much greater than that of the other two paralogs AhLAZY1-1 and AhLAZY1-2. GO annotation analysis indicated that AhLAZY1-3, not AhLAZY1-1/2, were annotated in the regulation of auxin polar transport. It is known that IGT genes regulate the gravitropic responses through asymmetrical auxin distribution1,15,52. In the meantime, only AhLAZY1-3 contained motif 3 which is a key domain of AtLAZY1 in Arabidopsis6. Hence, it is reasonable to conjecture that AhLAZY1-3 might participate in gravity sensing and shaping peanut architecture. However, this prediction needs to be tested by more experimental evidence. A much higher expression level of all AhTAC1 genes was found in the gynophore. Gynophore in peanuts is specialized to develop peg which is essential for burying the fertilized ovary into the soil. Positive gravitropism is the most typical features of pegs26. Whether AhTAC1s participate in peg penetration into the soil is another interesting project supported by further experimental evidence in the future.
Cis-element analysis showed that light-responsive elements accounted for most cis-acting elements in the promoter of AhIGTs. Several recent studies have reported that the signals of light and gravity function together in shaping plant architecture21,22,23. In Arabidopsis, the expression of TAC1 gradually declined once plants were transferred into the dark and returned to their normal levels 48 h after it was transferred back into the light24. The expression of TAC1, which plays an important role in regulating plant architecture in response to light signals, was light dependent24. qRT-PCR revealed that the expression of AhTAC1-2/4 was also light dependent but with a different pattern. AhTAC1-2/4 expression increased in the dark and then decreased sharply once transferred back in the light. Hence, it is speculated that AhTAC1–2/4 may have regulated plant architecture in response to light signals.
Conclusions
In this study, a genome-wide analysis of IGT family genes was performed in the genome of cultivated. A total of 13 AhIGT genes, which were found to be unevenly distributed on 9 chromosomes and classified into three groups, were identified. Gene structure, conserved motifs, and cis-element were examined to gain insights into IGT genes in peanuts. Their expression profiles across different tissues, and under different light and dark conditions were also evaluated. Results revealed that AhLAZY1-3 may play roles in shaping peanut architecture, AhTAC1 could potentially be involved in the process of peg penetration into the soil, and several AhIGT genes, like AhTAC1-2/4 may regulate plant architecture in response to gravity signals. The results provide a theoretical basis for further characterization of the biological functions of the AhIGT genes in shaping plant architecture.
Data availability
The data used in this article are open and publicly available. The links to the databases are listed below. Peanut Genome Resource (http://peanutgr.fafu.edu.cn/Download.php). The Arabidopsis Information Resource (TAIR) database (https://www.arabidopsis.org/). The PeanutBase (https://peanutbase.org). WoLF PSORT (https://www.genscript.com/wolf-psort.html). PlantCARE website (http://bioinformatics.psb.ugent.be/webtools/plantcare/html). ProtParam tool of ExPaSy (https://web.expasy.org/protparam/).
References
Waite, J. M. & Dardick, C. The roles of the IGT gene family in plant architecture: Past, present, and future. Curr. Opin. Plant Biol. 59, 101983. https://doi.org/10.1016/j.pbi.2020.101983 (2021).
Yoshihara, T. & Spalding, E. P. Switching the direction of stem gravitropism by altering two amino acids in AtLAZY11. Plant Physiol. 182, 1039–1051. https://doi.org/10.1104/pp.19.01144 (2019).
Yoshihara, T., Spalding, E. P. & Iino, M. AtLAZY1 is a signaling component required for gravitropism of the Arabidopsis thaliana inflorescence. Plant J. 74, 267–279. https://doi.org/10.1111/tpj.12118 (2013).
Dardick, C. et al. PpeTAC1 promotes the horizontal growth of branches in peach trees and is a member of a functionally conserved gene family found in diverse plants species. Plant J. 75, 618–630. https://doi.org/10.1111/tpj.12234 (2013).
Nakamura, M., Nishimura, T. & Morita, M. T. Bridging the gap between amyloplasts and directional auxin transport in plant gravitropism. Curr. Opin. Plant Biol. 52, 54–60. https://doi.org/10.1016/j.pbi.2019.07.005 (2019).
Yoshihara, T. & Spalding, E. P. Switching the direction of stem gravitropism by altering two amino acids in AtLAZY1. Plant Physiol. 182, 1039–1051. https://doi.org/10.1104/pp.19.01144 (2020).
Ashraf, A. et al. Evolution of deeper rooting 1-like homoeologs in wheat entails the C-terminus mutations as well as gain and loss of auxin response elements. PLoS One 14, e0214145. https://doi.org/10.1371/journal.pone.0214145 (2019).
Guseman, J. M., Webb, K., Srinivasan, C. & Dardick, C. DRO1 influences root system architecture in Arabidopsis thaliana and Prunus species. Plant J. 89, 1093–1105. https://doi.org/10.1111/tpj.13470 (2017).
Furutani, M. et al. Polar recruitment of RLD by LAZY1-like protein during gravity signaling in root branch angle control. Nat. Commun. 11, 76. https://doi.org/10.1038/s41467-019-13729-7 (2020).
Jones, J. W. & Roy, A. C. A "LAZY" mutation in rice. J. Hered. 8 (1938).
Vanoverbeek, J. "LAZY,"an a-geotropic form of maize. J. Hered. (1936).
Yoshihara, T. & Iino, M. Identification of the gravitropism-related rice gene LAZY1 and elucidation of LAZY1-dependent and -independent gravity signaling pathways. Plant Cell Physiol. 48, 678–688. https://doi.org/10.1093/pcp/pcm042 (2007).
Li, P. et al. LAZY1 controls rice shoot gravitropism through regulating polar auxin transport. Cell Res. 17, 402–410. https://doi.org/10.1038/cr.2007.38 (2007).
Dong, Z. et al. Maize LAZY1 mediates shoot gravitropism and inflorescence development through regulating auxin transport, auxin signaling, and light response. Plant Physiol. 163, 1306–1322. https://doi.org/10.1104/pp.113.227314 (2013).
Yoshihara, T. & Spalding, E. P. LAZY genes mediate the effects of gravity on auxin gradients and plant architecture. Plant Physiol. 175, 959–969. https://doi.org/10.1104/pp.17.00942 (2017).
Uga, Y., Okuno, K. & Yano, M. Dro1, a major QTL involved in deep rooting of rice under upland field conditions. J. Exp. Bot. 62, 2485–2494. https://doi.org/10.1093/jxb/erq429 (2011).
Kitomi, Y. et al. Root angle modifications by the DRO1 homolog improve rice yields in saline paddy fields. Proc Natl Acad Sci USA 117, 21242–21250. https://doi.org/10.1073/pnas.2005911117 (2020).
Yu, B. et al. TAC1, a major quantitative trait locus controlling tiller angle in rice. Plant J. 52, 891–898. https://doi.org/10.1111/j.1365-313X.2007.03284.x (2007).
Hollender, C. A., Hill, J. L. Jr., Waite, J. & Dardick, C. Opposing influences of TAC1 and LAZY1 on lateral shoot orientation in Arabidopsis thaliana. Sci. Rep. 10, 6051. https://doi.org/10.1038/s41598-020-62962-4 (2020).
Basu, U. & Parida, S. K. Restructuring plant types for developing tailor-made crops. Plant Biotechnol. J. https://doi.org/10.1111/pbi.13666 (2021).
Digby, J. & Firn, R. D. Light modulation of the gravitropic set-point angle (GSA). J. Exp. Bot. 53, 377–381. https://doi.org/10.1093/jexbot/53.367.377 (2002).
Vandenbrink, J. P., Kiss, J. Z., Herranz, R. & Medina, F. J. Light and gravity signals synergize in modulating plant development. Front. Plant Sci. 5, 563. https://doi.org/10.3389/fpls.2014.00563 (2014).
Roychoudhry, S. et al. The developmental and environmental regulation of gravitropic setpoint angle in Arabidopsis thaliana and bean. Sci. Rep. 7, 42664. https://doi.org/10.1038/srep42664 (2017).
Waite, J. M. & Dardick, C. TILLER ANGLE CONTROL 1 modulates plant architecture in response to photosynthetic signals. J. Exp. Bot. 69, 4935–4944. https://doi.org/10.1093/jxb/ery253 (2018).
Waite, J. M. & Dardick, C. IGT/LAZY family genes are differentially influenced by light signals and collectively required for light-induced changes to branch angle. bioRxiv, https://doi.org/10.1101/2020.07.15.205625 (2020).
Kumar, R. et al. Peg biology: deciphering the molecular regulations involved during peanut peg development. Front. Plant Sci. 10, 1289. https://doi.org/10.3389/fpls.2019.01289 (2019).
Zhao, J. et al. Characteristics of members of IGT family genes in controlling rice root system architecture and tiller development. Front. Plant Sci. 13, 961658. https://doi.org/10.3389/fpls.2022.961658 (2022).
Sun, C. et al. Genome-wide identification and characterization of the IGT gene family in allotetraploid rapeseed (Brassica napus L.). DNA Cell Biol. 40, 441–456. https://doi.org/10.1089/dna.2020.6227 (2021).
Wang, L. et al. The isolation of the IGT family genes in Malus × domestica and their expressions in four idiotype apple cultivars. Tree Genet. Genomes 14, 46. https://doi.org/10.1007/s11295-018-1258-9 (2018).
Zhuang, W. et al. The genome of cultivated peanut provides insight into legume karyotypes, polyploid evolution and crop domestication. Nat. Genet. 51, 865–876. https://doi.org/10.1038/s41588-019-0402-2 (2019).
Rozewicki, J., Li, S., Amada, K. M., Standley, D. M. & Katoh, K. MAFFT-DASH: Integrated protein sequence and structural alignment. Nucleic Acids Res. 47, W5–W10. https://doi.org/10.1093/nar/gkz342 (2019).
Potter, S. C. et al. HMMER web server: 2018 update. Nucleic Acids Res. 46, W200-w204. https://doi.org/10.1093/nar/gky448 (2018).
Bertioli, D. J. et al. The genome sequences of Arachis duranensis and Arachis ipaensis, the diploid ancestors of cultivated peanut. Nat. Genet. 48, 438–446. https://doi.org/10.1038/ng.3517 (2016).
Wilkins, M. R. et al. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 112, 531–552. https://doi.org/10.1385/1-59259-584-7:531 (1999).
Horton, P. et al. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 35, W585-587. https://doi.org/10.1093/nar/gkm259 (2007).
Hu, B. et al. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 31, 1296–1297. https://doi.org/10.1093/bioinformatics/btu817 (2015).
Bailey, T. L. et al. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 37, W202-208. https://doi.org/10.1093/nar/gkp335 (2009).
Chen, C. et al. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 13, 1194–1202. https://doi.org/10.1016/j.molp.2020.06.009 (2020).
Voorrips, R. E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J Hered 93, 77–78. https://doi.org/10.1093/jhered/93.1.77 (2002).
Larkin, M. A. et al. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948. https://doi.org/10.1093/bioinformatics/btm404 (2007).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. https://doi.org/10.1093/molbev/msw054 (2016).
He, Z. et al. Evolview v2: An online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Res. 44, W236-241. https://doi.org/10.1093/nar/gkw370 (2016).
Krzywinski, M. et al. Circos: An information aesthetic for comparative genomics. Genome Res. 19, 1639–1645. https://doi.org/10.1101/gr.092759.109 (2009).
Wang, Y. et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 40, e49. https://doi.org/10.1093/nar/gkr1293 (2012).
Wang, D. P., Wan, H. L., Zhang, S. & Yu, J. Gamma-MYN: A new algorithm for estimating Ka and Ks with consideration of variable substitution rates. Biol. Direct 4, 20. https://doi.org/10.1186/1745-6150-4-20 (2009).
Lescot, M. et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 30, 325–327. https://doi.org/10.1093/nar/30.1.325 (2002).
Huerta-Cepas, J. et al. eggNOG 5.0: A hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 47, D309–D314. https://doi.org/10.1093/nar/gky1085 (2019).
Babicki, S. et al. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 44, W147-153. https://doi.org/10.1093/nar/gkw419 (2016).
Vennapusa, A. R., Somayanda, I. M., Doherty, C. J. & Jagadish, S. V. K. A universal method for high-quality RNA extraction from plant tissues rich in starch, proteins and fiber. Sci. Rep. 10, 16887. https://doi.org/10.1038/s41598-020-73958-5 (2020).
Zhu, H. et al. A novel salt inducible WRKY transcription factor gene, AhWRKY75, confers salt tolerance in transgenic peanut. Plant Physiol. Biochem. 160, 175–183. https://doi.org/10.1016/j.plaphy.2021.01.014 (2021).
Jiao, Y. et al. Ancestral polyploidy in seed plants and angiosperms. Nature 473, 97–100. https://doi.org/10.1038/nature09916 (2011).
Jiao, Z., Du, H., Chen, S., Huang, W. & Ge, L. LAZY gene family in plant gravitropism. Front. Plant Sci. 11, 606241. https://doi.org/10.3389/fpls.2020.606241 (2020).
Xu, G., Guo, C., Shan, H. & Kong, H. Divergence of duplicate genes in exon-intron structure. Proc Natl Acad Sci USA 109, 1187–1192. https://doi.org/10.1073/pnas.1109047109 (2012).
Hu, W., Ren, Q., Chen, Y., Xu, G. & Qian, Y. Genome-wide identification and analysis of WRKY gene family in maize provide insights into regulatory network in response to abiotic stresses. BMC Plant Biol. 21, 427. https://doi.org/10.1186/s12870-021-03206-z (2021).
Funding
This work was supported by the National Natural Science Foundation of China (NSFC; No. 32301908).
Author information
Authors and Affiliations
Contributions
W.C.and W.N. conceptualized and designed the experiment; W.C., T.J., and S.W. conducted the experiments and analyzed data; W.C. prepared the first draft of the manuscript; X.Z. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chu, W., Zhu, X., Jiang, T. et al. Genome-wide identification of peanut IGT family genes and their potential roles in the development of plant architecture. Sci Rep 13, 20400 (2023). https://doi.org/10.1038/s41598-023-47722-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47722-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.