Abstract
Recent research has confirmed the efficiency of insectivorous bats as pest suppressors, underlining the ecological services they offer in agroecosystems. Therefore, some efforts try to enhance bat foraging in agricultural landscapes by acting upon environmental factors favouring them. In this study, we monitored a Miniopterus schreibersii colony, in the southern Iberian Peninsula. We intensively sampled their faeces and analysed them by metabarcoding to describe how the bent-winged bat diet would change with time, and to test whether their most-consumed prey would seasonally depend on different landscapes or habitats. Our results confirm that M. schreibersii are selective opportunist predators of moths, dipterans, mayflies, and other fluttering insects, shifting their diet to temporary peaks of prey availability in their foraging range, including both pest and non-pest insects. Supporting our hypothesis, throughout the year, M. schreibersii consume insects linked to diverse open habitats, including wetlands, grassland, diverse croplands, and woodland. The importance of each prey habitat varies seasonally, depending on their insect phenology, making bats indirectly dependent on a diverse landscape as their primary prey source. Bats' predation upon pest insects is quantitatively high, consuming around 1610 kg in 5 months, of which 1467 kg correspond to ten species. So, their suppression effect may be relevant, mainly in patchy heterogeneous landscapes, where bats' foraging may concentrate in successive outbursts of pests, affecting different crops or woodlands. Our results stress that to take advantage of the ecosystem services of bats or other generalist insectivores, keeping the environmental conditions they require to thrive, particularly a heterogeneous landscape within the colony's foraging area, is crucial.
Similar content being viewed by others
Introduction
Insectivorous bats prey upon a wide variety of arthropods, consuming between 30 and 80% of their body mass each night due to their high metabolic rate1,2. Not surprisingly, even before the morphological techniques used in diet studies could identify the consumed prey beyond the family level, scientists foresaw the likely beneficial effect of these mammals' predatory behaviour in the fight against pest arthropods3,4,5,6. This idea flourished long before the term Ecosystem Services was coined7.
The recent development of molecular methods based on DNA metabarcoding and High Throughput Sequencing (HTS) offers superior detection ability and accurate identification of the consumed prey to the species level [e.g.,8,9,10]. During the last decade, these methods have been widely applied to broad studies of trophic ecology11 and provided comprehensive diet descriptions for a vast list of animals [e.g.,12,13,14,15,16,17].
In the case of insectivorous bats, many molecular studies have described their food habits in detail [e.g.,18,19,20,21,22,23,24,25]. Moreover, several pieces of research have confirmed the predation of bats upon a wide array of agroforestry pests, underlining the ecological services that these mammals offer as consumers—and likely suppressors—of them [e.g.,26,27,28,29,30,31,32]. Not surprisingly, molecular diet studies on bats in agroecosystems have become an up-and-coming research field. These studies delve deeper into the ecological relationships between those mammals and the environment. As applied research, the authors aim to take advantage of the ecosystem services bats most likely provide [e.g.,33,34,35].
Insect pests have increased during the last century due to the intensification of agriculture and invasive forest management practices36. In the previous decades, though, regulating of chemical pesticides in agriculture has become tighter while pests are rapidly developing resistance to them37. In this scenario, the biological suppression of agroforestry pests appears as a suitable solution38,39. Many authors have supported the efficiency of bats as pest controllers31,40,41,42,43,44. Moreover, Boyles et al.33 valued the ecosystem services bats provided at $22.9 billion per year on agroecosystems in the United States; Wanger et al.44 estimated $1.2 million yearly in rice plantations in Thailand. Thus, some efforts have tried to promote ecosystem services by bats by providing supplementary roost availability or studying which environmental factors favour bat foraging in agricultural landscapes. For example, after the installation of bat boxes in rice paddies and their occupation by soprano pipistrelles (Pipistrellus pygmaeus), the striped rice borer moth (Chilo suppressalis) declined below the chemical treatment threshold at Delta del Ebre in Catalonia42.
Moths comprise major agricultural pests damaging crops worldwide45. They are the primary prey of many insectivorous bats, including several moth specialist species: e.g., Rhinolophus euryale 19,46, Tadarida teniotis47, Plecotus sp.18,48, or the open space forager Miniopterus schreibersii26.
As is well known, insectivorous bats are highly adaptive and respond to a wide variety of prey, adjusting their diet to changes in availability [e.g.,30,49,50]. Most agroforestry pest insects occur in cyclic outbreaks51, which may be a consequence of their population density dynamics52,53,54,55, environmental pressures56,57, or even an answer to global change58. Therefore, bats must have other trophic resources available throughout time, apart from pests, to set colonies that will thrive. Furthermore, bats will shift their foraging grounds toward those with higher prey availability49. There, they often consume prey that originated outside the habitats where they are hunted19. Consequently, management measures addressing bats' foraging requirements should also consider the prey’s ecological requirements throughout their life stages.
The bent-winged bat M. schreibersii, is a cave-dwelling bat responsible for the largest colonies in Europe: up to 60,000 individuals were reported roosting in the same place in Bulgaria59. They perform annual migrations to roosts of ideal microclimatic conditions60. Due to their eco-morphological characteristics and high-speed flight61, nightly, the bent-winged bats fly up to 30 km from their roost to forage, showing one of the most extensive home ranges in European bats62. Despite such foraging ranges, they use proportionally small individual hunting areas, suggesting an uneven distribution of resources62.
The traits mentioned above make bent-winged bats optimal candidates to efficiently suppress pest insects in agroecosystems. Accordingly, an extensive molecular diet study at a continental scale26 showed that M. schreibersii consumes up to 44 crop insect species in Europe, likely being a valuable asset for biological pest suppression in various agricultural productions. The study addressed in detail neither the seasonal variation of the species' diet in the same locality nor the primary habitat of the main prey they consumed at the time or locality.
In this study, we analysed the changes in the diet of a large colony of M. schreibersii in an agricultural landscape across their active season in the Iberian Peninsula, paying attention to the seasonal variations in diet and the habitats on which the most consumed preys depend. Our primary hypotheses were: (1) The bent-winged bat diet will change with time, showing a seasonally shifting most-consumed prey sequence; (2) Those most-consumed prey will depend on different landscapes or habitats, causing seasonal changes in the required environmental resources for the bats. And (3), we aim to identify the main pest insects these bats prey upon in this agricultural landscape and quantitatively assess the magnitude order in which such consumption might move.
Material and methods
Study area
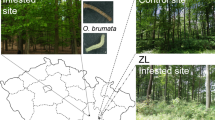
We conducted this study in a roost of M. schreibersii located at the Southernmost point of the Iberian Peninsula, near the cities El Puerto de Santa Maria and Jerez de la Frontera (Andalusia), approximately 15 km from the coast. Las Colmenas (90 m asl) is an artificial cave built to be an underground sandstone quarry in Sierra de San Cristobal range. Stone exploitation began in the Phoenician and Roman periods and peaked in the XVI and XVII centuries. The galleries, used as an ammunition dump in the second half of the XX century, fell into disuse at the beginning of the XXI. Since 2015, it has been included in Special Areas of Conservation (SAC No. ES6120030) due to the populations of cave-dwelling bats Myotis myotis, Myotis blythii, M. schreibersii roosting during the breeding season63.
The SAC Sierra de San Cristóbal holds 47.95 ha around a hill with 129 m asl. The vegetation is Mediterranean scrubland, where broom prevails (Retama sphaerocarpa), interspersed with scattered pine Pinus pinea 64. Highly modified croplands and urban areas encircle it. In a 40 km radius, sea occupies approximately 30% of the surface (Fig. 1). Diverse croplands cover two-thirds of the land surface; natural vegetation (pastures, scrubland, and woodland), marshes and dams, and urbanized and industrial lands cover the remaining third (Table 1). The landscape comprises smooth hills, with about 90% of the surface below 100 m asl and the highest at 412 m at the Eastern zone.
Regarding the croplands, 150,000 ha are devoted to rainfed annual crops, primarily cereals (wheat) and, to a lesser extent, sunflower. Non-irrigated wood crops comprise 15,000 ha, including 12,000 ha of vineyards and 3,000 ha of olive groves. Irrigated crops occupy 50,000 ha, mostly cotton and, to a lesser extent, corn, sunflower, tomato, rice and other horticultural ones. With less than 10,000 ha, pine woodland ranges from high density to scattered trees. The majority is located further than 25 km from the roost, with a main continuous patch in the North (within Doñana National Park) and smaller scattered patches closer to the seashore. Holm oak (Quercus ilex) and/or cork oak (Quercus suber) woodlands comprise around 6,000 ha in scattered patches, mainly further than 20 km from the roost, in the SE quadrant. Below Sierra de San Cristobal, the Guadalete River, its estuary and marshes (Bahía de Cádiz Natural Parc) offer diverse wetland habitats suitable for different insects (mostly Diptera and mayflies), which are putative prey for bats.
Sample collection
The M. schreibersii colony occupies the same roost from April to November, so sampling was carried out every two weeks from early May to early November 2018.
Each sampling night, we monitored the colony size, video-recording the emergence of bats by a near IR camera (SONY Handycam HDR-PJ780VE), lighted with an infrared torch Raytec VAR-I2-1 and connected to a frequency-division ultrasound detector Pettersson Elektronik D-240 as a microphone. The cave has a single entrance at ground level; the camera was set 8 m away from it, laterally to the bats' flight-way, fully covering the entrance. Recordings began after the first bat emerged (usually about 15 min after sunset) and continued for one hour, ensuring the full emergence was captured. Video and ultrasound recordings were analysed manually, simultaneously playing the video and ultrasound spectrograms (with BatSound v. 4.0, Pettersson Elektronik).
To collect the faecal samples, bats were captured entering the roost after the night foraging, using mist nets, and kept in individual cloth bags until they defecated. Ten samples of eight to ten faecal pellets each, taken from ten individual bats, were collected each night and stored for analysis; these yielded 140 samples in 14 nights. Afterwards, bats were immediately released to their roost to minimise their stress.
Ethics statement
We performed the captures under license from the Autonomous Government of Andalusia (resolution of 15/4/2019 by Dirección General de Medio Natural, Biodiversidad y Espacios Protegidos). The methods were performed with protocols that followed the guidelines for the treatment of animals in research and teaching67, in compliance with the ARRIVE guidelines, and approved by the Ethical committee at the EBD–CSIC (Estación Biologica de Doñana, Consejo Superior de Investigaciones Científicas).
DNA extraction, PCR amplification and sequencing
Extractions were performed in the Biological Station of Doñana (EBD-CSIC, Spain) using the DNA extraction kit DNeasy PowerSoil Kit (Qiagen), following the manufacturer’s instructions. We included extraction blanks in every extraction round. All samples were amplified with a combination of two primers that target the COI gene: (1) Zeale ZBJ-ArtR2c and ZBJ-ArtF1c, in the future called ‘Zeale’68; and (2) modified LepF1 and EPT-long-univR, from now on ‘Gillet’69. These two primer pairs complement each other and allow the detection of a broader spectrum of prey species70,71 compared to using one primer pair alone. PCR products migrated in agarose gel electrophoresis to test the success of the amplification process. Subsequently, a second PCR reaction was performed to attach a unique combination of tags and Illumina sequencing adapters to each amplicon using the Nextera XT Index Kit72. Finally, samples were pooled and sequenced using Illumina MiSeq technology. DNA library construction and sequencing processes were done at the Genomics and Proteomics General Service (SGIker) of the University of the Basque Country.
Sequence analysis and library building
Using Usearch v.1073, paired-end reads were merged in those sequences retrieved from Illumina. Then, a quality filter was applied to discard possible sequencing errors, using the Q30 quality value as a threshold. Reads were then demultiplexed according to primer sequences, primers were trimmed, and the remaining sequences were selected according to the appropriate length for each marker using Cutadapt74. Singleton sequences were set aside, and the remaining sequences were clustered into ZOTUs (Zero-Radius Operational Taxonomic Units), using the -unoise3 command in Usearch. This step implements denoising, i.e., error correction, to the sequence clustering step and maintains better biological resolution75. Then, for each sample, the ZOTUs with frequencies lower than 1% were removed using Usearch’s -otutab_norm and -otutab_trim commands. For the Gillet dataset, the first ZOTU, which belonged to the predator, was removed for this step because the relative read abundance of this ZOTU was considerably higher than the rest and limited the recoverable diversity in each sample. Finally, we compared each ZOTU against the online databases BOLD Systems and GenBank, following identification criteria by Clare et al.9 and Vesterinen et al.76.
Diet description and analysis
All statistical analyses were conducted in R version 4.0.477. The percentage of occurrence of each prey item was weighted by the number of prey items identified in that sample (wPOO:78).
We used distance-based redundancy analysis (db-RDA:79) to study the multivariate relationship of the diet with each of the sampling dates, which enabled us to select up to 26 prey species: the consumption of 22 of them was correlated with a specific sampling day, while four were often consumed but not related to seasonality. This analysis is further described in Supplementary Material 1. The consumption of the 22 species important for the constrained axes was modelled against date with Generalized Additive Mixed Models (GAMM) using function gamm in package mgcv80. We calculated the wPOO values of the 22 prey species per sampling date, log-transformed them, and modelled them as a day-of-the-year non-linear function representing the sampling date. Since the consumption of each species is different throughout the season, we should model a smoother for each species. Nonetheless, there needs to be more data points to do so reliably. Instead, an exploratory analysis showed that species could be paired together as their consumption followed similar trends. Therefore, the final model has eleven groups of species and a random intercept, which allows for different variances for each prey species. Given the temporality of the data, we considered adding a temporal correlation structure to the model. Still, based on Akaike’s information criterion (AIC) it did not improve, leaving it out of the final model.
The final model formulation is the following:
where:
sp, each prey species added to the model.
date, day of the year the sampling was conducted.
group, groups of prey species modelled together because they show similar trends.
For smoothing curves, p-values near 0.05 should be interpreted cautiously81, so we set the smoother significance threshold at a p-value of 0.01.
Characterisation of prey’s source habitats
We gathered information on the habitat preferences of the 26 prey species selected from the db-RDA analysis from several bibliographic sources and online databases (references available in Supplementary Material 2). We then summarised this information in a binary table composed of vegetation characteristics (15 categories) and vegetation density (3 classes) based on the habitat types available within 40 km around the colony.
We built a sampling date × habitat matrix using the prey × habitat binary matrix and the wPOO values for each prey (Supplementary Material l 3a). The prey × habitat table was first weighted to account for the differences in coverage of the habitats around the colony. Then, we multiplied it with the wPOO matrix, resulting in a sampling date × habitat matrix (Supplementary material 3b). Finally, average values for each habitat were calculated across all sampling dates to get a profile of the preferred habitats of the most consumed prey of M. schreibersii, accounting for their consumption—hereafter referred to as “prey’s source habitats”—. These “prey’s source habitats” were compared against the area of different types of habitats available around the colony—hereafter “available habitats”—using a Chi-square test of independence and simulated p-values by Monte Carlo test (2000 replicates) due to the low frequencies of some habitat categories.
Pest insects’ classification
First, we checked all the species to which the ZOTUs had been ascribed in the European and Mediterranean Plant Protection Organization (EPPO) Global Database82 to test which of them had been categorised as a pest in different degrees. Second, we classified them more conservatively following the Ministry of Agriculture of Spain (Supplementary Material 4), determining which major or minor pests were considered for different crops or woodlands.
A quantitative approach to pest insect consumption
To get a first quantitative approach to the pest insect consumption by the studied bat colony within their foraging grounds, we estimated the consumption of each of the selected pest items in every sampling date using the following formula:
where:
c: Kurta et al.1 estimated that in the temperate insectivorous bat Myotis lucifugus, individuals should daily eat about 70% of their body weight in insects to fulfil their requirements during pregnancy and lactation.
meanweight: 340 individual M. schreibersii bats (170 males and 170 females) captured in the study area in June–August 2018 weighted 11.92 g on average (SD = 0.75).
colsize: We monitored the colony size each sampling session during the study—above—.
wPOOPest: We relied on wPOO78,83 as the best proxy for translating molecular diet results to quantitative assessment of diet composition.
Based on the data collected every fifteen days during 2018, we predicted the consumption of relevant pest species every day throughout the sampling season using a LOESS smoother (span = 0.5). Based on that and weighting the total consumption of each pest species with the surface of their habitat in the study area, we calculated the predation intensity upon them, in kg/ha.
Ethical approval
The authors performed the captures under license from the Autonomous Government of Andalusia (resolution of 15/4/2019 by Dirección General de Medio Natural, Biodiversidad y Espacios Protegidos) with protocols that followed the published guidelines for the treatment of animals in research and teaching (66).
Results
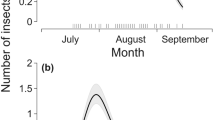
The colony size ranged from 3200 to 7244 individuals during the study period, with a minimum size in October and a maximum in late August (Fig. 2).
DNA extraction and amplification were successful in all the faecal samples chosen for analysis. After sequencing and bioinformatic process, with the Gillet primers, we recovered 214 ZOTUs: we ascribed 169 out of them to potential prey species, three to parasite species, 14 to environmental pollution DNA (e.g., fungi), and 89 could not be identified; ZOTUs assigned to M. schreibersii were amplified in all faecal samples. With the Zeale primers, we got 325 ZOTUs, of which 275 were ascribed to potential prey, and the rest were left unassigned. Combining Gillet and Zeale primers, we identified 187 different arthropods at the species level in bats' faeces, including the bat parasite Nycteribia schmidtii (Diptera, Hippoboscidae). We discarded 17 species out of them because they were previously recorded neither in the Iberian Peninsula nor within the 1000 km range in North Africa (Supplementary Material 5). Therefore, we classified 169 species as prey consumed by M. schreibersii (Supplementary Material 6).
Most species were consumed only once (71 spp) or twice (29 spp), and only 24 had frequency of occurrences (FOO) equal to or above 10%. Regarding species richness and wPOO, the most relevant prey groups at the order level were Lepidoptera, followed by Diptera, Hemiptera and Ephemeroptera (Table 2). Only 30 species showed wPOO ≥ 1% (Supplementary Material 6). They included 20 moth species (prevailing Noctuidae), six Diptera, three Ephemeroptera and one Hemiptera. Following wPOO, the most consumed species were the crambid moth Thopeutis gallerielus, followed by noctuids Agrotis segetum, A. ipsilon and Leucania loreyi, and the cranefly Symplecta pilipes (Diptera, Limoniidae), respectively. Besides, the most frequently consumed—in terms of FOO—were the noctuid moth A. segetum, followed by Ephemeroptera Caenis luctuosa, Ephoron virgo and Chroroterpes picteti, and the noctuid moths L. loreyi, Autographa gamma and A. ipsilon, respectively.
Temporal variations in diet
The diet of M. schreibersii changed seasonally in terms of total consumed species richness, the average number of species per faecal sample, and the most consumed prey (Table 3). The diet richness was higher in spring, late summer, and autumn. The most frequently consumed prey species changed every sampling session, except for T. galleriellus, which showed the highest wPOO in three consecutive sets from 18 June to 16 July.
To further study the temporality of the diet, we chose those with the highest and lowest scores in the significant axes (22 species in total) and the first two unconstrained axes (four additional species) of the db-RDA (Table 4, Supplementary Material 1). The consumption of all 26 species is portrayed in Fig. 3 using a LOESS smoother (span 0.7). The consumption of these species was variable throughout the year, and each one followed a different tendency. Seasonally, the most consumed prey in early May were the noctuids Noctua pronuba and M. vitellina, followed by the mayfly E. virgo and the mosquito C. pipiens. They are gradually substituted in late May and early June by the citric pest moth P. citri. In early summer, the geometrid G. rufifasciata, the crambid moth T. galleriellus and the nolid moth N. squalida sequentially replaced the cranefly D. ventralis. Throughout August, consumption of the mayflies E. virgo and C. picteti is overcome by solid predation upon the pine moth T. pityocampa (Notodontidae), leading to second consumption peaks of the noctuids N. pronuba and M. vitellina in autumn.
Observed wPOO values of 26 species selected from the canonical and uncanonical axes of the db-RDA: the points represent the observed wPOO values, and the trend is represented with LOESS smoother (span 0.7). Plots are ordered roughly according to the seasonality of the consumption peak (from left to right) and the range of wPOO values (from top to bottom).
The 22 species influential in the canonical axes of the db-RDA were grouped and modelled against date as the continuous factor day-of-the-year in a Generalized Additive Mixed Model (Eq. 1, in Material and Methods). Five of the eleven smoothers were significant at the 0.01 value: A. ipsilon + L. loreyi, M. vitellina + N. pronuba, S. pilipes + P. citri, T. pityocampa + L. anella and T. galleriellus + G. rufifasciata (Table 5). These species show the highest variability in their consumption throughout the sampling season, being only identified in a particular sampling period. The estimated wPOO values of the five significant smoothers (Fig. 4) confirm the tendencies portrayed in Fig. 3. There is substantial turnover in the prey species consumed by M. schreibersii. The remaining species in the GAMM are still essential food items for our colony, even though we could not model a significant consumption pattern against time. Species like A. segetum, Peridroma saucia or Palpita vitrealis are consumed in great numbers throughout the year, providing a more constant food source for M. schreibersii, albeit without overshadowing more punctual, ephemeral resources like the ones mentioned above.
Landscape requirements of the main prey
Assessing the environmental requirements of M. schreibersii based on their prey’s source habitats, we must conclude that they depend on very diverse habitat types, from water bodies and rice paddies to sparsely vegetated areas and scrublands, different types of cropland, and diverse woodlands as well. Moreover, the weight of those habitats in their most consumed prey varies seasonally (Fig. 5).
The mosaic plot in Fig. 6 portrays the differences between available and prey’s source habitats. The Chi-square test of independence showed no difference between the available and prey's source habitat types (Fig. 6A) according to the vegetation density (X-squared = 4.5692, df = NA, p-value = 0.2054). For the vegetation coverage (Fig. 6B), significant differences were found (X-squared = 21.357, df = NA, p-value = 0.03448), albeit not very pronounced. Pearson’s residuals showed that "Water Bodies" contributed to 28.8% of the differences in the Chi-square statistic. This category was the only one significantly different, being more important as prey source by M. schreibersii than expected from its availability around the colony. This suggests that the colony studied frequently consumed prey associated with water bodies such as C. luctuosa, E. virgo, C. pipiens or L. nubeculosa, which could be hunted close to drinking spots. No other vegetation type shows a significant difference between the two datasets, indicating that M. schreibersii exploits prey of all habitats equally around the colony.
Mosaic plots of available habitat types versus source habitats of the most consumed prey species. Habitat types are classified according to (A) the vegetation density and (B) the vegetation type. The bars’ width represents the combined percentage of that category in the dataset, and colours show Pearson’s correlation coefficients.
Pest consumption
Notably, 39 species identified as consumed are categorized as a pest in different degrees. Consulting the results of the db-RDA and the GAMM models, we identified ten pest species consumed in significant numbers by this colony of M. schreibersii. We calculated their overall consumption based on wPOO for that specific prey and the colony size, resulting in different consumption patterns for each pest species (Fig. 7). Estimation was possible from the 11th of May to the 10th of October 2018, when we monitored the colony size.
Quantitatively, the consumption of agricultural pest insects by bats summed up to 1610.3 kg in 5 months, of which 1467.3 kg correspond to ten species. The most consumed was A. segetum—234 kg—with a steady consumption year-round and higher peaks in mid-June and autumn (Fig. 7). The following most consumed pests were A. gamma (195 kg), C. pallidus (186 kg), T. pityocampa (173 kg) and A. ipsilon (163 kg). The consumption of C. pallidus was relatively uniform. Contrarily, predation upon T. pityocampa was limited to its flying season, starting in August: in a single day, the colony consumed up to 6 kg of T. pityocampa. With lower numbers, P. saucia (142 kg), P. vitrealis (141 kg) and P. citri (109 kg) were also heavily consumed. While the bats preyed upon the first two throughout the sampling season, P. citri was only eaten during its flying season in spring. N. pronuba (86 kg) was only consumed in early May, and C. fagiglandana (32 kg) primarily in the late season. Finally, C. pipiens (73 kg) was consumed mostly during May and August.
Assessed by weighting the total consumption with the surface of prey-habitat surface in the study area, the highest predation intensity occurs upon the more habitat-specialist pest species:
-
P. vitrealis, 4.7 kg/100 ha in olive groves.
-
T. pityocampa, 1.73 kg/100 ha in conifer plantations.
-
C. fagiglandana, 0.53 kg/100 ha in Quercus woodland.
-
C. pallidus, 0.37 kg/100 ha in irrigated crops.
On the contrary, the pests listed above with the highest consumption numbers inhabit varying habitat types, and consequently, their predation intensity will be relatively lower.
Discussion
In this study, we combined the population monitoring of a colony of M. schreibersii with an intensive sampling and thorough metabarcoding analysis of the faecal samples to show that: (1) the bats’ diet continuously varies, showing a succession of peak prey, which includes both pest and non-pest insects; (2) many of those most-consumed-species have different habitat preferences/dependences (Fig. 5), bats positively selecting water bodies but somehow using—or taking benefit from—all of the other habitat types as well; (3) bats’ predation upon pest insects is quantitatively high; and (4) their suppression effect may be relevant, mainly in patchy heterogeneous landscapes, where foraging bats may concentrate in successive outbursts of pests, affecting to different crops or woodlands.
Diet diversity and variation
Our results fit well with M. schreibersii being a fast-flying, open-space-forager61,84 and aerial-hawking bat [e.g.,85,86], specialised in fluttering flying insects, such as moths, dipterans, and others. Our analysis confirms moth —mostly Noctuidae, but also Crambidae, Geometridae, Pyralidae, Notodontidae and others—constitute their staple diet, in agreement with both the morphological87,88 and molecular studies26 hitherto published.
Among the most consumed prey, the noctuid moths A. gamma, A. segetum, A. ipsilon, N. pronuba, P. saucia, M. vitellina or Nomophila noctuella are widespread and abundant insects, which seasonally constitute the staple food of many moth-specialist bats: namely Plecotus sp.18,48; Rhinolophus euryale89; Tadarida teniotis47; and also M. schreibersii26. The notodontid pine moth T. pityocampa is a frequent prey of several moth-specialist bats as well, including not only forest bats but also M. schreibersii and other open-space-foragers28. Similarly, the main Diptera consumed are common prey of insectivorous bats: e.g., L. nubeculosa is consumed by R. hipposideros and R. ferrumequinum70,90, T. teniotis47, Myotis daubentonii23,76, Myotis dasycneme23, and Plecotus auritus48; S. pilipes by M. daubentonii90 and R. hipposideros27; and D. ventralis by R. hipposideros27 and M. daubentonii90.
Noteworthy, some of the most frequent prey in this study were seldom or never reported as bats' prey. Among these, the most frequently consumed species in this study, T. galleriellus91, is a crambid moth only known from very few distribution data in southern Europe (including the nearby Doñana National Park), the Middle East and Asia92,93. Like many other crambid moths, it seems linked chiefly to wet grassland and marshes. Similarly, the nolid moth N. squalida and C. pallidus, a hemipteran pest from the family Miridae, had not been recorded as bats prey before. These findings indicate that new research on insectivorous bats' diets in different localities and seasons will unveil other relevant prey species, helping complete our knowledge of the trophic webs35.
Also notable is the intense consumption of Ephemeroptera, only punctually reported as prey for this bat26,88. In our study, mayflies C. luctuosa, E. virgo and C. picteti were relatively important prey, showing several peaks throughout the year (Fig. 4), in accordance with M. schreibersii foraging most actively over rivers and wetlands94,95. In contrast, we detected neither Trichoptera nor Chrysopidae (Neuroptera), previously reported as prey26,87,88.
Insectivorous bats are selective opportunistic predators that actively search areas with abundant prey sources30,96,97,98,99, adjusting their diet to prey abundance. Slight differences in M. schreibersii diet composition have been described among localities and seasonally. The metabarcoding analysis of M. schreibersii's faeces across Europe showed their diet varied among localities linked to habitat, indicating that the dietary diversity was negatively related to the area of intensive agricultural fields26. Moreover, by morphological identification of prey remains in faeces at the order and family levels, a study carried out in a single roost in Slovenia throughout the year showed M. schreibersii as a moth specialist that opportunistically switches among other available prey, with a more diverse diet in spring and autumn87.
With a detailed identification of prey at the species level, our study shows M. schreibersii hunting more opportunistically in spring and from late summer onwards but more selectively in summer. This pattern is consistent with the behaviour described for several insectivorous bats: e.g., species of genus Plecotus18, Pipistrellus kuhlii100, or R. ferrumequinum101.
Moreover, according to our first hypothesis, our results confirm M. schreibersii gradually shifts from one peak-prey species to another, most likely tracking varying insect availability. Many of those prey species show one single consumption peak throughout the studied period (e.g., T. galleriellus, S. pilipes, T. pityocampa or C. pallidus, in Fig. 4). Others show two maxima, likely answering to availability. Among the later, some are migratory species, more abundant in spring and autumn (e.g., N. pronuba, A. gamma or A. ipsilon). Others produce two or more broods throughout the year (e.g., A. gamma, A. segetum, N. noctuella)102,103,104,105.
Habitat requirements
Supporting our second hypothesis, many of the most consumed M. schreibersii prey depend on different landscapes or habitats, which implies seasonal changes in the required environmental resources for the bats (Fig. 5). While some prey species may inhabit varying habitats and crops (e.g., A. gamma, Agrotis sp., Spodoptera sp.), others are more clearly linked to specific landscape elements: e.g., Ephemeroptera to water bodies; limoniid and psychodid Diptera to wetlands and damp spots; T. pityocampa to conifer woodland; P. citri to citrus plantations; C. flagigandana to Quercus and beech forests; T. galleriellus to wet grasslands and marshes, et cetera (see Table 4).
In insectivorous bats, habitat selection is a hierarchical decision-making process49: the species' morpho-ecological specializations drive it first, and subsequently, factors linked to optimal foraging prevail, e.g., prey availability49,99,100,106, distance to the roost107, landscape features101, or interspecific competition108,109.
For high-flying, open-space foragers like M. schreibersii, the importance of the habitat types underlying their hunting grounds is challenging to ascertain beyond the prey availability above them (e.g., Vespertilio murinus110,111; Eptesicus nilssonii112; Nyctalus lasiopterus113; Hypsugo savii114. Radiotracking studies on M. schreibersii in France have shown high habitat flexibility: they mostly forage over urban areas, followed by open spaces, woodlands, orchards and parks62,115; meanwhile, they use water bodies less frequently than expected from availability. On the contrary, in Italy, M. schreibersii forage in riverine forests94. They mainly use open areas and water bodies, followed by lighted urban areas, oak forests, and water lines in Portugal95,107. This foraging plasticity fits well with the habitat diversity of the principal prey in the present study (Table 4). Moreover, it also mirrors the main habitats available in the colony's foraging range (Fig. 1) and is consistent with our data for the whole active season, revealing no habitat selection (Fig. 6).
Our data do not reveal whether the bats foraged in any specific habitat but link each prey species to some of them. In fact, bats may often hunt prey originating in habitats different from where they are hunted19. Thus, bats' foraging requirements are not constrained to their foraging habitats, but also encompass their prey requirements116,117,118. Consequently, in our case study, M. schreibersii will seasonally rely on the diverse habitat around their roost, if not to directly forage therein, at least as sources of the prey they mostly hunt.
Several pieces of research showed the foraging activity of insectivorous bats in agroecosystems is linked to habitat heterogeneity [e.g.,98,119,120,121]. This correlation has been explained because farmland heterogeneity is a crucial predictor of overall biodiversity122,123,124 and of bats’ prey insects in particular98,125,126. Therefore, landscape heterogeneity increases spatial niche partitioning and thereby reduces the effects of interspecific competition among bat species127. Beyond that, our results indicate that habitat heterogeneity in agroecosystems is of great value in itself, also for single bat species like M. schreibersii, allowing them to shift from one insect outburst to the next, sequentially occurring in different habitat types. Moreover, they point out that aerospace is the true foraging habitat for high-flying, open-space forager bats. The underlying habitat types may be seasonally relevant either as source of prey outbursts—e.g., crops, forest, water bodies or other habitats—, as foreseeable prey attractors —streetlights or waterbodies—, or because they provide linear landscape elements or landmarks helpful in commuting or foraging.
Ecosystem services: which ones and how much?
Based on their extensive diet study across Europe, Aizpurua et al.26 pointed out that M. schreibersii might be a valuable asset as a pest suppressor. Our results confirm their forecast, as several agroforestry pests—and two disease vectors—are among the most consumed prey by this bat. Thus, among the 39 consumed species clategorized as pests in the EPPO Global Database82, in Spain 22 are major pests of several crops and woodland, and 18 are minor pests (Supplementary Material 4).
Moreover, M. schreibersii also preyed upon the hemipteran Neophilaenus campestris, which, if not directly regarded as a crop pest, is a potential vector of Xylella fastidiosa, a bacterium causing significant economic losses, especially in olive crops128,129. Noteworthy, we also recorded the nematoceran C. pipiens within the most consumed prey (Table 4), reaching 3.25% wPOO in mid-May. This mosquito is a vector of the Nile Fever disease, with several cases detected in the area in 2020, including one deceased130.
The insectivorous bats' diet metabarcoding studies have confirmed that these mammals prey upon many pest species [e.g.,26,27,28,29,30,33,131]. Nevertheless, deciphering to which extent bats consume them within intensive agroecosystems is crucial to talk about pest regulation or effectively assess the actual value of bat insectivory as an ecosystem service35. Even more to manage the negative impacts of pests through consumption by insectivorous bats27.
Quantitative assessment of diet composition is always challenging, implying several assumptions132. With the coming of metabarcoding techniques, additional difficulties appeared in turning the genomic analysis output into reliable quantitative diet data [e.g.,30,78,133,134,135,136]. Therefore, the assumptions underlying the quantitative analysis presented herein are debatable since they may either overestimate or underestimate specific consumptions. On the first hand, bats' total amount of insects consumed each night is open to discussion, ranging from 30 to 100% of their body weight [e.g.,1,2,4]. Therefore, our choice of 70% (following 1) might lead to overestimation, mostly because our sampling did not only happen during the breeding season and a high percentage of the samples belonged to males. Moreover, we did not consider that, due to meteorological factors, bats do not hunt in all nights—even if the climate is highly benign in the study area. On the other hand, the wPOO used to measure the importance of each prey in the diet tends to sub-estimate the largest or most abundantly consumed items78,83.
All in all, our quantitative approach determines the order of magnitude of the suppression a colony this size exerts on pests and disease vector insects in its foraging range. Besides, they will similarly affect the quantitative assessment of most main prey consumption. The exception will be the mosquitoes Culex pipiens, for wPOO may estimate the same consumed proportion, the bats having eaten anything from one single individual to hundreds of mosquitoes—so, we excluded them from the further discussion.
Notwithstanding the mentioned limitations, our data show that M. schreibersii heavily preys upon many pests. The generalist noctuid moths A. segetum, A. gamma and A. ipsilon quantitatively prevail. All are major pests of several horticultural crops and Zea mays, and minor of many others. It is also worth mentioning the high consumption of C. pallidus, a common pest in cotton lands and Solanaceae in the study area (Table 4; Supplementary Material 4).
To evaluate the ecosystem services bats provide consuming these pest insects, we must go beyond the absolute consumption numbers and consider the extension of croplands and woodland they affect in the colony's foraging range. Taking that into account, it is noteworthy that in our study the highest predation intensity happened upon woodland pests such as P. vitrealis (pest of olive groves), T. pityocampa (pest of conifers), or C. fagiglandana (pest of Quercus forests). Obviously, forestry pests such as C. fagiglandana are common prey of the forest bats B. barbastellus or P. auritus137. At first glance, the vigorous predation intensity on woodland pests observed in our study by an open space forager as M. schreibersii is more surprising. It indicates that these bats readily answer to the hot spots caused by the pest outbreaks, even in forests and other wooded areas, heavily hunting them, maybe above the canopy, along the forest edges, in clearings, or during insects commuting among patches. This intense predation upon forest pests is more noticeable considering that some of the host habitat patches likely exploited are far away from their roost—e.g., pine plantations for T. pityocampa, 28–30 km far. Others, such as the Quercus forests inhabited by C. flagigandana, are lacking in the 40 km around the roost, and we cannot discard that those bats fly further to profit from them (Fig. 1). The frequent consumption of forest pests by M. schreibersii and other open space foraging bats had been previously reported, e.g., consuming the pine moth T. pityocampa28 or outbreaks of the Asian gipsy moths (Lymantria dispar) in holm-oak woodlands62.
Our quantitative data suggest that such predation may be strong enough to restrain or even reduce pest damage, mainly when it affects patches of limited size. Moreover, the swift shift of their primary prey —and their habitats— suggests that M. schreibersii track pest abundance, increasing foraging activity when pests become more numerous and consequently decrease pest numbers, a requirement to exert control over pest populations35.
Accordingly, the bat population peak we observed in late August does not correspond to the incorporation of yearling individuals, who most likely began their foraging flights in early July (Fig. 2). Otherwise, this short-lasting rise might be due to the joining of bats from other roosts outside the study area, attracted to outbursts of prey such as T. pytiocampa and L. anella, whose consumption peaks are entirely coincidental in time.
This link between the spatial and social ecology and the availability of trophic resources was already foreseen by Rodrigues and Palmeirim60, who suggested that at least part of the seasonal displacements among roosts by M. schreibersii would answer to differential prey availability. Consequently, the tracking of pests and other outburst of insects by M. schreibersii would not only happen at the colony level but at a larger territorial scale, involving other nearby colonies of the metapopulation.
Conclusions
This study confirms that M. schreibersii are selective opportunist predators of moths and other fluttering insects in the aerospace, shifting their diet to temporary peaks of prey availability in their foraging range. Throughout the year, they consume insects linked to diverse open habitats, including wetlands, grassland, and diverse croplands, but woodland as well. The importance of each prey habitat varies seasonally, depending on the insect phenology therein, making bats indirectly dependent on a diverse landscape for their primary prey source. Besides, M. schreibersii consumes high numbers of several agroforestry pests and at least two disease vector insects, confirming the value of this bat as a suppressor and potential regulator of detrimental insect populations. Thus, our results stress that it is crucial to preserve the funcionality of bats or other generalist insectivores, keeping the environmental conditions they require to thrive, particularly a heterogeneous landscape within the colony’s foraging area. Moreover, farmers and land managers should look for combining different crops and land patches whose major insect assemblies would present non-overlapping outbursts, based on their expected phenology.
Data accessibility
The dataset generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Kurta, A., Bell, G. P., Nagy, K. A. & Kunz, T. H. Energetics of pregnancy and lactation in free-ranging little brown bats (Myotis lucifugus). Physiol. Zool. 62, 804–818. https://doi.org/10.1086/physzool.62.3.30157928 (1989).
Kunz, T. H., Whitaker, J. O. Jr. & Wadanoli, M. D. Dietary energetics of the insectivorous Mexican free-tailed bat (Tadarida brasiliensis) during pregnancy and lactation. Oecologia 101(4), 407–415. https://doi.org/10.1007/BF00329419 (1995).
Jones, G., Jacobs, D. S., Kunz, T. H., Willig, M. R. & Racey, P. A. Carpe noctem: The importance of bats as bioindicators. Endanger. Spec. Res. 8, 93–115. https://doi.org/10.3354/esr00182 (2009).
Kunz, T. H., Braun de Torrez, E., Bauer, D., Lobova, T. & Fleming, T. H. Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 1223, 1–38. https://doi.org/10.1111/j.1749-6632.2011.06004.x (2011).
Wenny, D. G. et al. The need to quantify ecosystem services provided by birds. Auk 28(1), 1–14. https://doi.org/10.1525/auk.2011.10248 (2011).
Whitaker, J. O. Jr. Food of the big brown bat Eptesicus fuscus from maternity colonies in Indiana and Illinois. Am. Midl. Nat. 134(2), 346–360. https://doi.org/10.2307/2426304 (1995).
Constanza, R. et al. The value of the world’s ecosystem services and natural capital. Nature 387(15), 253–260. https://doi.org/10.1016/S0921-8009(98)00020-2 (1997).
Bohmann, K. et al. Molecular diet analysis of two african free-tailed bats (Molossidae) using high throughput sequencing. PLoS ONE 6(6), e21441. https://doi.org/10.1371/journal.pone.0021441 (2011).
Clare, E. L., Symondson, W. O. C. & Fenton, M. B. An inordinate fondness for beetles? Variation in seasonal dietary preferences of night-roosting big brown bats (Eptesicus fuscus). Mol. Ecol. 23, 3633–3647. https://doi.org/10.1111/mec.12519 (2014).
Razgour, O. et al. High-throughput sequencing offers insight into mechanisms of resource partitioning in cryptic bat species. Ecol. Evol. 1(4), 556–570. https://doi.org/10.1002/ece3.49 (2011).
Carreon-Martinez, L. & Heath, D. D. Revolution in food web analysis and trophic ecology: Diet analysis by DNA and stable isotope analysis. Mol. Ecol. 19(1), 25–27. https://doi.org/10.1111/j.1365-294X.2009.04412.x (2010).
Biffi, M. et al. Novel insights into the diet of the Pyrenean desman (Galemys pyrenaicus) using next-generation sequencing molecular analyses. J. Mamm. 98(5), 1497–1507. https://doi.org/10.1093/jmammal/gyx070 (2017).
Deagle, B. E., Krikwood, R. & Jarman, S. N. Analysis of Austrlian fur seal diet by pyrosequencing prey DNA in faeces. Mol. Ecol. 18, 2022–2038. https://doi.org/10.1111/j.1365-294X.2009.04158.x (2009).
Esnaola, A., Arrizabalaga-Escudero, A., González-Esteban, J., Elosegi, A. & Aihartza, J. Determining diet from faeces: Selection of metabarcoding primers for the insectivore Pyrenean desman (Galemys pyrenaicus). PLoS ONE 13(12), e0208986. https://doi.org/10.1371/journal.pone.0208986 (2018).
Kartzinel, T. R. et al. DNA metabarcoding illuminates dietary niche partitioning by African large hervibores. PNA 112(26), 8019–8024. https://doi.org/10.1073/pnas.1503283112 (2015).
Murray, D. C. et al. DNA-based faecal dietary analysis: A comparison of qPCR and high throughput sequencing approaches. PLoS ONE 6(10), e25776. https://doi.org/10.1371/journal.pone.0025776 (2011).
Rytkönen, S. et al. From feces to data: A metabarcoding method for analysing consumed and avaliable prey in a bird-insect food web. Ecol. Evol. 9(1), 631–639. https://doi.org/10.1002/ece3.4787 (2018).
Andriollo, T., Michaux, J. R. & Ruedi, M. Food for everyone: Differential feeding habits of cryptic bat species inferred from DNA metabarcoding. Mol. Ecol. 30, 4584–4600. https://doi.org/10.1111/mec.16073 (2021).
Arrizabalaga-Escudero, A. et al. Trophic requirements beyond foraging habitats: The importance of prey source habitats in bat conservation. Biol. Conserv. 191, 512–519. https://doi.org/10.1016/j.biocon.2015.07.043 (2015).
Burgar, J. M. et al. Who is for dinner? High-throughput sequencing reveals bat dietary differentiation in a biodiversity hotspot where prey taxonomy is largely undescribed. Mol. Ecol. 23, 3605–3617. https://doi.org/10.1111/mec.12531 (2014).
Hope, P. R. et al. Second generation sequencing and morphological faecal analysis reveal unexpected foraging behaviour by Myotis nattereri (Chiroptera, Vespertilionidae) in winter. Front. Zool. 11, 39. https://doi.org/10.1186/1742-9994-11-39 (2014).
Ibáñez, C., Popa-Lisseanu, A. G., Pastor-Beviá, D., García-Mudarra, J. L. & Juste, J. Concealed by darkness: Interactions between predatory bats and nocturnally migrating songbirds illuminated by DNA sequencing. Mol. Ecol. 25(20), 5254–5263. https://doi.org/10.1111/mec.13831 (2016).
Krüger, F. et al. An integrative approach to detect subtle trophic niche differentiation in the sympatric bat species Myotis dasycneme and Myotis daubentonii. Mol. Ecol. 23(15), 3657–3671. https://doi.org/10.1111/mec.12512 (2014).
Krüger, F., Clare, E. L., Symondson, W. O. C., Keišs, O. & Petersons, G. Diet of the insectivorous bat Pipistrellus nathusii during autumn migration and summer residence. Mol. Ecol. 23, 3672–3683. https://doi.org/10.1111/mec.12547 (2014).
Vesterinen, E. J., Puisto, A. I. E., Blomberg, A. S. & Lilley, T. M. Table for five, please: Dietary partition in boreal bats. Ecol. Evol. 8, 10914–10937. https://doi.org/10.1002/ece3.4559 (2018).
Aizpurua, O. et al. Agriculture shapes the trophic niche of a bat preying on multiple pest arthropods across Europe: Evidence from DNA metabarcoding. Mol. Ecol. 27, 815–825. https://doi.org/10.1111/mec.14474 (2018).
Baroja, U. et al. Pest consumption in a vineyard system by the lesser horseshoe bat (Rhinolophus hipposideros). PLoS ONE 14(7), e0219265. https://doi.org/10.1371/journal.pone.0219265 (2019).
Garin, I. et al. Bats from different foraging guilds prey upon the pine processionary moth. PeerJ 7, e7169. https://doi.org/10.7717/peerj.7169 (2019).
Kemp, J. et al. Bats as potential suppressors of multiple agricultural pests: A case study from Madagascar. Agric. Ecosyst. Env. 269, 88–96. https://doi.org/10.1016/j.agee.2018.09.027 (2019).
McCracken, G. et al. Bats track and exploit changes in insect pest populations. PLoS ONE 7(8), e43839. https://doi.org/10.1371/journal.pone.0043839 (2012).
Murphy, F. & Ament, J. Pluralistic valuation of codling moth regulation by Brown long-eared bats in English Apple orchards. Sustainability 14, 11966. https://doi.org/10.3390/su141911966 (2022).
Taylor, P. J., Grass, I., Alberts, A. J., Joubert, E. & Tscharntke, T. Economic value of bat predation services—a review and new estimates from macadamia orchards. Ecosyst. Serv. 30, 372–381. https://doi.org/10.1016/j.ecoser.2017.11.015 (2018).
Boyles, J. G., Cryan, P. M., McCracken, G. F. & Kunz, T. H. Economic importance of bats in agriculture. Science 332(6025), 41–42. https://doi.org/10.1126/science.1201366 (2011).
Cleveland, C. J. et al. Economic value of the pest control service provided by Brazilian free-tailed bats in south-central Texas. Front. Ecol. Env. 4(5), 238–243. https://doi.org/10.1890/1540-9295(2006)004[0238:EVOTPC]2.0.CO;2 (2006).
Russo, D., Bosso, L. & Ancillotto, L. Novel perspectives on bat insectivory highlight the value of this ecosystem service in farmland: Research frontiers and management implications. Agric. Ecosyst. Env. 266, 31–38. https://doi.org/10.1016/j.agee.2018.07.024 (2018).
Singh, T. & Satyanarayana, J. Insect outbreaks and their management. In Integrated Pest Management: Innovation-Development Process (eds Peshin, R. & Dhawan, A. K.) (Springer, 2009). https://doi.org/10.1007/978-1-4020-8992-3_13.
Jensen, D. F., Karlsson, M., Sarrocco, S. & Vannacci, G. Biological control using microorganisms as an alternative to disease resistance. In Plant Pathogen Resistance Biotechnology (ed. Collinge, D. B.) 341–363 (Wiley, 2016). https://doi.org/10.1002/9781118867716.ch18
Naranjo, S. E., Ellsworth, P. C. & Frisvold, G. B. Economic value of biological control in intergrated pest management of managed plant systems. Annu. Rev. Entomol. 60, 621–645. https://doi.org/10.1146/annurev-ento-010814-021005 (2015).
Zehnder, G. et al. Arthropod pest management in organic crops. Annu. Rev. Entomol. 52(1), 57–80. https://doi.org/10.1146/annurev.ento.52.110405.091337 (2007).
Maas, B. et al. Bird and bat predation services in tropical forests and agroforestry landscapes. Biol. Rev. 91, 1081–1101. https://doi.org/10.1111/brv.12211 (2016).
Maine, J. J. & Boyles, J. C. Bats initiate vital agroecological interactions in corn. PNAS 112(40), 12438–12443. https://doi.org/10.1073/pnas.1505413112 (2015).
Puig-Montserrat, X. et al. Pest control service provided by bats in Mediterranean rice paddies: Linking agroecosystems structure to ecological functions. Mamm. Biol. 80(3), 237–245. https://doi.org/10.1016/j.mambio.2015.03.008 (2015).
Williams-Guillén, K., Perfecto, I. & Vandermeer, J. Bats limit insects in a neotropical agroforestry system. Science 320, 70. https://doi.org/10.1525/auk.2011.10248 (2008).
Wanger, T. C., Darras, K., Bumrungsri, S., Tscharntke, T. & Klein, A. M. Bat pest control contributes to food security in Thailand. Biol. Conserv. 171, 220–223. https://doi.org/10.1016/j.biocon.2014.01.030 (2014).
Alford, D. V. A Textbook of Agricultural Entomology (Blackwell Science Ltd., 1999).
Arrizabalaga-Escudero, A. et al. Trait-based functional dietary analysis provides a better insight into the foraging ecology of bats. J. Anim. Ecol. 88, 1587–1600. https://doi.org/10.1111/1365-2656.13055 (2019).
Mata, V. A. et al. How much is enough? Effects of technical and biological replication on metabarcoding dietary analysis. Mol. Ecol. 28, 165–175. https://doi.org/10.1111/mec.14779 (2018).
Andriollo, T., Gillet, F., Michaux, J. R. & Ruedi, M. The menu varies with metabarcoding practices: A case study with the bat Plecotus auritus. PLoS ONE 14(7), e0219135. https://doi.org/10.1371/journal.pone.0219135 (2019).
Almenar, D., Aihartza, J., Goiti, U., Salsamendi, E. & Garin, I. Hierarchical patch choice by an insectivorous bat through prey availability components. Behav. Ecol. Sociobiol. 67(2), 311–320. https://doi.org/10.1007/s00265-012-1451-z (2013).
Napal, M., Garin, I., Goiti, U., Salsamendi, E. & Aihartza, J. Past deforestation of Mediterranean Europe explains the present distribution of the strict forest dweller Myotis bechsteinii. For. Ecol. Manag. 293, 161–170. https://doi.org/10.1016/j.foreco.2012.12.038 (2013).
Cooke, B. J., Nealis, V. G. & Regniere, J. Insect defoliators as periodic disturbances in northern forest ecosystems. In Plant Disturbance Ecology: The Process and the Response (eds. Johnson, E. A. & Miyanashi, K. ) 487–525 (Elsevier, 2007).
Volterra, V. Variations and fluctuations of the number of individuals in animal species living together. In Chapman RN (ed. Ecology, A.) 31–113 (McGraw-Hill, 1926).
Turchin, P. Rarity of density dependence or population regulation with lags?. Nature 344, 660–663. https://doi.org/10.1038/344660a0 (1990).
Kendall, B. E. et al. Why do populations cycle? A synthesis of statistical and mechanistic modeling approaches. Ecology 80(6), 1789–1805. https://doi.org/10.1890/0012-9658(1999)080[1789:WDPCAS]2.0.CO;2 (1999).
Haynes, K. J., Liebhold, A. M. & Johnson, D. M. Spatial analysis of harmonic oscillation of gypsy moth outbreak intensity. Oecologia 159, 249–256. https://doi.org/10.1007/s00442-008-1207-7 (2009).
Nelson, W. A., Bjørnstad, O. N. & Yamanaka, T. Recurrent insect outbreaks caused by temperature-driven changes in system stability. Science 341, 796–799. https://doi.org/10.1126/science.1238477 (2013).
Reddy, G. V. P. et al. The seesaw effect of winter temperature change on the recruitment of cotton bollworms Helicoverpa armigera through mismatched phenology. Ecol. Evol. 5, 5652–5661. https://doi.org/10.1002/ece3.1829 (2015).
Walter, J. A., Ives, A. R., Tooker, J. F. & Johnson, D. M. Life history and habitat explain variation among insect pest populations subject to global change. Ecosphere 9(5), e02274. https://doi.org/10.1002/ecs2.2274 (2018).
Benda, P. et al. Bats (Mammalia: Chiroptera) of the eastern Mediterranean, part 3, review of bat distribution in Bulgaria. Acta Soc. Zool. Bohem. 67(4), 245–357 (2003).
Rodrigues, L. & Palmeirim, J. M. Migratory behaviour of the Schreiber’s bat: When, where and why do cave bats migrate in a Mediterranean region?. J. Zool. 274, 116–125. https://doi.org/10.1111/j.1469-7998.2007.00361.x (2007).
Norberg, U. M. & Rayner, J. M. V. Ecological morphology and flight in bats (Mammalia; Chiroptera): Wing adaptations, flight performance, foraging strategy and echolocation. Philos. Trans. R. Soc. 316, 335–427. https://doi.org/10.1098/rstb.1987.0030 (1987).
Vincent, S., Nemoz, M. & Aulagnier, S. Activity and foraging habitats of Miniopterus schreibersii (Chiroptera, Miniopteridae) in southern France: Implications for its conservation. Hystrix It. J. Mamm. 22(1), 57–72. https://doi.org/10.4404/hystrix-22.1-4524 (2011).
BOJA (2015, accessed 300 Nov 2022). http://www.juntadeandalucia.es/eboja/2015/50/BOJA15-050-00045-4371-01_00065608.pdf.
Consejería de Medio Ambiente y Ordenación del Territorio. Valores ambientales de la Zona de Especial Conservación, Cuevas de la Mujer y de las Colmenas (ES6120030). Junta de Andalucía (2015).
Qgis v 3.24 (2022, accessed 30 Nov 2022) https://www.qgis.org/en/site/about/index.html.
Junta de Andalucía. Mapa de usos del suelo y coberturas vegetales de Andalucía: 2007 (2017, accessed 30 Nov 2022). https://www.juntadeandalucia.es/datosabiertos/portal/dataset/mapa-de-usos-del-suelo-y-coberturas-vegetales-de-andalucia-2007.
Sherwin, C. E. Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 83, 301–309. https://doi.org/10.1016/j.anbehav.2011.10.031 (2012).
Zeale, M. R., Butlin, R. K., Barker, G. L., Lees, D. C. & Jones, G. Taxon-specific PCR for DNA barcoding arthropod prey in bat faeces. Mol. Ecol. Resourc. 11(2), 236–244. https://doi.org/10.1111/j.1755-0998.2010.02920.x (2011).
Gillet, F. et al. A new method to identify the endangered Pyrenean desman (Galemys pyrenaicus) and to study its diet, using next generation sequencing from faeces. Mamm. Biol. 80(6), 505–509. https://doi.org/10.1016/j.mambio.2015.08.002 (2015).
Aldasoro, M. et al. Gaining ecological insight on dietary allocation among horseshoe bats through molecular primer combination. PLoS ONE 14(7), e0220081. https://doi.org/10.1371/journal.pone.0220081 (2019).
Esnaola, A. et al. It is the ambience, not the menu. Prey availability does not drive habitat selection by the endangered Pyrenean desman. Aquat. Conserv. Mar. Freshw. Ecosyst. 31, 1859–1872. https://doi.org/10.1002/aqc.3563 (2021).
Illumina, I. 16S Metagenomic sequencing library preparation. Preparing 16S Ribosomal RNA Gene Amplicons for the Illumina MiSeq System 1–28 (2013, accessed 30 Nov 2022). https://support.illumina.com/downloads/16s_metagenomic_sequencing_library_preparation.html.
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 16(19), 2460–2461. https://doi.org/10.1093/bioinformatics/btq461 (2010).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17(1), 10–12. https://doi.org/10.14806/ej.17.1.200 (2011).
Edgar, R. C. UNOISE2: Improved error-correction for Illumina 16S and ITS amplicon sequencing. BioRxiv 2016, 081257. https://doi.org/10.1101/081257 (2016).
Vesterinen, E. J., Lilley, T., Laine, V. N. & Wahlberg, N. Next generation sequencing of fecal DNA reveals the dietary diversity of the widespread insectivorous predator Daubenton’s bat (Myotis daubentonii) in Southwestern Finland. PLoS ONE 8(11), e0201763. https://doi.org/10.1371/journal.pone.0082168 (2013).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2021). https://www.R-project.org/
Deagle, B. E. et al. Counting with DNA in metabarcoding studies: How should we convert sequence reads to dietary data?. Mol. Ecol. 28, 391–406. https://doi.org/10.1111/mec.14734 (2019).
Legendre, P. & Anderson, M. Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 69, 1–24. https://doi.org/10.1890/0012-9615(1999)069[0001:DBRATM]2.0.CO;2 (1999).
Wood, S. N. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J. Am. Stat. Assoc. 99, 673–686. https://doi.org/10.1198/016214504000000980 (2004).
Zuur, A., Ieno, E. N., Walker, N., Saveliev, A. A. & Smith, G. M. Mixed Effects Models And Extensions In Ecology with R (Springer, 2009).
European and Mediterranean Plant Protection Organization (EPPO) Global Database (2022). https://gd.eppo.int/.
Cuff, J. P., Windsor, F. M., Tercel, M. P. T. G., Kitson, J. J. N. & Evans, D. M. Overcoming the pitfalls of merging dietary metabarcoding into ecological networks. Methods Ecol. Evol. 13(3), 545–559. https://doi.org/10.1111/2041-210X.13796 (2022).
Constant, P. & Cannonge, B. Evaluation de la vitesse du vol des miniopteres. Mammalia 21, 301–302 (1957).
Ahlen, I. Identification of bats in flight (Swedish Society for Conservation of Nature, 1990).
Barataud, M. Acoustic ecology of European bats: Species identification, study of their habitats and foraging behaviour (National Museum of Natural History, Paris, 2015).
Lugon, A. Le régime alimentaire du Minioptère de Schreibers: Premiers résultats. In Eco-conseil, La Chaux de Fonds, Switzerland (1998).
Presetnik, P. & Aulagnier, S. The diet of Schreiber’s bent-winged bat, Miniopterus schreibersii (Chiroptera: Miniopteridae), in northeastern Slovenia (Central Europe). Mammalia 77(3), 297–305. https://doi.org/10.1515/mammalia-2012-0033 (2013).
Arrizabalaga-Escudero, A. et al. Assessing niche parititioning of co-ocurring sibling bat species by DNA metabarcoding. Mol. Ecol. 27(5), 1273–1283. https://doi.org/10.1111/mec.14508 (2018).
Galan, M. et al. Metabarcoding for the parallel identification of several hundred predators and their prey: Application to bat species diet analysis. Mol. Ecol. Resourc. 18, 474–489. https://doi.org/10.1111/1755-0998.12749 (2018).
Ragonot,. Cephis Galleriullus. Pp: 295-296 + Plate III In: Staudinger, O. Lepidopteren aus Tunis. Deutsche Entomol. Zeitschr. Iris 5(2), 277–300 (1892).
Agenjo, R. Thopeutis galleriella (Ragonot, 1892) género y especie nuevos para la Península Ibérica. EOS, Rev. Españ. Entomol. 53, 7–8 (1979).
Sociedad Gaditana de Historia Natural. VI BIOBLITZ de la SGHN Marisma de Trebujena (Cádiz). 22 y 23 de mayo de 2021. Soc. Gad. Hist. Nat. 9, 20–32 (2021).
Russo, D. & Jones, G. Use of foraging habitats by bats in a Mediterranean area determined by acoustic surveys: Conservation implications. Ecography 26(2), 197–209. https://doi.org/10.1034/j.1600-0587.2003.03422.x (2003).
Rainho, A. Summer foraging habitats of bats in a Mediterranean region of the Iberian Peninsula. Acta Chiropterol. 9(1), 171–181. https://doi.org/10.3161/1733-5329(2007)9[171:SFHOBI]2.0.CO;2 (2007).
Goiti, U., Vecin, P., Garin, I., Saloña, M. & Aihartza, J. R. Diet and prey selection in Kuhl’s pipistrelle Pipistrellus kuhlii (Chiroptera: Vespertilionidae) in south-western Europe. Acta Theriol. 48, 457–468 (2003).
Heim, O. et al. Landscape and scale-dependent spatial niches of bats foraging above intensively used arable fields. Ecol. Process. 6, 24. https://doi.org/10.1186/s13717-017-0091-7 (2017).
Kolkert, H., Andrew, R., Smith, R., Rader, R. & Reid, N. Insectivorous bats selectively source moths and eat mostly pest insects on dryland and irrigated cotton farms. Ecol. Evol. 10, 371–388. https://doi.org/10.1002/ece3.5901 (2020).
Lee, Y. F. & McCracken, G. F. Dietary variation of Brazilian free-tailed bats links to migratory populations of pest insects. J. Mammal. 86, 67–76. https://doi.org/10.1644/1545-1542(2005)086%3c0067:DVOBFB%3e2.0.CO;2 (2005).
Cohen, Y., Bar-David, S., Nielsen, M., Bohmann, K. & Korine, C. An appetite for pests: Synanthropic insectivorous bats exploit cotton pest irruptions and consume various deleterious arthropods. Mol. Ecol. 29(6), 1185–1198. https://doi.org/10.1111/mec.15393 (2020).
Tournaire, O. et al. eDNA metabarcoding reveals a core and secondary diets of the greater horseshoe bat with strong spatio-temporal plasticity. Env. DNA 3(1), 277–296. https://doi.org/10.1002/edn3.167 (2020).
Chinery, M. Collins Guide to the Insects of Britain and Western Europe (Collins, 1991).
Showers, W. B. Migratory ecology of the black cutworm. Annu. Rev. Entomol. 42, 393–425. https://doi.org/10.1146/annurev.ento.42.1.393 (1997).
Waring, P. & Townsend, M. Field Guide to the Moths of Great Britain and Ireland (Bloomsbury Publishing, 2017).
Sullivan, M. & Molet, T. CPHST Pest Datasheet for Autographa gamma. USDA-APHIS-PPQ-CPHST (2007, accessed 30 Nov 2022). https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjBr7XExI7-AhWDPwKHfAaD54QFnoECAgQAQ&url=http%3A%2F%2Fdownload.ceris.purdue.edu%2Ffile%2F3026&usg=AOvVaw1AqCmOxQfKSubcv2qS16I.
Goiti, U., Garin, I., Almenar, D., Salsamendi, E. & Aihartza, J. Foraging by Mediterranean horseshoe bats (Rhinolophus euryale) in relation to prey distribution and edge habitat. J. Mammal. 89(2), 493–502. https://doi.org/10.1644/07-MAMM-A-054R2.1 (2008).
Rainho, A. & Palmeirim, J. The importance of distance to resources in the spatial modelling of bat foraging habitat. Plos One 6(4), e19227. https://doi.org/10.1371/journal.pone.0019227 (2011).
Salsamendi, E., Garin, I., Arostegui, I., Goiti, U. & Aihartza, J. What mechanism of niche segregation allows the coexistence of sympatric sibling rhinolophus bats?. Front. Zool. 9(30), 1–12. https://doi.org/10.1186/1742-9994-9-30 (2012).
Salinas-Ramos, V. B., Ancillotto, L., Bosso, L., Sánchez-Cordero, V. & Russo, D. Interspecific competition in bats: State of knowledge and research challenges. Mammal. Rev. 50, 68–81. https://doi.org/10.1111/mam.12180 (2019).
Rydell, J. & Baagøe, H. J. Vespertilio murinus. Mammal. Spec. 467, 1–6. https://doi.org/10.2307/3504265 (1994).
Jaberg, C., Leuthold, C. & Blant, J. D. Foraging habitats and feeding strategy of the parti-coloured bat Vespertilio murinus L. 1758 in western Switzerland. Myotis 36, 51–61 (1998).
Haupt, M., Menzler, S. & Schmidt, S. Flexibility of habitat use in Eptesicus nilssonii: Does the species profit from anthropogenically altered habitats?. J. Mammal. 87(2), 351–361. https://doi.org/10.1644/05-MAMM-A-193R1.1 (2006).
Popa-Lisseanu, A. G., Bontadina, F. & Ibáñez, C. Giant noctule bats face conflicting constraints between roosting and foraging in a fragmented and heterogeneous landscape. J. Zool. 278(2), 126–133. https://doi.org/10.1111/j.1469-7998.2009.00556.x (2009).
Kipson, M. et al. Foraging habitat, home-range size and diet of a Mediterranean bat species, Savi’s pipistrelle. Acta Chiropterol. 20(2), 351–369. https://doi.org/10.3161/15081109ACC2018.20.2.007 (2018).
Lugon, A., Bilat, Y. & Roué, S. Y. Etude d'incidence de la LGV Rhin-Rhône sur le site Natura 2000 Mine d'Ougney, sur mandat de Réseau Ferré de France, Mission TGV Rhin-Rhône, Besançon. In Eco-conseil, La Chaux de Fonds, Switzerland (2004).
Moreno-Mateos, D. et al. The long-term restoration of ecosystem complexity. Nat. Ecol. Evol. 4, 676–685. https://doi.org/10.1038/s41559-020-1154-1 (2020).
Carr, A. et al. Moths consumed by the barbastelle Barbastella barbastellus require larval host plants that occur within the bat’s foraging habitats. Acta Chiropterol. 22(2), 275–269. https://doi.org/10.3161/15081109ACC2020.22.2.003 (2021).
Froidevaux, J. et al. Bat responses to changes in forest composition and prey abundance depend on landscape matrix and stand structure. Sci. Rep. 11, 10586. https://doi.org/10.1038/s41598-021-89660-z (2021).
García-Estrada, C. G., Damon, A., Sanchez-Hernández, C., Soto-Pinto, L. & Ibarra-Núñez, G. Bat diversity in montane rainforest and shaded coffee under different management regimes in southeastern Chiapas, Mexico. Biol. Conserv. 132, 351–361. https://doi.org/10.1016/j.biocon.2006.04.027 (2006).
Fuentes-Montemayor, E., Goulson, D. & Park, K. J. Pipistrelle bats and their prey do not benefit from four widely applied agri-environment management prescriptions. Biol. Conserv. 144, 2233–2246. https://doi.org/10.1016/j.biocon.2011.05.015 (2011).
Monck-Whipp, L., Martin, A. E., Francis, C. M. & Fahrig, L. Farmland heterogeneity benefits bats in agricultural landscapes. Agric. Ecosyst. Env. 253, 131–139. https://doi.org/10.1016/j.agee.2017.11.001 (2018).
Benton, T. G., Vickery, J. A. & Wilson, J. D. Farmland biodiversity: Is habitat heterogeneity the key?. Trends Ecol. Evol. 18, 182–188. https://doi.org/10.1016/S0169-5347(03)00011-9 (2003).
Duelli, P. & Obrist, M. K. Regional biodiversity in an agricultural landscape: The contribution of seminatural habitat islands. Basic Appl. Ecol. 4, 129–138. https://doi.org/10.1078/1439-1791-00140 (2003).
Turner, M. G., Gardner, R. H. & O’Neill, R. V. Landscape Ecology in Theory and Practice: Pattern and Process (Springer, 2001).
Bertrand, C., Burel, F. & Baudry, J. Spatial and temporal heterogeneity of the crop mosaic influences carabid beetles in agricultural landscapes. Landsc. Ecol. 31, 451–466. https://doi.org/10.1007/s10980-015-0259-4 (2016).
Fahrig, L. et al. Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol. Lett. 14, 101–112. https://doi.org/10.1111/j.1461-0248.2010.01559.x (2011).
Tilman, D. & Kareiva, P. Spatial Ecology: The Role of Space in Population Dynamics and Interspecific Interactions (Princeton University Press, 1997).
Hopkins, D. L. Xylella fastidiosa: Xylem-limited bacterial pathogen of plants. Annu. Rev. Phytopathol. 27, 271–290. https://doi.org/10.1146/annurev.py.27.090189.001415 (1989).
Lago, C. et al. Dispersal of Neophilaenus campestris, a vector of Xylella fastidiosa, from olive groves to over-summering hosts. J. Appl. Entomol. 2021, 1–12. https://doi.org/10.1111/jen.12888 (2021).
Rodríguez-Alarcón, L. G. S. M. et al. Unprecedented increase of West Nile virus neuroinvasive disease, Spain, summer 2020. Eurosurveillance 26(19), 2002010. https://doi.org/10.2807/1560-7917.ES.2021.26.19.2002010 (2021).
Taylor, P. J. et al. Diet determined by next generation sequencing reveals pest consumption and opportunistic foraging by bats in macadamia orchards in South Africa. Acta Chiropterol. 19(2), 239–254. https://doi.org/10.3161/15081109ACC2017.19.2.003 (2017).
Litvaitis, J. A. Investigating food habits of terrestrial vertebrates. In Research Techniques in Animal Ecology (eds Boitani, L. & Fuller, T. K.) 165–190 (Columbia University Press, 2000).
Alberdi, A., Aizpurua, O., Gilbert, M. T. P., Bohmann, K. & Mahon, A. Scrutinizing key steps for reliable metabarcoding of environmental samples. Methods Ecol. Evol. 9, 134–147. https://doi.org/10.1111/2041-210X.12849 (2018).
Elbrecht, V. & Leese, F. Can DNA-based ecosystem assessment quantify species abundance? Testing priomer bias and biomass-sequence relationships with an innovative metabarcoding protocol. PLoS ONE 10(7), e0130324. https://doi.org/10.1371/journal.pone.0130324 (2015).
Piñol, J., Senar, M. A. & Symondson, W. O. C. The choice of universal primers and the characteristics of the species mixture determines when DNA metabarcoding can be quantitative. Mol. Ecol. 28(2), 407–419. https://doi.org/10.1111/mec.14776 (2018).
Pompanon, F. et al. Who is eating what: Diet assessment using next generation sequencing. Mol. Ecol. 21, 1931–1950. https://doi.org/10.1111/j.1365-294X.2011.05403.x (2012).
Ancillotto, L. et al. Bats as suppressors of agroforestry pests in beech forests. For. Ecol. Manag. 522(120467), 1–7. https://doi.org/10.1016/j.foreco.2022.120467 (2020).
Acknowledgements
We are grateful to the INVIED OA of the Ministry of Defense for facilitating access to the faeces collection and allowing us to conduct experiments on their estates in Sierra de San Cristóbal. Special gratitude to the Sequencing and Genotyping Unit—Genomic Facilities—SGIker (UPV/EHU/ERDF, EU) for the technical support and the Molecular Ecology Laboratory of the Estación Biológica de Doñana (LEM-EBD) for logistical support. Aitor Larrañaga's advice was of great help to improve statistics. Stewart Finlayson and Tyson Lee Holmes, from Gibraltar National Museum, assisted in fieldwork.
Funding
The Spanish Ministry of Economy and Competitiveness (CGL-2015-69069P and PID2019-108123GB-I00) and the Government of the Basque Country (IT1163-19 and IT1571-22) funded this project. The Spanish Ministry of Economy and Competitiveness and the University of the Basque Country UPV/EHU granted NV (FPU18/02701) and MA (PIF20/131), respectively.
Author information
Authors and Affiliations
Contributions
Joxerra Aihartza, Nerea Vallejo, Urtzi Goiti and Carlos Ibáñez conceived the ideas and designed the methodology. Carlos Ibáñez and Jesus Nogueras collected the samples and monitored the colony. Nerea Vallejo and Juan L. García-Mudarra produced the sequences. Nerea Vallejo performed the bioinformatic analyses, and Nerea Vallejo, Joxerra Aihartza and Miren Aaldasoro performed the data analysis. Urtzi Goiti and Joxerra Aihartza managed the research funding. Joxerra Aihartza led the writing of the manuscript. All authors critically contributed to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aihartza, J., Vallejo, N., Aldasoro, M. et al. Aerospace-foraging bats eat seasonably across varying habitats. Sci Rep 13, 19576 (2023). https://doi.org/10.1038/s41598-023-46939-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46939-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.