Abstract
The sex profile estimation of pre-historic communities is often complicated by the commingled and scattered nature of skeletal assemblages. Demographic profiles are usually lacking and provide very truncated representations of these populations but proteomic analysis of sex-specific amelogenin peptides in tooth enamel brings new promise to these studies. The main objective was to obtain the sex profile of the human assemblage recovered from the Neolithic cave-necropolis of Escoural (Montemor-o-Novo, southern Portugal) through liquid chromatography-mass spectrometry. The secondary objective was to analyse sex-specific linear enamel hypoplasias (LEH), and to test the reliability of canine odontometric sex estimation. Sex estimation through peptide analysis was carried out in 36 left permanent canines which were macroscopically examined for the presence of LEH. The canine buccolingual diameter was used for odontometric sex estimation. The obtained sex ratio (0.5:1, M:F) is biased to female individuals, probably due to cultural factors since the natural sex ratio of the human population falls between 0.95:1 and 1.02:1 (M:F). A high frequency of LEH was observed, but with no significant sexual differences (p = 0.554). The mean LEH age of onset occurred at 3 years of age, with no significant differences between the sexes (p = 0.116), and was possibly related to the weaning process. Odontometric sex estimation revealed a correct classification of 80%, with a high number of males mistakenly attributed to females. This study is one of the largest samples subjected to peptide analysis, and thus demonstrates its usefulness on the research of commingled and scattered skeletal assemblages.
Similar content being viewed by others
Introduction
Biological profiling of individuals from prehistoric necropolises often poses challenges to biological anthropologists due to the usual commingled, fragmented, and scattered disposition of human skeletal remains1,2,3,4,5,6,7,8. In such cases, sex estimation is relatively challenging because it must combine sexually dimorphic skeletal element that simultaneously provides the highest possible minimum number of individuals (MNI). This is a common scenario in prehistoric funerary contexts due to complex social, environmental, and biological taphonomic factors9 and is also the case of the Neolithic Escoural Cave with a MNI of 109 (42 immatures and 67 matures) located in the Alentejo (Montemor-o-Novo, southern Portugal), for which the sex profile is discussed in this paper.
In both biological and forensic anthropology, sex estimation is a key element in the investigation of the biological profile, along with age at death, stature, and ancestry10,11. In turn, age at death, stature and ancestry estimates are sex-dependant12,13,14, which makes sex assessment even more relevant. Its importance relies also in the fact that it is critical for palaeodemographic reconstructions and provides better insight about the activities of human past populations, namely funerary practices3,4,5,6,7,15,16,17,18,19,20.
Ancient DNA still does not constitute an easy and widespread solution to the sex profiling of collective necropolises since it requires a high sample consumption which does not contribute to the preservation of archaeological materials, and is highly sensitive to the DNA contamination during the excavation, material manipulation, and analysis, besides being too expensive, time-consuming, and requiring good preservation of the material21,22. Therefore, conventional skeletal sex estimation is still the most accessible approach to study the sex profile of ancient populations. Metric and morphognostic skeletal sex estimations are based on sexual dimorphism in size and shape at the intra- and inter-population levels23 due to genetic and hormonal sexual differences starting early during foetal development at 7–9 gestational weeks11,24,25.
However, the sex estimation reliability of immature remains is much lower than that of adult remains and is therefore often under-studied leading to truncated sex profiles, i.e. excluding non-adult. The development of more reliable sex estimation methods applied to immature individuals is hampered by the usual reduced size of available reference samples25. Nevertheless, both metric and morphognostic26,27,28,29,30 methods have been proposed to estimate the sex of non-adults, with varied accuracies and a tendency towards better classification of males to the detriment of females (see review in25).
Metric methods have a serious drawback since they are population-specific and applying them to other populations can be problematic31,32,33. Another issue is that the most dimorphic portions of long bones, the epiphyses34, are often poorly preserved in archaeological contexts thus limiting their use. Immature remains are also known to present worse preservation. In turn, morphognostic methods are based on qualitative assessments of sexual indicators and/or their expressivity thus being more susceptible to intra and interobserver errors32,35,36,37,38,39. Sexual dimorphism is population-specific both at the temporal and spatial scales40,41,42 besides being also variable at the intra-population level43. Although the hip bone, which is the most sexually dimorphic bone, is not as much affected by these hindrances41, it is often not sufficiently preserved in collective tombs with commingled, fragmented, and scattered remains to allow for the widespan estimation of sex.
Preservation problems are less of an issue with teeth, which tend to preserve better than bones2,5,7,44,45,46,47. Pilloud and Scott48 showed that, among different populations, the most dimorphic measurement was the buccolingual diameter of the lower canine along with, to a smaller degree, the same measurement on the upper canine. In non-adults, these were also demonstrated to be the most sexually dimorphic features27. Therefore, and despite the limitations mentioned above, odontometry appears to be one potential option to achieve sex estimation of commingled, scattered, and poorly preserved remains in large assemblages, although only rarely this claim has been put to test in archaeological remains since no DNA or peptides confirmation are usually carried out in large scale49.
The ground-breaking potential of sex estimation through tooth enamel peptides has recently been demonstrated50,51,52,53,54,55,56,57,58 and encompasses several advantages. It is reliable, fast, inexpensive, and minimally destructive50,58,59. As mentioned above, tooth enamel is very resistant to taphonomic and diagenetic factors owing to its larger bioapatite crystals and higher mineral content compared to bone. Teeth are indeed among the skeletal elements providing highest MNIs owing to their better preservation5,7,60,61,62.
The advantages of teeth are not limited to sex estimation. Teeth record infancy’s non-specific physiological stress events through linear enamel hypoplasias (LEH hereafter) often caused by severe illness and/or nutritional deficiencies63. Since tooth crowns do not suffer remodelling, LEH are a good indicator of those events64 occurring during amelogenesis between the 6th gestational month and 18.5 years65 even though LEH age estimation is only roughly achieved, since dental wear is difficult to assess and measure64,66,67.
The main objective of this paper was to obtain the most complete as possible sex profile of the collection of human remains from the Neolithic cave-necropolis of Escoural, based on amelogenin analyses. Secondary objectives were to assess potential sex differences regarding LEH-monitored physiological stress events, and to test the reliability of canine odontometric sex estimation by comparing it with the sex classifications obtained through the peptides analysis. As a result, this paper provides a comprehensive portrait of the sex profile of the Escoural Cave-necropolis, which is inclusive of both adult and non-adult individuals, a feat never before achieved for Neolithic human populations inhabiting this Portuguese inner Alentejo region.
Site description
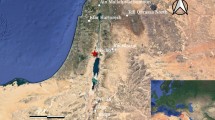
To date, the Escoural Cave is the only karst system known in the inner Alentejo (Montemor-o-Novo, southern Portugal) (Fig. 1). It documents archaeological remains from the Middle Palaeolithic (short-term hunting camp), the Upper Palaeolithic (rock art) and the Neolithic (necropolis)68. A fortified Chalcolithic settlement was established above Neolithic rock carvings on the top of the hill where the entrance of the cave is. The latter is also surrounded by numerous megalithic monuments (it is in fact the richest region in the country in megalithic architecture), although most of them have not preserved human remains due to the acidity of the granite soils that characterizes the region69,70. Additionally, the excavation strategies carried out in those monuments during the 19th and the early twentieth centuries were not the most adequate, especially with regard to the recovery and recording of materials45,70,71,72. Owing to the cultural affinities between cave-necropolis and those monuments68,73, the same people most probably used the Escoural Cave and built the megalithic monuments. Therefore, Escoural appears to be the best chance of achieving more comprehensive biocultural knowledge of these somewhat cryptic megalithic builders.
The Escoural Cave is divided into three main floors, with the necropolis occupying the intermediate and largest one (Fig. 2) (see Supplementary data S1 for historical details). This floor includes three rooms and several galleries. The main room (Room 1) has access from the current entrance; Room 2 is located on the opposite side, near the original entrance; Room 3 is located at the confluence of galleries 11 and 3 (Fig. 2). Human remains were exposed on the surface of the main room and adjacent galleries, covered by a thick layer of calcite carbonate and associated with diverse grave goods (pottery, polished stones, bone tools, adornments, etc.)68.
Generally, the human remains were commingled on the surface layers and were clustered in “groups” (Fig. 3), attaining a MNI of 109 (42 immatures and 67 matures). Apparently, bones from each individual were not scattered onto multiple clusters, since bone pairs were always found intra-group.
Exceptionally, an adult female individual in partial anatomical connection was present in Group 1 (Fig. 4). She was deposited at the surface on her left flank, with the legs flexed and associated to three ceramic bowls and a probable female cranium, also on her left side. This cranium was not in anatomical connection, being unclear if it belonged to a primary or a secondary deposition. Both depositions were surrounded by several secondarily deposited immature and mature bones. Other two exceptions to the secondary surface depositions comprised apparent secondary depositions of crania in shelves and niches (Figs. 5 and 6).
Santos74,75 also mentions an inhumation in a pit, but there is no graphic or photographic documentation of this putative funerary practice in the archives of the National Museum of Archaeology. Human remains were found also outside the cave, next to the south-western natural entrance. Radiocarbon results from bone samples retrieved in this outer area do not differ from those obtained inside the cave, pointing to its funerary use during the Late Neolithic (~ 3600 to ~ 3000 cal BC). Moreover, some skeletal remains display on their surfaces the same carbonate concretions that characterize the material from inside the cave, suggesting that their primary origin was also most likely this same. Therefore, both samples from inside and outside the cave were brought together and studied as one for the purpose of this study.
Material and methods
Peptide analysis
Sex estimation through peptide analysis was carried out in 36 lower left permanent canines (LLPCs), for which enamel mineralization occurs at 1.5–6.5 years65. Three of these canines were not completely formed (root mineralization was ¼ complete in two teeth while initial root formation with diverse edges was presented by another tooth), eight had a broken root and 25 were mature or in mature mandibles.
Enamel fragments (ca. 10 mg) were washed with MilliQ using an ultrasonic bath and leached for a few minutes using 5% HCl. Then, enamel peptides were extracted submerging enamel fragments in 250 µl of 5% HCl for 1 h. The supernatant was collected and purified through C18 in-house stage tips. Resin-bounded peptides were eluted using 50 μL of 60% acetonitrile in 0.1% formic acid. The whole laboratory protocol was performed at the Proteomic facility of the BONES Lab (University of Bologna). Extracted peptides were dried and resuspended in a mixture of water:acetonitrile:formic acid 95:3:2, before the nanoLC-MS/MS measures. Analyses were conducted using Nano UHPLC Ultimate 3000 coupled to an Exploris™ 480 Hybrid Quadrupole-Orbitrap™ Mass Spectrometer via an EASY-Spray source interface, housed at the Centro Interdipartimentale Grandi Strumenti of the University of Modena and Reggio Emilia76. Peptides were loaded onto a 300 µm I.D. × 5 mm cartridge, packed with Acclaim PepMap100 C18, 5 µm, 100 Å beads then separated on a 75 μm I.D. × 150 mm EASY-Spray column, packed with Acclaim PepMap100 C18, 3 µm, 100 Å beads. The nanoLC-MS/MS system was controlled with Standard Instrument Integration (SII) for Xcalibur software. All hardware and software for data acquisition were from Thermo Fisher Scientific. The sample (2 µL) was injected onto the precolumn cartridge kept 30 °C using a 10 µL/min flow of a 0.1% formic acid in water solution. The loading step was performed for 2 min then the pre-concentrated peptides were directed toward the Easy-spray column for separation using a reverse 900 nL/min flow at the starting gradient composition. The mobile phase components were (A) 0.1% formic acid in water and (B) 0.1% formic acid in acetonitrile:water (97:3 v/v). Chromatographic separation was performed at 40 °C with B% kept constant at 4% for 3′, then linearly increased from 4 to 26% in 38 min and again from 26 to 50% in 4’; B% was then brought to 95% in 1 min and kept at 95% B for 3 min, before the reconditioning step. Each sample required a total run time of 65 min. The electrospray source was operated in positive ionization mode with the ion transfer tube temperature at 275 °C and a spray voltage of 1600 V. Mass spectrometric detection of peptides was performed using a Data-Dependent Acquisition strategy. An inclusion list with the main endogenous enamel peptides was also included in the method i.e., SM(ox)IRPPY (AMELY; [M+2H]2+ 440.2233 m/z); SYEVLTPLK (AMELX,Y; [M+2H]2+ 525.2975 m/z), SIRPPYPSY (AMELX; [M+2H]2+ 540.2796 m/z); PYFGYFGYH (ENAM; [M+2H]2+ 575.7533 m/z); YEVLTPLKWY (AMELX,Y; [M+2H]2+ 656.3528 m/z)54,55. Ion chromatograms were manually inspected using Xcalibur™ (Thermo Scientific) searching for specific AMELX (amelogenin X isoform 1; H. sapiens Q99217) and AMELY (amelogenin Y isoform 2; H. sapiens Q99218) peptides, following Stewart et al.58 and Lugli et al.54 for sex estimation. No AMEL-peaks were detected in the extraction blank, processed and analysed along with the samples. Raw chromatograms (.raw) are deposited in Zenodo (https://zenodo.org/record/8188927).
Linear enamel hypoplasias analysis
All 36 lower left permanent canines were macroscopically examined for the presence of LEH. When present, the distance between the LEH and the cementum-enamel junction (CEJ) was measured with a dental caliper. To estimate the age at which these stressful episodes occurred, the recommendations of Cares-Henriquez and Oxenham77 and Cares-Henriquez78 were adopted whenever the occlusal wear was equal or inferior to grade 2 using the classification from Smith79 adapted by Silva80. The wear was described as 10% if the tooth presented a grade-2 wear, as 5% if it presented a grade-1 wear and 0 if no wear was present.
Odontometric analysis
The buccolingual diameter was measured in the 36 LLPCs and the work of Cardoso27 was used as reference (sex discriminating cut-off point: 7.73 mm) for odontometric sex estimation. Measurements were taken three times and only the median value was used for subsequent analyses. The intra- and inter-observer variations were calculated based on the measurements obtained by two researchers (RG & DG). In the case of the intra-observer variation, measurements were obtained on two different moments with a one-year interval. Observer variation was obtained by calculating the relative technical error of measurement (%TEM)81,82.
Statistical analysis
Sex differences regarding the occurrence of LEH were tested using the Chi-square statistic. Sex differences in mean LEH age of onset were tested using an unpaired Student’s t-test. The agreement between sex classifications obtained through peptide analyses vs odontometric analyses was tested using the Kappa Cohen statistics. The intra– and inter-observer variations of the buccolingual diameter of the lower left permanent canine were calculated using the technical error of measurement on a sample of 25 specimens81,82.
Results
Sex profile through peptide analysis
Proteomics results are presented in Table 1 (see Supplementary Table S1 for details) and examples of male and female chromatograms are given in Fig. 7a and b. Sex estimation was possible in all cases, i.e. amelogenin peptides were present in all the enamel samples. 12 out of 36 samples showed the presence of AMELY specific peptides, indicating unambiguously male sex. On the other hand, 23 individuals were identified as likely females, because of the lack of AMELY and high ion signals of AMELX (~ 106–107). One sample from inside the cave (Room 1), likely due to taphonomic alterations, resulted in lower spectrum quality and ion intensity (i.e. AMELX 540.2796 m/z signal ~ 105), hampering a confident sex estimation. Since the absence of AMELY_HUMAN can be due to the female sex, low-resulting signals of AMELY peptides, or AMELY deletion83, we prefer to treat the result of sample nº 983.1308.50 with caution, being AMELX-peptide ~ 100 times lower in signal compared to the other individuals (see Fig. 7c).
Ion chromatograms of individuals estimated as (a) male (GDE 3, nº 984.98.266) and (b) female (GDE 2, nº 983.340.45). Chromatograms search was performed using Xcalibur software (Thermo Scientific) with a mass tolerance of 5 ppm. The black peak is peptide SM(ox)IRPPY (AMELY; [M+2H]2+ 440.2233 m/z); the green peak is peptide M(ox)IRPPY (AMELY; [M+2H]2+ 396.7073 m/z), while the red peak is peptide SIRPPYPSY (AMELX; [M+2H]2+ 540.2796 m/z); retention times are as expected (see, Lugli et al.54). (c) Maximum ion intensities of peptide SM(ox)IRPPY (AMELY; [M+2H]2+ 440.2233 m/z) vs peptide SIRPPYPSY (AMELX; [M+2H]2+ 540.2796 m/z), this latter reported as log10; males (as estimated through proteomics) are orange dots while (possibly) females are dark-green dots; the red dashed line is the male with the lowest 540.2796 m/z signal.
Based on this result, the overall sex ratio of 0.52:1 in favour of females. Bearing in mind that the sample outside the cave was relatively small, a higher number of males was observed outside the cave compared to females. A more complete sex profile of the assemblage was obtained by adding one other female individual whose sex estimation was achieved through the hip bone morphology of the individual in anatomical connection. As a result, a more female-prone sex ratio of 0.5:1 (M:F) was obtained overall.
Linear enamel hypoplasias
The majority of the individuals (24/36, 67%) present at least one enamel defect, a trend observed in both females (15/23, 65%) and males (9/12, 75%) with no significant sexual differences found between both sexes (χ2 = 350; p = 0.554). The probable female, who did not present any LEH, was not included in this analysis. Most individuals displaying only one LEH defect (females: 9/15, 60%; males: 6/12, 50%). Two defects were observed in 27% of females (4/15) and 17% of males (2/12) while three defects were observed in 13% of females (2/15) and 8% of males (1/12).
The mean LEH age of onset was 3.5 years in 12 females and 3.9 years in 7 males, the difference being non-significant (p = 0.116). The youngest age of onset was 2.6 years in females and 3.2 years in males while the oldest age of onset was 4.4 years and 4.6 years, respectively. In both sexes, the highest number of LEH cases occurred during the third year of life.
LEH frequency per sex
If the LEH frequency is analysed by sex, it is possible to conclude that there are no significant differences between sexes (p = 0.554). Apparently, both sexes were equally affected by physiological stress events.
Odontometric sex estimation
The intra- and inter observer variations of the buccolingual diameter provided %TEM below 2%, demonstrating the good replicability and repeatability of this standard measurement (see Supplementary Table S2 for details). The high coefficient of reliability (≥ 93%) indicated that only a small portion of the measurement variance in the sample was the result of measurement error.
Assuming that peptide-based sex estimations are correct, the reliability of odontometric sex estimation was calculated. The buccolingual diameter allowed for 80% correct sex classification of the overall sample. Based on the Kappa Cohen statistics, the agreement between the peptide and the odontometric sex estimations was moderate (0.53; p = 0.001) and 91.3% of females (21/23) and only 58.3% of males (7/12) were allocated to the correct sex.
Discussion
Sex profile
In human societies, natural sex ratios usually range from about 0.95:1 to 1.02:1 (M:F), with exceptions being reported in cases such as heavy warfare losses or intense immigration84. Sex ratios of ≤ 0.9:1 and ≥ 1.05:1 (M:F) are considered to be extreme values84. Therefore, the sex ratio observed in the Escoural Cave (0.5:1, M:F) can be assumed to have been extremely unbalanced in favour of females and, most probably, the reason for that pattern cannot be attributed to natural dynamics. The Escoural Cave was almost totally excavated, so the unbalanced sex ratio is probably not the result of excavation-related bias either (e.g., differential sieving procedures). On the other hand, differential sex-related preservation of skeletal elements does not seem to explain it as well since that usually benefits male representation due to their more robust physique85. It should be noted that all but one of the male teeth presented dental wear incompatible with a non-adult age.
Therefore, the well-marked imbalance in the sexual profile of the dead deposited in the Escoural Cave seems to be more related to anthropogenic choices of a socio-cultural nature and may reflect: (i) the contemporary demographic pattern, also unbalanced or (ii) choices dictated by cultural perceptions regarding the dead and death.
In contrast to the Escoural Cave, a more sex-balanced ratio ranging from 0.96:1 to 0.9:1 (M:F) was proposed for the Bom Santo cave, located in the Estremadura region and also used as a necropolis, but during the Middle Neolithic (3800–3400 cal BC)4,86. However, in the case of this funerary cave, sex-ratio was exclusively obtained through odontometric evaluation; so, no definitive conclusions can be obtained.
Other Portuguese cave-necropolises offer little data in terms of sex-ratio. For instance, most were used not only during the Early, Middle and Late Neolithic, but also during the Copper, Bronze and Iron ages, making the analysis even more difficult. Additionally, the number of individuals on which sex estimation was achieved is much lower than the minimum number of individuals (MNI) recorded in each of these cave-contexts. This problem becomes even more complex if the discrepancy in the assessment methods used for sex estimation (and not always fully available) is added to the debate. Consequently, the set of cave-necropolises recorded for Portuguese archaeology does not meet the conditions to support the hypothetical sex-ratio imbalance suggested by the data from the Escoural Cave. Its absolute chronology, based on several radiocarbon results (more than 30), points to their use mainly during the Late Neolithic (~ 3600 to ~ 3000 cal BC), although three additional values deviate from this chrono-cultural framework (this subject deserves a much deeper discussion, which does not fit into this paper). Once again, Bom Santo remains the best reference for comparisons, although it is somewhat older than Escoural. To reinforce this view, both have very similar material cultures.
Cases of very unbalanced sex ratios in caves have also been reported for other parts of the Iberian Peninsula. Fernández-Crespo and de-la-Rúa87 studied the sex profile of Late Neolithic past populations from the northern Spain and found sex ratios varying from 1.1:1 to 2:1 (M:F) in dolmens, whereas the caves yielded ratios ranging from 0.08:1 to 0.5:1 (M:F). It was argued that cultural factors may have played an important role in the origin of the bias87. Unfortunately, due to preservation issues, Portuguese dolmens usually have small amounts of human bones thus it is difficult to investigate potential unbalanced sex ratios88,89,90,91. Amelogenin sex estimation may help circumventing this obstacle since dental remains tend to preserve better and even allow higher MNI in some cases7,44.
On the other hand, Cintas-Peña and Sanjuán92 and Cintas-Peña and Herrero-Corral93 detected a higher proportion of male (or possible male) over female (or possible female) individuals in Iberian Neolithic assemblages (n = 119 –[23.11%] vs 79 –[15.34%]). However, the large proportion of individuals with indeterminate sex (n = 207 [61.56%]) prevents reliable conclusions. Moreover, these authors used several types of sepulchres on their analysis, with a large predominance of pit necropolises. In her study of seven Late Neolithic/Chalcolithic collective tombs (including dolmens, tholos, natural and artificial caves), Silva6,7 also detected a higher proportion of females, based on metric analyses of femurs, humeri, calcanei and tali. However, these studies suffer from the issues mentioned above and/or are from later chronologies.
As for hypothetical scenarios explaining the unbalanced sex profile in the Escoural Cave, no archaeological evidence is particularly instructive. We were unable to build a comprehensive age and health profile for this assemblage, so it was not possible to assess if the male population was particularly old or unhealthy compared to females. If so, this could be suggestive of a scenario in which younger, healthier, or more active men were dying somewhere distant from the cave (and the settlement). This would possibly be the case in situations involving warfare or transhumance, assuming that those activities were mainly performed by males, which is not at all clear. So far, no strontium isotope analyses were performed to check for possible migration patterns, which could have contributed to the sex profile here observed.
Apparently, the Escoural Cave was not restricted to one of the sexes since both female and male individuals were found in it. However, the possibility of a sex restriction rule having occurred at some point of its utilization as a necropolis cannot be entirely discarded. Such occurrence, even if implemented during a shorter period could result in the unbalanced sex profile obtained by our forcibly synchronic analysis. If a sex restriction was in place at some point in time, this would mean that specific funerary ordinances were applied to segments of the community. As mentioned before, the dolmens in the region did not preserve, in most cases, organic material that can be used for comparative purposes.
LEH
The Escoural assemblage presented an unusually high frequency of LEH in both female and male individuals. That becomes clear from both diachronic and synchronic comparisons with other prehistoric necropolises in the Portuguese territory reflecting a wide chronology from the 7th to the 3th millennia cal BC. Table 2 provide chrono-culturally homogeneous sites for which LEH observations per individual were possible.
The LEH frequency in the Portuguese prehistoric necropolises is below 42% in most of the sites. The ones with the highest LEH frequencies (above 60%) are also the ones where fewer individuals were assessed (below 40% of the MNI), so interpretation must be cautious.
Regarding Neolithic caves (Escoural and Caldeirão), LEH was observed in more than two thirds of the individuals. On the other hand, these frequencies appeared all to be under or equal to 30% in dolmens. However, due to the scarcity of data is not possible, for now, to draw inferences.
Regarding the Escoural’s LEH onset ages, values are very close to the weaning end age (3.3 ± 0.7 years old) estimated through isotopes by Fernández-Crespo et al.106 in several Spanish Late Neolithic cave necropolises. However, in the case of Escoural, it was not possible to estimate the age of 16 individuals because teeth presented a wear grade higher than 279,80, thus precluding the estimation of LEH onset age.
Odontometric sex estimation
The canine buccolingual diameter to estimate the sex of the individuals from the Escoural Cave was somewhat reliable. It allowed correct classification of four in every five individuals, even though the reference cut-off point used for that purpose was obtained from a recent Portuguese population who lived five millennia after the Escoural population27. Therefore, metric differences due to secular trend and population variability are to be expected as deduced from the very variable odontometric profiles of varied populations27,107,108,109,110,111,112,113. Indeed, although sex estimation accuracy was high, it was relatively unbalanced between the two sexes thus suggesting that the reference sex discriminating cut-off point (7.73 mm) was not entirely adjusted to the Escoural assemblage. Looking at the average canine buccolingual diameter in the latter (sex pooled mean = 7.54 mm; weighed sex pooled mean = 7.43 mm), it became clear that the reference recent population presented larger mean canine metric features. As a result, the reference cut-off point led to the high number of males mistakenly attributed to the female sex. The Escoural sex pooled mean could have been used as a sample-specific sex discriminating cut-off point, an approach used previously in archaeological research4,114 but we chose not to do it because the required sample was smaller than the 40 specimens recommended by Albanese et al.31. Only used here as an illustration of the benefit of using sample-specific metric references that are more adjusted to the population under study, the informal testing of the sample-specific cut-off point would result in a more balanced correct sex classification (females: 77%; males: 75%).
As expected, the odontometric approach does not guarantee a reconstitution of the sex profile as reliable as those obtained from DNA and peptide analyses. Some degree of error should be expected from sex ratios obtained through canine buccolingual diameters. 20 to 25% of the sample was incorrectly sex estimated following the application of sample-specific cut-off points in the Escoural assemblage. Therefore, the use of intervals taking into account such magnitudes of variation appears to be recommendable.
Conclusion
This research sheds new light on the way of life of the Neolithic community that used the Escoural Cave 5000 years ago. To date, this is the largest Portuguese sample subjected to peptide sex estimation. Possibly, the cave played a differential role in the funerary processing of women and men of this Neolithic community which mirrors the one reported for communities from northern Iberia. Nonetheless, the LEH analysis suggested that stress physiological episodes in the infancy were probably equally frequent and transverse to both sexes. The large presence of LEH can possibly be explained by a stressful weaning process which equally affected all analysed individuals at similar ages independently of their sex.
The new and important data gathered in this research were only possible to obtain thanks to the uniquely good preservation of the numerous human remains of this archaeological site which is culturally homogenous. Moreover, the large number of recovered teeth enabled the proteomic analysis of sex-specific amelogenin peptides. This new methodology is ground-breaking and overcomes problematic issues related to the sex estimation of immature and mature individuals, especially in contexts where remains are commingled, scattered, and/or fragmented, as is often the case in prehistorical necropolises. Peptide analysis allowed us to go further in terms of a more holistic assessment of funerary practices, demographic data or even pathologies. Therefore, this approach can promote the active re-visitation of other emblematic Portuguese Neolithic sites and it would be interesting to extend this approach to the individuals deposited in other coeval tombs, as dolmens and caves, to replicate the study presented here.
Peptide analysis appears to have an advantage over the forcibly reductive osteological examination of commingled and scattered remains as well as over the considerably more expensive aDNA analysis thus constituting an appealing resource for bioarchaeological research.
Data availability
The generated during and/or analysed during the current study are available in the Zenodo repository (https://zenodo.org/record/8188927).
References
Carvalho, A. F., Antunes-Ferreira, N. & Valente, M. J. A gruta-necrópole neolítica do Algar do Barrão (Monsanto, Alcanena). Rev. Port. Arqueol. 6, 101–119 (2003).
Evangelista, L. E. S. Resting in peace or in pieces? Tomb I and death management in the 3rd millennium BC at the Perdigões enclosure (Reguengos de Monsaraz, Portugal). Preprint at http://hdl.handle.net/10316/81204 (2017).
Fernández-Crespo, T. & de-la-Rúa, C. Demographic evidence of selective burial in megalithic graves of northern Spain. J. Archaeol. Sci. 53, 604–617 (2015).
Gonçalves, D., Granja, R., Cardoso, F. A. & Carvalho, A. F. Sample-specific sex estimation in archaeological contexts with commingled human remains: A case study from the Middle Neolithic cave of Bom Santo in Portugal. J. Archaeol. Sci. 49, 185–191 (2014).
Granja, R., Gonçalves, D. & Alves-Cardoso, F. Osteological sample profile. In Bom Santo Cave (Lisbon) and the Middle Neolithic societies of Southern Portugal (ed. Carvalho, A. F.) 101–119 (Promontoria Monográfica 17, Universidade do Algarve, 2014).
Silva, A. M. Portuguese populations of Late Neolithic and Chalcolithic periods exhumed from collective burials: An overview. Anthropologie 41, 55–64 (2003).
Silva, A. M. Antropologia funerária e paleobiologia das populações portuguesas (litorais) do Neolítico final/Calcolítico (Fundação Calouste Gulbenkian, 2012).
Silva, A. M. et al. Mortuary practices in Perdigões (Reguengos de Monsaraz, Portugal): Bio-anthropological approach to Tomb 2. Menga 8, 71–86 (2017).
Suárez, M. V. et al. Burial taphonomy and megalithic ritual practices in Iberia: The Panoría cemetery. Archaeol. Anthropol. Sci. 15, 18. https://doi.org/10.1007/s12520-023-01716-5 (2023).
Boldsen, J. L. & Milner, G. R. Age estimation (skeleton). In The International Encyclopedia of Biological Anthropology (ed. Trevathan, W.) 1–5 (Wiley, 2018).
Marino, R., Tanganelli, V., Pietrobelli, A. & Belcastro, M. G. Evaluation of the auricular surface method for subadult sex estimation on Italian modern (19th to 20th century) identified skeletal collections. Am. J. Phys. Anthropol. 174, 792–803 (2021).
Coqueugniot, H. & Weaver, T. D. Brief communication: infracranial maturation in the skeletal collection from Coimbra, Portugal: New aging standards for epiphyseal union. Am. J. Phys. Anthropol. 134, 424–437 (2007).
Dunn, R. R., Spiros, M. C., Kamnikar, K. R., Plemons, A. M. & Hefner, J. T. Ancestry estimation in forensic anthropology: A review. WIREs. Forensic Sci. 2, e1369. https://doi.org/10.1002/wfs2.1369 (2020).
Mendonça, M. C. Estimation of height from the length of long bones in a Portuguese adult population. Am. J. Phys. Anthropol. 112, 39–48 (2000).
Elliot, E., Saupe, T., Thompson, J. E., Robb, J. E. & Scheib, C. L. Sex bias in Neolithic megalithic burials. Am. J. Biol Anthropol 180, 196–206 (2022).
Fernández-Crespo, T. & Schulting, R. J. Living different lives: Early social differentiation identified through linking mortuary and isotopic variability in Late Neolithic/Early Chalcolithic north-central Spain. PLoS ONE 12, e0177881. https://doi.org/10.1371/journal.pone.0177881 (2017).
Fowler, C. et al. A high-resolution picture of kinship practices in an Early Neolithic tomb. Nature 601, 584–587 (2022).
Klales, A. R. Sex Estimation of the Human Skeleton (Academic Press, 2020).
Sánchez-Quinto, F. et al. Megalithic tombs in western and northern Neolithic Europe were linked to a kindred society. PNAS 116, 9469–9474. https://doi.org/10.1073/pnas.1818037116 (2019).
Ubelaker, D. H. & DeGaglia, C. M. Population variation in skeletal sexual dimorphism. Forensic Sci. Int. 278(407), e1-407.e7 (2017).
Mikšík, I., Morvan, M. & Brůžek, J. Peptide analysis of tooth enamel—A sex estimation tool for archaeological, anthropological, or forensic research. J. Sep. Sci. 46, 2300183 (2023).
Skoglund, P., Storå, J., Götherström, A. & Jakobsson, M. Accurate sex identification of ancient human remains using DNA shotgun sequencing. J. Archaeol. Sci. 40, 4477–4482 (2013).
Klales, A. R. Introduction to sex estimation and this volume. In Sex Estimation of the Human Skeleton, History, Methods, and Emerging Techniques (ed. Klales, A.) xxxi–xli (Academic Press, 2020).
Nikitovic, D. Sexual dimorphism (humans). In The International Encyclopedia of Biological Anthropology (eds Trevathan, W. et al.) 1–4 (Wiley, 2018).
Stull, K. E., Cirillo, L. E., Cole, S. J. & Hulse, C. N. Subadult sex estimation and KidStats. In Sex Estimation of the Human Skeleton, History, Methods, and Emerging Techniques (ed. Klales, A. R.) 219–242 (Academic Press, 2020).
Calleja, A. M. M., Aranda, C. M., Santos, A. L. & Luna, L. H. Evaluation of the auricular surface method for non-adult sex estimation on the Lisbon documented collection. Am. J. Phys. Anthropol. 172, 1–11 (2020).
Cardoso, H. F. V. Sample-specific (universal) metric approaches for determining the sex of immature human skeletal remains using permanent tooth dimensions. J. Archaeol. Sci. 35, 158–168 (2008).
Luna, L. H., Aranda, C. M. & Santos, A. L. New method for sex prediction using the human non-adult auricular surface of the ilium in the collection of identified skeletons of the University of Coimbra. Int. J. Osteoarchaeol. 27, 898–911 (2017).
Schutkowski, H. Sex determination of infant and juvenile skeletons: 1. Morphognostic features. Am. J. Phys. Anthropol. 90, 199–205 (1993).
Wilson, L. A., MacLeod, N. & Humphrey, L. T. Morphometric criteria for sexing juvenile human skeletons using the ileum. J. Forensic Sci. 53, 269–278 (2008).
Albanese, J., Cardoso, H. & Saunders, S. Universal methodology for developing univariate sample-specific sex determination methods: An example using the epicondylar breadth of the humerus. J. Archaeol. Sci. 32, 143–152 (2005).
Brůžek, J. & Murail, P. Methodology and reliability of sex determination from the skeleton. In Forensic Anthropology and Medicine (eds Schmitt, A. et al.) 225–242 (Humana Press, 2006).
Franklin, D., Cardini, A., Flavel, A. & Kuliukas, A. The application of traditional and geometric morphometric analysis for forensic quantification of sexual dimorphism: Preliminary investigations in a Western Australian population. Int. J. Legal Med. 126, 549–558 (2012).
Spradley, M. K. & Jantz, R. L. Sex estimation in forensic anthropology: Skull versus postcranial elements. J. Forensic Sci. 56, 289–296 (2011).
Brůžek, J. A method for visual determination of sex, using the human hip bone. Am. J. Phys. Anthropol. 117, 157–168 (2002).
Ferembach, D., Schwidetzky, I. & Stloukal, M. Recommendations for age and sex diagnoses of skeletons. J. Hum. Evol. 9, 517–549 (1980).
Phenice, T. W. A newly developed visual method of sexing the os pubis. Am. J. Phys. Anthropol. 30, 297–301 (1969).
Pretorius, E., Steyn, M. & Scholtz, Y. Investigation into the usability of geometric morphometric analysis in assessment of sexual dimorphism. Am. J. Phys. Anthropol. 129, 64–70 (2006).
Rösing, F. et al. Recommendations for the forensic diagnosis of sex and age from skeletons. HOMO- J. Comp. Hum. Biol. 58, 75–89. https://doi.org/10.1016/j.jchb.2005.07.002 (2007).
Guyomarc’h, P. et al. Impact of secular trends on sex assessment evaluated through femoral dimensions of the Czech population. Forensic Sci. Int. 262(284), e1-284.e6 (2016).
Brůžek, J., Santos, F., Dutailly, B., Murail, P. & Cunha, E. Validation and reliability of the sex estimation of the human os coxae using freely available DSP2 software for bioarchaeology and forensic anthropology. Am. J. Phys. Anthropol. 164, 440–449 (2017).
Spradley, M. K. & Stull, K. E. Sex estimation (skeleton). In The International Encyclopedia of Biological Anthropology (eds Trevathan, W. et al.) 1–3 (Wiley, 2018).
Boldsen, J. L., Milner, G. R. & Ousley, S. D. Paleodemography: From archaeology and skeletal age estimation to life in the past. Yearb. Phys. Anthropol. 178, 115–150 (2021).
Boaventura, R. As antas e o Megalitismo da região de Lisboa. Preprint at https://repositorio.ul.pt/bitstream/10451/587/1/21498_Vol%201%20As%20Antas%20e%20Megalitismo%20de%20Lisboa.pdf (2009)
Boaventura, R., Ferreira, M. T., Neves, M. J. & Silva, A. M. Funerary practices and anthropology during the middle-late Neolithic (4th and 3rd Millenia BCE) in Portugal: Old bones, new insights. Anthropologie 52, 183–205 (2014).
Thompson, A. R. Odontometric determination of sex at Mound 72, Cahokia. Am. J. Phys. Anthropol. 151, 408–419 (2013).
Viciano, J., D’Anastasio, R. & Capasso, L. Odontometric sex estimation on three populations of the Iron Age from Abruzzo region (central-southern Italy). Arch. Oral Biol. 60, 100–115 (2015).
Pilloud, M. A. & Scott, G. R. Dentition in the estimation of sex. In Sex Estimation of the Human Skeleton, History, Methods, and Emerging Techniques (ed. Klales, A.) 149–169 (Academic Press, 2020).
Jusić, B. et al. Sex determination of medieval skeletal remains: Evaluation of anthropological, odontological and genetic methods. J. Bioanthropol. 2, 37–44. https://doi.org/10.54062/jb.2.1.2 (2022).
Buonasera, T. et al. A comparison of proteomic, genomic, and osteological methods of archaeological sex estimation. Sci. Rep. 10, 11897. https://doi.org/10.1038/s41598-020-68550-w (2020).
Cintas-Peña, M. et al. Amelogenin peptide analyses reveal female leadership in Copper Age Iberia (c. 2900–2650 BC). Sci. Rep. 13, 9594. https://doi.org/10.1038/s41598-023-36368-x (2023).
Froment, C. et al. Analysis of 5000 year-old human teeth using optimized large-scale and targeted proteomics approaches for detection of sex-specific peptides. J. Proteomics 211, 103548 (2020).
Gasparini, A. et al. Biological sex vs. archaeological gender: Enamel peptide analysis of the horsemen of the Early Middle age necropolises of Campochiaro (Molise, Italy). J. Archaeol. Sci. Rep. 41, 103337 (2022).
Lugli, F. et al. Enamel peptides reveal the sex of the Late Antique ‘Lovers of Modena’. Sci. Rep. 9, 13130. https://doi.org/10.1038/s41598-019-49562-7 (2019).
Lugli, F. et al. Sex-related morbidity and mortality in non-adult individuals from the Early Medieval site of Valdaro (Italy): The contribution of dental enamel peptide analysis. J. Archaeol. Sci. Rep. 34, 102625 (2020).
Parker, G. J. et al. Sex estimation using sexually dimorphic amelogenin protein fragments in human enamel. J. Archaeol. Sci. 101, 169–180 (2019).
Rebay-Salisbury, K. et al. Gendered burial practices of early Bronze Age children align with peptide-based sex identification: A case study from Franzhausen I, Austria. J. Archaeol. Sci. 139, 105549 (2022).
Stewart, N. A., Gerlach, R. F., Gowland, R. L., Gron, K. J. & Montgomery, J. Sex determination of human remains from peptides in tooth enamel. PNAS 114, 13649–13654. https://doi.org/10.1073/pnas.1714926115 (2017).
Brůžek, J. et al. Undertaking sex assessment of human remains within cultural heritage: Applicability of minimally-invasive methods for proteomic sex estimation from enamel peptides.https://doi.org/10.2139/ssrn.4439221.
Hillson, S. Teeth (Cambridge University Press, 2005).
Kinaston, R., Willis, A., Miszkiewicz, J. J., Tromp, M. & Oxenham, M. F. The dentition: Development, disturbances, disease, diet, and chemistry. In Ortner’s Identification of Pathological Conditions in Human Skeletal Remains (ed. Buikstra, J. E.) 749–797 (Elsevier Academic Press, 2019).
Scott, G. R. Dental morphology. In Biological Anthropology of the Human Skeleton (eds Katzenberg, M. A. & Saunders, S. R.) 265–298 (Wiley, 2008).
Hillson, S. Health, stress and evolution: Case studies in bioarchaeology and palaeoanthropology. In Tooth Development in Human Evolution and Bioarchaeology (ed. Hillson, S.) 198–227 (Cambridge University Press, 2014).
Bereczki, Z., Teschler-Nicola, M., Marcsik, A., Meinzer, N. J. & Baten, J. Growth disruption in children. Linear enamel hypoplasias. In The Backbone of Europe. Health, Diet, Work and Violence Over Two Millennia (eds Steckel, R. H. et al.) 175–197 (Cambridge University Press, 2018).
AlQahtani, S. J., Hector, M. P. & Liversidge, H. M. Brief communication: The London atlas of human tooth development and eruption. Am. J. Phys. Anthropol. 142, 481–490 (2010).
Hillson, S. Dental markers of disease and malnutrition. In Tooth Development in Human Evolution and Bioarchaeology (ed. Hillson, S.) 162–197 (Cambridge University Press, 2014).
Katzenberg, M. A., Herring, D. A. & Saunders, S. R. Weaning and infant mortality: Evaluating the skeletal evidence. Yearb. Phys. Anthropol. 39, 177–199 (1996).
Araújo, A. C. & Lejeune, M. Gruta do Escoural: necrópole neolítica e arte rupestre paleolítica (Trabalhos de Arqueologia 8, Instituto Português do Património Arquitectónico e Arqueológico, 1995).
Rocha, L. Datações absolutas para contextos funerários do Sul de Portugal: algumas reflexões em torno das arquiteturas e dos espólios. Scientia Antiquitatis 4, 81–104 (2020).
Rocha, L. & Duarte, C. Megalitismo funerário no Alentejo Central: os dados antropológicos das escavações de Manuel Heleno. In Investigaciones histórico-médicas sobre salud y enfermedad en el pasado (eds Cerdá, M. P. & García-Próper, E.) 763–781 (Grupo Paleolab e Sociedad Española de Paleopatología, 2009).
Boaventura, R., Ferreira, M. T. & Silva, A. M. Perscrutando espólios antigos: a anta de Sobreira 1 (Elvas). Revista Portuguesa de Arqueologia 16, 63–79 (2013).
Gordon, C. C. & Buikstra, J. E. Soil pH, bone preservation, and sampling bias at mortuary sites. Am. Antiq. 46, 566–571 (1981).
Carvalho, A. F. On mounds and mountains “Megalithic behaviours” in Bom Santo Cave, Montejunto Mountain Range (Lisbon, Portugal). In Megalithic Monuments and Cult Practices. Proceedings of the Second International Simposium (eds Markov, V. et al.) 114–123 (Neofit Rilski University Press, 2016).
Santos, F. S. Manifestações votivas da necrópole da Gruta do Escoural. In Actas do II Congresso Nacional de Arqueologia 95–97 (Ministério da Educação Nacional, Junta Nacional de Educação, 1971).
Santos, F. S. Manifestações neolíticas no contexto dos testemunhos pré-históricos do Outeiro da Herdade da Sala (Escoural. Montemor-o-Novo, Portugal). In Actas do XVII Congreso Nacional de Arqueologia 135–142 (Secretaría General de los Congresos Arqueológicos Nacionales, 1985).
Peripoli, B. et al. Exploring prenatal and neonatal life history through dental histology in infants from the Phoenician necropolis of Motya (7th–6th century BCE). J. Archaeol. Sci. Rep. 49, 104024 (2023).
Cares-Henriquez, A. & Oxenham, M. F. New distance-based exponential regression method and equations for estimating the chronology of linear hypoplasia (LEH) defects on the anterior dentition. Am. J. Phys. Anthropol. 168, 510–520 (2018).
Cares-Henriquez, A. LEH chronology calculator (Version 1.0). https://www.lehtools.com (2019).
Smith, B. H. Patterns of molar wear in hunter-gatherers and agriculturalists. Am. J. Phys. Anthropol. 63, 39–56 (1984).
Silva, A. M. O Hipogeu de Monte Canelas I (IV–III milénios a.C.): estudo paleobiológico da população humana exumada (University of Coimbra, 1996).
Ulijaszek, S. J. & Lourie, J. A. Intra-and inter-observer error in anthropometric measurement. In Anthropometry: The Individual and the Population (eds Ulijaszek, S. J. & Mascie-Taylor, C. G. N.) 30–55 (Cambridge University Press, 1994).
Ulijaszek, S. J. & Kerr, D. A. Anthropometric measurement error and the assessment of nutritional status. Br. J. Nutr. 82, 165–177 (1999).
Parker, G. J. et al. AMELY deletion is not detected in systematically sampled reference populations: A reply to Štamfelj. J. Archaeol. Sci. 130, 105354 (2021).
Hobbs, F. Age and sex composition. In The Methods and Materials of Demography (eds Siegel, J. S. & Swanson, D. A.) 125–173 (Elsevier Academic Press, 2004).
Ferreira, M. T. S. Para lá da morte: Estudo tafonómico da decomposição cadavérica e da degradação óssea e implicações na estimativa do intervalo pós-morte. Preprint at http://hdl.handle.net/10316/21839 (2012).
Carvalho, A. F. et al. Hunter-gatherer genetic persistence at the onset of megalithism in western Iberia: New mitochondrial evidence from Mesolithic and Neolithic necropolises in central-southern Portugal. Quat. Int. https://doi.org/10.1016/j.quaint.2023.03.015 (2023).
Fernández-Crespo, T. & de-la-Rúa, C. Demographic differences between funerary caves and megalithic graves of northern Spanish Late Neolithic/Early Chalcolithic. Am. J. Phys. Anthropol. 160, 284–297 (2016).
Silva, A. M. The Megalithic builders: New data on old bones from the Megalitho do Facho (Figueira da Foz, Portugal). Doc. Praehist. 47, 390–403 (2020).
Silva, A. M. Insights into the funerary practices in the dolmen of Cabecinha (Figueira da Foz, Portugal). Doc. Praehist. 48, 328–339 (2021).
Silva, A. M., Sousa, A. C., Boaventura, R. & Scarre, C. The forgotten bones of the dolmen of Carrascal (Agualva, Sintra, Portugal). Examining old human remains. Trab. de Prehist. 76, 345–356 (2019).
Silva, A. M., Sousa, A. C. & Scarre, C. A closer look at the forgotten bones of the dolmen of Pedras Grandes (Odivelas, Portugal). Examining old human remains. SPAL 30(2), 20–46. https://doi.org/10.12795/spal.2021.i30.16 (2021).
Cintas-Peña, M. & Sanjuán, L. G. Gender inequalities in Neolithic Iberia: A multi-proxy approach. Eur. J. Archaeol. 22, 499–522 (2019).
Cintas-Peña, M. & Herrero-Corral, A. M. Missing prehistoric women? Sex ratio as an indicator for analyzing the population of Iberia from the 8th to the 3rd millennia BC. Archaeol. Anthropol. Sci. 12, 263 (2020).
Cardoso, F. A. Problemas de crescimento no Mesolítico português. Contribuição de alguns indicadores de stress. https://doi.org/10.13140/RG.2.1.4804.4566 (2001).
Lubell, D. & Jackes, M. Mesolithic-Neolithic continuity: evidence from chronology and human biology. In Actas da I Reunião do Quaternário Ibérico (ed. Grupo de trabalho português para o estudo do Quartenário & Grupo español de trabajo del Cuartenario) 113–133 (Fundação Calouste Gulbenkian, 1985).
Lubell, D., Jackes, M., Schwarcz, H., Knyf, M. & Meiklejohn, C. The Mesolithic-Neolithic transition in Portugal: Isotopic and dental evidence of diet. J. Archaeol. Sci. 21, 201–216 (1994).
Martins, J. M. M., Carvalho, A. F. & Soares, A. M. M. A calibração das datas de radiocarbono dos esqueletos humanos de Muge. Promontoria 6, 73–93 (2008).
Meiklejohn, C., Jackes, M. & Lubell, D. Radiocarbon dating of human skeletal material from two sites in Portugal. Mesolith. Misc. 7, 4–6 (1986).
Peyroteo-Stjerna, R. Death in the Mesolithic or the mortuary practices of the last hunter-gatherers of the south-western Iberian Peninsula, 7th-6th millenium BCE. Preprint at http://hdl.handle.net/10451/30962 (2016).
Cunha, E. & Cardoso, F. New data on Muge shell middens: a contribution to more accurate numbers and dates. Estudos Arqueológicos de Muge 1, 171–83 (2002–2003).
Cunha, E., Cardoso, F. & Umbelino, C. Inferences about Mesolithic life style on the basis of anthropological data. The case of the Portuguese shell middens. In Mesolithic on the Move: Papers Presented at the Sixth International Conference on the Mesolithic in Europe (eds Larsson, L. et al.) 184–188 (Oxbow Books, 2003).
Jackes, M., Alvim, P., Anacleto, J. A. & Roksandic, M. New photographic evidence on the 1954 excavations at Moita do Sebastião, Muge, Portugal. In The Cultural Dynamics of Shell-Matrix Sites (eds Roksandic, M. et al.) 131–149 (University of New Mexico Press, 2014).
Jackes, M. & Lubell, D. The Early Neolithic human remains from Gruta do Caldeirão. In Gruta do Caldeirão: O Neolítico antigo (ed. Zilhão, J.) 259–295 (Trabalhos de Arqueologia 6, Instituto Português do Património Arquitectónico e Arqueológico, 1992).
Zilhão, J. Cronologia absoluta. In Gruta do Caldeirão, o Neolítico Antigo (ed. Zilhão, J.) 78–81 (Trabalhos de Arqueologia 6, Instituto Português do Património Arquitectónico e Arqueológico, 1992).
Valera, A. C., Silva, A. M. & Márquez Romero, J. E. The temporality of Perdigões enclosures: Absolute chronology of the structures and social practices. SPAL 23(11–26), 6 (2014).
Fernández-Crespo, T. et al. Multi-isotope evidence for the emergence of cultural alterity in Late Neolithic Europe. Sci. Adv. 6, eaay2169. https://doi.org/10.1126/sciadv.aay2169 (2020).
Ditch, L. E. & Rose, J. C. A multivariate dental sexing technique. Am. J. Phys. Anthropol. 37, 61–64 (1972).
Yamada, Y. & Sakai, T. Sexual dimorphism in tooth crown diameter of the cook Islanders. In Structure, Function and Evolution of Teeth (eds Smith, P. & Tchernov, E.) 437–450 (Freund Publishing House, 1992).
Ișcan, M. Y. & Kedici, P. S. Sexual variation in bucco-lingual dimensions in Turkish dentition. Forensic Sci. Int. 137, 160–164 (2003).
Vodanovic, M., Demo, Z., Njemirovskij, V., Keros, J. & Brkic, H. Odontometrics: A useful method for sex determination in an archaeological skeletal population?. J. Archaeol. Sci. 34, 905–913 (2007).
Rai, B., Dhattarwal, S. K. & Anand, S. C. Sex determination from tooth. Med. Legal Update 8, 3–5 (2008).
Ruengdit, S., Riengrojpitak, S., Tiensuwan, M. & Santiwong, P. Sex Determination from Teeth Size in Thais. Preprint at https://forensic.sc.mahidol.ac.th/proceeding/52_Sittiporn.pdf (2011).
Zorba, E., Moraitis, K. & Manolis, S. K. Sexual dimorphism in permanent teeth of modern Greeks. Forensic Sci. Int. 210, 74–81 (2011).
Tuttösí, P. & Cardoso, H. F. V. An assessment of sexual dimorphism and sex estimation using cervical dental measurements in a Northwest Coast archaeological sample. J. Archaeol. Sci. Rep. 3, 306–312 (2015).
Acknowledgements
Raquel Granja has received research support from Portuguese Foundation for Science and Technology through a PhD grant (grant SFRH/BD/146991/2019). The authors thank the director and all technical staff of the National Museum of Archaeology, in Lisboa, Portugal, for providing access to the collections and for allowing us to carry out the sampling programme for these investigations. The authors also thank Rita Peyroteo-Stjerna for reviewing the manuscript, improving it, and for her efforts in taking the MoreEscoural forward. The authors thank the ‘Fondazione Cassa di Risparmio di Modena’ for funding the nLC-MS system at the Centro Interdipartimentale Grandi Strumenti of UNIMORE. Dr. Diego Pinetti and Dr. Filippo Genovese are deeply thanked for the assistance during LC-MS analyses.
Funding
Raquel Granja has received research support from Portuguese Foundation for Science and Technology through a PhD grant (SFRH/BD/146991/2019). This work was financed by Portuguese funds through FCT - Foundation for Science and Technology, I.P., within the scope of the projects UIDB/00283/2020, UIDB/00698/2020 and UIDP/00698/2020.
Author information
Authors and Affiliations
Contributions
D.G., A.C.A., A.M.S. and R.G. contributed to the study conception and design. Material preparation, data collection and analysis were performed by R.G., D.G., A.C.A., F.L., S.S. and A.M.S. The first draft of the manuscript was written by R.G. and D.G. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Granja, R., Araújo, A.C., Lugli, F. et al. Unbalanced sex-ratio in the Neolithic individuals from the Escoural Cave (Montemor-o-Novo, Portugal) revealed by peptide analysis. Sci Rep 13, 19902 (2023). https://doi.org/10.1038/s41598-023-47037-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47037-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.