Abstract
Chromium is a hazardous compound from industrial processes, known for its toxicity, mutagenicity, teratogenicity, and carcinogenicity. Chemical methods are efficient but cost-effective alternatives with reduced sludge are sought. Electro-coagulation, utilizing low-cost iron plate electrodes, was explored for factual tannery wastewater treatment in this manuscript. Operating parameters such as initial chromium concentration, voltage, electrode number, operating time, agitation speed and current density has been studied to evaluate the treatment effeciency. Under optimal conditions (15 V, 0.4 mA/cm2, 200 rpm, 330 ppm chromium, 8 iron electrodes with a total surface area of 0.1188 m2, 3 h), chromium elimination was 98.76%. Iron anode consumption, power use, and operating cost were 0.99 gm/L, 0.0143 kW-h/L, and 160 EGP/kg of chromium eliminated, respectively. Kinetics studies were pursued first-order reaction (97.99% correlation), and Langmuir isotherms exhibited strong conformity (Langmuir R2: 99.99%). A predictive correlation for chromium elimination (R2: 97.97%) was developed via statistical regression. At HARBY TANNERY factory in Egypt, industrial sewage treatment achieved a final chromium disposal rate of 98.8% under optimized conditions.
Similar content being viewed by others
Introduction
Wastewater treatment is no longer a mere option but an imperative necessity to safeguard the environment, ecosystems, and public health1,2,3. The tannery industry, one of the oldest worldwide, generates a significant volume of hazardous waste, laden with chemicals, salts, dyes, and other pollutants4. Among these pollutants, chromium stands out as a major inorganic contaminant, notably in tannery effluents with concentrations ranging from 10 to 1000 mg/L, far exceeding discharge limits5,6. The oxidation of chromium to its toxic form, Cr (VI), poses severe health and environmental risks7,8,9. Tannery effluents contain a cocktail of substances, including dyes, organic compounds, acids, alkalis, tannins, and various chemicals, which are not completely bound to the skins and consequently remain in the wastewater. A substantial portion of these effluents, around 90%, finds its way into the environment and the prove for this truth that the landfill leachate that formed after digestion of municipal solid waste contain a percent of chrouim10,11.
To address this critical issue, various methods have been employed for chromium removal from wastewater including ion exchange, chemical reduction and precipitation, reverse osmosis, photocatalytic processes, and adsorption12,13,14. The coagulation/flocculation process is one of them and has grown in popularity due to its ease of use, however its major drawback is that it employs too many chemicals, which results in secondary contamination15,16. Adsorption and ion exchange methods are expensive and have limited removal capacity. Thus, there is a need for cost-effective treatment methods that can handle the high chromium loads in tannery wastewater without secondary pollution. Among these methods, electro-coagulation (EC) has gained attention due to its eco-friendliness, cost-effectiveness, unlike traditional procedures, and minimal chemical usage, reducing the risk of secondary contamination17,18,19. EC involves the generation of coagulants during the degradation of sacrificial anodes due to the applied current, coupled with the production of hydrogen at the cathode, facilitating pollutant removal through precipitation and flotation. The prime drawback of this technique is the high energy consumption, which we overcme in our investigation by adding electrolyte to enhance solution conductivity and reduce energy use during electro-coagulation.
This technology has been applied successfully in treating wastewater from diverse industrial sectors, including tanneries, dairy wastewater, pharmaceutical wastewater, and distillery wastewater20,21,22,23. The efficiency of the EC process depends on several key parameters, such as electrode material, current density, wastewater pH, operating time, and initial chromium concentration. Studies have shown that optimizing these parameters can lead to significant chromium removal rates, offering potential for recycling chromium-rich sludge and using as a raw material in many industries, such as the ceramic industry and conversion of this sludge to a value-added product such as biogas energy which will also reduce any negative environmental effects24,25.
In previous studies, Nahid M. Genawi26 achieved complete chromium removal at 13 mA/cm2, pH 7, and a chromium concentration of 750 ppm, with XPS analysis showing 79.28% chromium oxides and 20.72% chromium hydroxides, indicating recycling potential26. Angel Villabona-Ortíz27 improved electro-coagulation with longer residence times, lower voltages, and increased electrode numbers, achieving a 92.9% removal rate using 10 electrodes at 20 V for 30 minutes27. Hamadan and El-Naas28,29 attained complete chromium removal at 7.9 mA/cm2 and enhanced the process using an EC column with a helical iron cathode and air injection for better mixing28,29. Other studies reports that increasing current density, enhancing electrode dissolution and removal rates, demonstrating effective chromium removal under both alkaline and acidic pH conditions30,31,32.
This manuscript focuses on the optimization of an electro-coagulation process for efficient and economical chromium elimination from simulated tannery wastewater using a simple batch electro-coagulation cell. The iron plates were utilized as electrodes for the electro-coagulation technique. The numerous operating factors like initial chromium concentration, applied voltage, current density, number of electrodes, agitation velocity, and treatment time were assessed to define the best chromium elimination. A kinetic study has been fulfilled to ensure the influence of numerous processing variables on chromium disposal. The manuscript also estimates electrode and energy consumption under ideal conditions and characterizes the deposited sludge resulting from the electro-coagulation process. After that, application of electro-coagulation on elimination of chromium from Factual tannery wastewater huddled from an effluent stream of leather tanning industry by HARBY TANNERY factory in Rubiki (Badr city) was explored under these optimized conditions. This research offers valuable insights into an environmentally friendly and efficient method for tackling chromium pollution in tannery effluents. In addition, the using of modeling isotherm with these such combinations of economical, kinetics and using real waste may decrease the effort and cost that could be paid for doing such pilot-scale work for electrochemical treatment of tannery wastewater.
Materials and methods
Materials
The reagents utilized in this experiment were potassium chromate of 98% purity, hydrochloric acid (HCl) of 30% purity, sodium hydroxide Pellets (NaOH) with a purity of 98% and sodium chloride (NaCl) of 98% purity. All reagents were obtained from El-Gomhouria Company for Trading Chemicals and Medical Appliances located at greater Cairo in Egypt.
Samples collection and classification
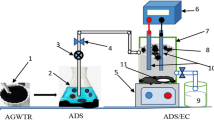
Factual tannery wastewater samples are assembled from a leather tanning factory in the industrial area of Rubiki (east of Cairo). These samples are transported and stored at 4 0C to be analyzed per APHA 2017 standards1. To assess disposal efficiency under varying conditions, we created a chromium stock solution by dissolving 98% pure potassium chromate salt in distilled water. We achieved the desired experimental concentrations through successive dilutions with distilled water. Table 1 reveals the factual tannery wastewater characterizations.
Electro-coagulation system
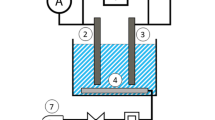
The experimental unit as shown in Fig. 1 includes a cylindrical glass container (reactor) with internal diameter of 10.5 cm and total a capacity about 1.2 L. This design allows setting 10 equidistant iron electrodes. Each iron electrode has a rectangular Sect. (13.5 cm × 5.5 cm) with thickness of 1 mm. Electrodes are vertically positioned, and arranged parallel to each other with a space of 1cm between them and installed at 2 cm from the bottom of the reactor. The electrodes operate in monopolar mode and connect to the positive and negative depots of the DC power supply (Range 230 V/50 A). The magnetic stirrer is utilized to maintain an unchanged composition and avoid the association of the clumps in the solution.
The anode efficient area (EA) can be calculated as shown in Eq. (1)33. The immersed length here will be 9 cm form plate height (13.5 cm).
Experimental proceeding
In each trial, 500 mL of the sample was placed in the glass reactor. Optimum pH for chromium elimination is preferred within the range 4–8, as suggested by previous studies34,35. The observed pH increase is owin to the generation of hydroxide ions (OH-) from the cathode through water electrolysis, as described in Eq. (3), which is used to produce hydroxides or bind to the sludge36. Elevated pH values, primarily Fe(OH)3, positively impact contaminant removal, consistent with many litrature. OH- ions can also undergo partial combination with Cr3+ ions, resulting in the formation of the insoluble hydroxide precipitate Cr (OH)3. Interestingly, when the initial pH was raised to 9.00, the efficiency of total Cr removal decreased because of the conversion of Cr ions to CrO42–, which in turn weakened the effectiveness of Cr disposal37. The solution pH is adjusted by adding HCl (10%) and/or NaOH (10%) solutions as required. A specific concentration of NaCl (98% purity) is added for supporting electrolyte and depending on the experiment to increase the conductivity. All experiments were carried out at room temperature (25 0C). At specified time intervals; 30 mL supernatant was withdrawn by a pipette from the top of the reactor for chromium measurement. The final concentration of chromium in the solution was determined by UV–VIS spectrophotometer; model UV-BK1900 with a 1 cm quartz cell at 540 nm. The produced sludge was analyzed by XRF analyzer. Table 2 indicates the removal efficiency combined to the change in a lot of factors to investigate the reaction optimum conditions. The chromium removal percent was appraised using next equation:
where C0 and Ct are the initial and the final value of chromium, (mg/L).
Chromium elimination mechanism
Elimination process of chromium is done according to the oxidation and reduction reaction at anode and cathode respectively according to the next equations
Economical study
In this manuscript, plate material and electrical power price were considered as main price terms in the processing costs estimation (EGP/gm chromium eliminated) utilizing the succeeding equation electrodes consumption can be calculated from the next Eq.38,39.
where I = current intensity (A), t = elimination time (s), M = iron atomic weight (55.845 gm/mole), z = number of electrons transmitted in the reaction Fe = Fe2+ + 2e-, F = Faraday’s constant (96,500 Cb/mole), and V = sewer water solution volume (L).
Power exhaustion, E is estimated from the below equation at the optimum processing factors40,41.
where I = current intensity (A), t = time (h), V = wastewater solution volume (L), and U = applied voltage (volt).
where a is the electrical power price, (EGP/kWh) and it taken as 1 kWh = 1.60 EGP, b is the plate material price, (EGP/tiron) the price 1 t of iron = 27,500 EGP.
Ethics approval
Not applicable. This manuscript does not involve researching about humans or animals.
Consent to participate
All of the authors consented to participate in the drafting of this manuscript.
Results and discussion
Influence of treatment time on chromium concentration
The required treatment time has a significant effect not only in effluent quality but also in the treatment cost and reactor volume42. The impact of reaction time on chromium elimination efficacy was inspected under several process time values ranging from 1 to 5 h, while other operating factors were kept at constant values; i.e. 15 V, 0.4 mA/cm2 current density, 330 ppm initial chromium concentration, 200 rpm, and 6 Fe electrodes. It is obvious as shown in Fig. 2 that the elimination efficacy rises with the increase in treatment time. 3 h were enough to achieve removal efficiency of 97.01% which also almost equal to maximum the removal efficiency after 4 h 97.25%. Economically, time reaction 3 hours should be considered. The elimination efficacy reduces with increasing time which may be because of the influence of the electro-coagulation attaining the saturation point. Thus, unrestricted progress is not accomplished by raising the reaction time. As time increases, metal sheets develop a thin protective passivation film, affecting the quantity of deteriorated Fe(II) plates, generating radicals, reducing ions, and decreasing flocculant levels, while also diminishing the oxidation effect. Another crucial factor to consider in electro-coagulation is power consumption, influenced by longer operation times, leading to higher treatment costs. This is mainly due to the rapid oxidation of Cr3+ and the formation of Cr(OH)3. Initially, fine particles tend to aggregate during electro-coagulation, but as treatment time extends, particle size increases, resulting in reduced chromium disposal efficiency43. One possible explanation is that after 8 h, deposited chromium returns to the liquid phase, leading to decreased chromium disposal efficiency and an increased ratio of total dissolved solids.
Influence of initial chromium concentration on treatment efficiency
Figure 3 indicates that the disposal efficiency reduces with a raise in an initial chromium concentration. This performance is a result of the lack of clumps for a sorption of extra chromium at elevated concentrations and likewise because of the minimum iron corrosion rates and rising iron surface passivation at elevated chromate concentrations44,45. The cause why the chromium elimination efficacy reduces with raising in its premier concentration is concluded from Faraday’s law. In line with Faraday's law, a fixed current density yields a steady release of Fe2+ into the solution through anodic electro-dissolution, which intensifies with prolonged electrolysis46,47. These ions play a a pivotal role in facilitating the reduction of Cr(VI) to Cr(III), leading to the formation of insoluble Cr(OH)3 and Fe(OH)3. Subsequently, when the initial chromium concentration rises, a substantial quantity of iron ions becomes essential during prolonged electrolysis periods to achieve complete chromium ion reduction29,48. Alternatively, one can increase either the current density or the electrode surface area proportionally to ensure sufficient Fe2+ production for effective Cr(VI) disposal from the effluent49.
Influence of applied voltage and current density on treatment efficiency
The applied voltage is a crucial parameter that plays a critical role in the electro-coagulation technique. The data depicted in Fig. 4 exhibits that an increase in applied voltage corresponds to a greater degree of chromium disposal.The maximum elimination percentage was 97.01% at voltage of 15 V then there was a decrease in the elimination percentage with additional voltage. For that reason, the applied voltage was elected 15 V for chromium elimination during the electro-coagulation technique. Hasan et al.42 reported the similar observation and achieved utmost chromium efficacy at 15 V.
Increasing current density enhances metal removal efficiency by boosting Fe2+ and OH- production40,50. Current density impacts electro-chemical metal dosing rate, electrolytic bubble production, and floc growth,while also influencing electrical energy consumption, electrode material usage, and overall operating costs in electro-coagulation51,52. The same results were in the current density as shown in Fig. 5. The chromium elimination percentage is raised with raising the current density. In accordance with Faraday’s law, this observation was respected owing to an anodic dissolution28,39,45. In contrast, when a current density of 0.54 mA/cm2 is applied, a slight reduction in the elimination percentage is noticed. This reduction can be attributed to the formation of a higher amount of hydrogen bubbles at the cathode, which causes the sludge to rise and hinders the formation of flocs28,53,54. As a result, the chromium removal percent is reduced. It is noticed that with raising the current density, the turbidity of wastewater was also raised.
As per Moussa et al. 's findings in (2017)55, this behavior can be ascribed to a critical current density threshold. Even when using higher current density, the treated water does not exhibit a significant improvement beyond this critical point. Elevated current densities result in the release of a notable quantity of Fe3+ ions through anode dissolution. As a result, the generated Fe(OH)3 molecules adhere to surfaces without the presence of contaminants due to the saturation of adsorption sites created by iron hydroxide56. The utilization of high working current values has been linked to the generation of residual energy, leading to an increase in water temperature7. For that reason, 0.4 mA/cm2 of current density believed the suitable current density for the technique electro-coagulation.
The impact of current desity on the power consumption was also elucidated under several values ranging from 0.15 to 0.54 mA/cm2 while other operating factors were kept at constant values; i.e. 15 V, 330 ppm initial chromium concentration, 200 rpm, and 8 Fe electrodes for 3 h. It is obvious as depicted in Fig. 6 that the power consumption is directly related to the current density. Increasing current density from 0.15 to 0.4 mA/cm2 boosted chromium disposal efficacy from 89.84% to 98.76%, with power consumption rising from 0.0053 to 0.0143 kW-h/L. In a separate study, raising current density from 0.42 to 0.94 mA/cm2 improved chromium removal efficiency from 74.35% to 100%, alongside an energy consumption increase from 0.24 to 0.94 kW-h/m3.
Effect of magnetic rotational speed and number of fe electrodes on treatment efficiency
Rotational speed is very important as it ensures that the flocculants from the dissolved electrodes are homogeneously dispersed in the reactor. Referring to Fig. 7, the suitable magnet agitation velocity can be 200 rpm. In the chromium elimination process, iron and hydroxyl ions combine to create highly absorbent iron hydroxide that binds to contaminants. Iron hydroxide also forms aggregates with a network structure, effectively removing contaminants from the liquid. Excessive agitation can disrupt these aggregates, releasing contaminants57. Also, high agitation speeds lead to increased shear rates at the floc interface, resulting in irreversible floc breakage and preventing re-growth58.
The effects of rising electrode number on the elimination efficacy are elucidated in Fig. 8. From the figure, it was noticed that the disposal efficiency was raised with raising number of Fe electrodes from 4 to 8 electrodes. This elimination is owing to the destabilization of Cr in the solution through the hydrolysis products of iron, which permits accumulation and a superior segregation of the solution thru sedimentation or flotation.
It was noticed that the simultaneous elevate in the plates number improves disposal efficacy. This could be owing to the existence of ferrous hydroxides, which raises with the plates number. These composites perform as coagulants and trap contaminant molecules, perhaps thru the relative rise in Fe(II) hydroxides beside the plates number59. The outcomes also indicate that elevating the effective area enhanced the disposal efficiency and reduced processing time beloved, the elucidation of this observation as obeys: greater plate surface area led to a greater dispersion of bubbles everywhere the reactor, whilst a minimal plate surface area led to a massive bubbles source inside the reactor, and as the bubbles dispersion inside the reactor, the likelihood of collision between the bubbles and coagulant rises causing to rise the elimination efficacy.
The outcomes also indicated that the elimination efficiency relatively constant in case of 10 electrodes applied. For that reason, the ideal number of electrodes was considered 8 electrodes for chromium elimination through the electro-coagulation process.
Kinetic investigation via treatment process
The kinetic models of Cr elimination from wastewater were fulfilled under the ideal processing factors (330 ppm premier chromium concentration, 0.4 mA/cm2 current density, elimination time of 3 h, 8 iron electrodes, 200 rpm, and applied voltage of 15 V). Adequate results of Cr elimination kinetics are revealed in Table 3. The linear formula of first and second order kinetic models can be introduced in the following equations:
where Co and Ct are the premier and final chromium concentrations, respectively. K1 and K2are first and second order rate constants in min−1 and L. gm−1 min−1, respectively, and t is the treatment time (in min). A plot lnCt and [\(\frac{1}{Ct} - \frac{1}{Co}\)] against time for each run leads to a straight line whose slope is K1 and K2, respectively. It can be observed that the correlation coefficient R2 of the pseudo first order kinetic model was better than that of the pseudo second order kinetic model. An identical examination has been mentioned before by37 in the elimination of chromium from tannery wastewater by electro-coagulation. Also, Bingül et al.60 and Lorgio Valdiviezo-Gonzales et al.7 reported the first order kinetic model with good correlation for chromium. The regression analysis of the concentration curves against treatment time denotes that the reaction rate can be depicted by means of first order kinetic model for chromium elimination. The end result is exhibited in Fig. 9.
Isothermal modeling investigation
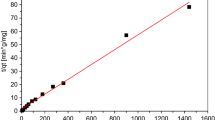
Isotherms are employed to depict the adsorption process under ideal factors ((330 ppm premier chromium concentration, 0.4 mA/cm2 current density, 8 iron electrodes, 200 rpm, and applied voltage of 15 V), and the Langmuir and Freundlich isotherms are widely used for this purpose. The Langmuir isotherm specifically characterizes adsorption at uniform sites, where a monolayer is formed. It is represented by a linear equation, given by the below Eq. (13)49:
The adsorption capacity qe (mg/gm) was calculated by means of the bellow Eq. (14):
where qe (mg/gm) is the equilibrium concentration of chromium, Ce (mg/L) is the equilibrium concentration in the liquid phase, V and W are volume of solution (L) and the weight of a sorbent (gm), individually. A linear plot of Ce/qe versus Ce indicates that the adsorption data reasonably fits the Langmuir isotherm, as exhibited in Fig. 10. The constants were obtained from the slope (1/qm) and intercept (1/KL) and are listed in Table 4. The Langmuir equation, expressed in terms of the dimensionless factor RL, is expressed by:
The value of RL, a positive number (0 < RL < 1), denotes the feasibility of the sorption process.
The Freundlich isotherm model is based on the assumption that adsorption takes place on heterogeneous surfaces with varying adsorption energies. The linear form of the Freundlich equation is expressed as:
where 1/n is the heterogeneity factor related to intensity, and Kf is the Freundlich constant. The slope and intercept of log qe against log Ce give 1/n and Kf values as elucidated in Fig. 10b.
The comparison of correlation values (R2) between the Langmuir and Freundlich adsorption isotherms indicates that the Langmuir isotherm outperforms the Freundlich isotherm. This indicates a robust correlation between the experimental data and the Langmuir model, suggesting that the Langmuir adsorption isotherm accurately describes the adsorption behavior in the studied system. The Langmuir isotherm is consistent with prior work61.
Sludge characteristics
The formed sludge was gathered, dried at 103 0C for 24 h, and then cooled in desiccators to assess the surface morphology of the deposited sludge throughout the XRF analysis. The XRF results showed that the chemical composition of the sludge ranged from 70–75% as weight of iron oxide and the percent of the chromium was ranged from 25–30% as weight from the simulated wastewater and the ferric and ferrous hydroxides formed during electro-coagulation later turn into magnetite. These results were in the same line of Un et al.30who stated that the percent of the chromium in the produced sludge in his study was 16.6% as weight and 74.3% as weight of chromium which confirms our finding and our hypothesis of iron oxide forming.
Statistical analysis for the proposed treatment process
To elucidate the influence of the operative factors on the chromium elimination efficiency, a mathematical correlation must be recommended. Statistical and least square multivariate regression techniques are extremely utilized for modeling and analysis of troubles in which a response of interested dependent variable is influenced by numerous independent variables. This model was utilized in an attempt to detect the mathematical correlation that can clarify the influence of the operative factors on the chromium disposal efficiency (ANOVA).
The values and p-values of the coefficients are provided in Table 5. The p-values decides if any given factor is momentous or not. The correlation terms having a p-value less than 0.0001 are momentous. The R2 was 97.97%; it exhibited that the changeability in the adsorption could be explicated by the model, with the cohesion between the experimental and predicted values being momentous inside the process. The acquired correlation in terms of momentous factors only has the following formula:
where CD is the current density, Co is the chromium initial concentration, AV agitation velocity, t is the elimination time, and N is the number of iron electrodes.
The normal probability of standardized residuals with average correlation errors of zero is demonstrated in Fig. 11. The linear distribution of the residual errors elucidates that the errors are normally distributed which denotes that the model prognoses are not prejudiced and for more clarity the comparison between the experimentally observed values of chromium removal percentage and predicted values is shown in Fig. 12. The figure exhibits a perfect convention between them. Table 6 clarifies the experimental observed and predicted chromium elimination percentage for the 19 runs. The chromium elimination percentage varied from 49.53% to 98.79%.
Application of electro-coagulation on chromium disposal from factual tannery wastewater with economical analysi
Chromium removal from sewage obtained from HARBY TANNERY in Rubiki (Badr city) was studied. The initial characteristics of the tannery sewage were 2700 mg/L total suspended solids, 1750 mg/L BOD5, 4025 mg/L COD, pH (6–9), NH4 (100 mg/L), TKN (250 mg/L), oil and gas (140 mg/L), sulphide (350 mg/L), and 3300 mg/L chromium. Electro-coagulation was conducted under optimized conditions (15 V applied voltage, 0.4 mA/cm2 current density, 200 rpm, and 330 ppm chromium initial concentration using 8 electrodes for 3 h). The real tannery wastewater concentration was reduced from 3300 to 330 ppm through dilution with distilled water. These results indicate a remarkable chromium elimination rate of 98.8% under these optimized conditions. A comparison with previous electro-coagulation studies on tannery wastewater treatment is exhibited in Table 7.
Economic studies have been used for the purpose of commercialization and many studies used the economical in their research to make the research easy use62,63,64. Based on the Eqs. (8–10), it was found that under optimized conditions (330 ppm initial chromium concentration, 0.4 mA/cm2 of current density, treatment time of 3 h, 8 iron electrodes, 200 rpm, and applied voltage of 15 V), the sacrificial iron anode exhaustion per liter of the effluent was 0.99 gm Fe per L. and the power exhaustion, E was 0.0143 kW-h/L of treated wastewater. The final Processing cost was (0.1546 EGP/gm (cr) removed).
A kilogram of pure chromium amounts to the equivalent of 4,500 EGP, and thus it is a profitable value for the idea of working the cell to remove and recover chromium in the future.
Conclusion
Electro-coagulation technique was used to remove chromium from wastewater of tanning and other leather processing. The recent study aimed to determine the optimum operation conditions which achieve high removal efficiency taken into account the economic concept and it concluded that:
-
For raw wastewater with 330 ppm of chromium, elimination efficacy of 98.76% can be achieved at the conditions of 15 V, 0.4 mA/cm2 current density, 200 rpm, 8 plate of electrodes and reaction time of 3 h.
-
The chromium elimination followed the 1st order reaction by kinetic analysis of electro-coagulation technique with a 97.99% correlation factor (R2).
-
The chemical composition of the deposited sludge after treatment ranged from 70–75% as weight of iron oxide and the percent of the chromium was ranged from 25–30% as weight from the simulated wastewater and the ferric and ferrous hydroxides formed during electro-coagulation later turn into magnetite.
-
The acquired correlation, considering significant factors only, can be expressed by the following formula:
$${\text{Removal }}\% \, = \, 167.24 \, + \, 13.68\;{\text{CD }}{-} \, 0.0687\;{\text{C}}_{0} {-} \, 0.04987\;{\text{AV}}{-}23.14\;{\text{N}} + 3.941 \, t{-}48.3636\;{\text{CD}}^{2} + 8.008\;t*N{-}6.506\;{\text{t}}^{2}$$ -
The maximum elimination percentage of chromium from sewage assembled from the effluent stream of the HARBY TANNERY factory in Rubiki (Badr city) reached 98.8% under the aforementioned optimal processing conditions. The 98.8% removal achievable exceeds most prior electro-coagulation studies, highlighting the optimization of operating conditions.
-
At optimized conditions, each 326 removed ppm from chromium consumes 0.99 gm/L from iron anode electrode, 0.0143 kw-h/L power and costs about 0.05 EGP.
-
In comparison to chemical coagulation, electro-chemical treatment is a faster and more efficient and cost-effective technique for wastewater treatment, providing a sustainable treatment alternative. It requires lower coagulant doses and shorter treatment times, making it an efficient and economical choice. In addition, the using of modeling isotherm with these combinations of economical, kinetics and using real waste may decrease the effort and cost that could be paid for doing such pilot-scale work for electrochemical treatment of tannery wastewater.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Abdelfattah, I. et al. Integrated system for recycling and treatment of hazardous pharmaceutical wastewater. Int. J. Environ. Sci. Technol. 20, 4101–4110 (2023).
Hellal, M. S., Al-Sayed, A., El-Liethy, M. A. & Hassan, G. K. Technologies for wastewater treatment and reuse in Egypt: Prospectives and future challenges. in Handbook of Advanced Approaches Towards Pollution Prevention and Control 275–310 (Elsevier, 2021).
El-Gawad, H. A., Ebrahiem, E. E., Ghaly, M. Y., Afify, A. A. & Mohamed, R. M. An application of advanced oxidation process on industrial crude oily wastewater treatment. Sci. Rep. 13, 3420 (2023).
Kumar, R. et al. Management of tannery waste effluents towards the reclamation of clean water using an integrated membrane system: A state-of-the-art review. Environ. Res. 115881 (2023).
Tessema, T. S., Adugna, A. T. & Kamaraj, M. Removal of Pb (II) from synthetic solution and paint industry wastewater using activated carbon derived from african arrowroot (Canna indica) stem. Adv. Mater. Sci. Eng. 2020, 1–10 (2020).
Laxmi, V. & Kaushik, G. Toxicity of hexavalent chromium in environment, health threats, and its bioremediation and detoxification from tannery wastewater for environmental safety. Bioremediation Ind. waste Environ. Saf. Vol. I Ind. waste its Manag. 223–243 (2020).
Valdiviezo Gonzales, L. G. et al. Kinetic study of electrocoagulation of tannery wastewater. (2023).
Castiblanco, Y., Perilla, A., Arbelaez, O., Velásquez, P. & Santis, A. Effect of the pH and the catalyst concentration on the removal of hexavalent chromium (Cr (VI)) during photocatalysis of wastewater from plating on plastics industry. Chem. Eng. Trans. 86, 679–684 (2021).
Gonzalez-Delgado, A., Tejada-Tovar, C. & Villabona-Ortiz, A. Computer-aided modeling and evaluation of a packed bed for chromium (vi) removal using residual biomass of Theobroma Cacao L. Chem. Eng. Trans. 92, 517–522 (2022).
Hassan, G. K., Gad-Allah, T. A., Badawy, M. I. & El-Gohary, F. A. Remediation of ammonia-stripped sanitary landfill leachate by integrated heterogeneous Fenton process and aerobic biological methods. Int. J. Environ. Anal. Chem. 1–14 (2021).
Hassan, G. K. & El-Gohary, F. A. Evaluation of partial nitritation/anammox process for reduction of pollutants from sanitary landfill leachate. Water, Air, Soil Pollut. 232, 1–12 (2021).
Kusku, O. et al. A comparative study of removal of Cr (VI) by ion exchange resins bearing quaternary ammonium groups. J. Chem. Technol. Biotechnol. 89, 851–857 (2014).
Koushkbaghi, S. et al. Aminated-Fe3O4 nanoparticles filled chitosan/PVA/PES dual layers nanofibrous membrane for the removal of Cr (VI) and Pb (II) ions from aqueous solutions in adsorption and membrane processes. Chem. Eng. J. 337, 169–182 (2018).
Obayomi, K. S., Auta, M. & Kovo, A. S. Isotherm, kinetic and thermodynamic studies for adsorption of lead (II) onto modified Aloji clay. Desalin. Water Treat. 181, 376–384 (2020).
Lefebvre, O. & Moletta, R. Treatment of organic pollution in industrial saline wastewater: A literature review. Water Res. 40, 3671–3682 (2006).
Kumar, D., Singh, J. & Baleanu, D. A new numerical algorithm for fractional Fitzhugh-Nagumo equation arising in transmission of nerve impulses. Nonlinear Dyn. 91, 307–317 (2018).
Ghernaout, D., Naceur, M. W. & Ghernaout, B. A review of electrocoagulation as a promising coagulation process for improved organic and inorganic matters removal by electrophoresis and electroflotation. Desalin. Water Treat. 28, 287–320 (2011).
Afify, A. A. et al. Electrochemical production of sodium hypochlorite from salty wastewater using a flow-by porous graphite electrode. Energies 16, 4754 (2023).
Aboutaleb, E., Kamel, G. & Hellal, M. Investigation of effective treatment techniques for olive mill wastewater. Egypt. J. Chem. 61, 415–422 (2018).
Makwana, A. R. & Ahammed, M. M. Continuous electrocoagulation process for the post-treatment of anaerobically treated municipal wastewater. Process Saf. Environ. Prot. 102, 724–733 (2016).
Khaldi, S., Lounici, H., Drouiche, M. & Drouiche, N. Treatment of ointment pharmaceutical wastewater by electrocoagulation process. Desalin. Water Treat. 71, 152–158 (2017).
Aitbara, A., Cherifi, M., Hazourli, S. & Leclerc, J.-P. Continuous treatment of industrial dairy effluent by electrocoagulation using aluminum electrodes. Desalin. water Treat. 57, 3395–3404 (2016).
Thakur, C., Srivastava, V. C. & Mall, I. D. Electrochemical treatment of a distillery wastewater: Parametric and residue disposal study. Chem. Eng. J. 148, 496–505 (2009).
Hassan, G. K. et al. Harnessing Cu@ Fe3O4 core shell nanostructure for biogas production from sewage sludge: Experimental study and microbial community shift. Renew. Energy 188, 1059–1071 (2022).
Xu, X. et al. Treatment of industrial ferric sludge through a facile acid-assisted hydrothermal reaction: Focusing on dry mass reduction and hydrochar recyclability performance. Sci. Total Environ. 869, 161879 (2023).
Genawi, N. M., Ibrahim, M. H., El-Naas, M. H. & Alshaik, A. E. Chromium removal from tannery wastewater by electrocoagulation: Optimization and sludge characterization. Water 12, 1374 (2020).
Villabona-Ortíz, Á., Tejada-Tovar, C. & Contreras-Amaya, R. Electrocoagulación como alternativa para eliminación de cromo (VI) en solución. Rev. Tecnura 25, NA-NA (2021).
Hamdan, S. S. & El-Naas, M. H. An electrocoagulation column (ECC) for groundwater purification. J. water Process Eng. 4, 25–30 (2014).
Hamdan, S. S. & El-Naas, M. H. Characterization of the removal of Chromium (VI) from groundwater by electrocoagulation. J. Ind. Eng. Chem. 20, 2775–2781 (2014).
Un, U. T., Onpeker, S. E. & Ozel, E. The treatment of chromium containing wastewater using electrocoagulation and the production of ceramic pigments from the resulting sludge. J. Environ. Manage. 200, 196–203 (2017).
Zaroual, Z., Chaair, H., Essadki, A. H., El Ass, K. & Azzi, M. Optimizing the removal of trivalent chromium by electrocoagulation using experimental design. Chem. Eng. J. 148, 488–495 (2009).
Akbal, F. & Camcı, S. Copper, chromium and nickel removal from metal plating wastewater by electrocoagulation. Desalination 269, 214–222 (2011).
Villabona-Ortíz, Á., Tejada-Tovar, C. & Contreras-Amaya, R. Electrocoagulation as an alternative for the removal of chromium (VI) in solution. Tecnura 25, 28–42 (2021).
Adhoum, N., Monser, L., Bellakhal, N. & Belgaied, J.-E. Treatment of electroplating wastewater containing Cu2+, Zn2+ and Cr (VI) by electrocoagulation. J. Hazard. Mater. 112, 207–213 (2004).
Vasudevan, S., Lakshmi, J. & Sozhan, G. Studies on the removal of iron from drinking water by electrocoagulation–A clean process. Clean-Soil, Air, Water 37, 45–51 (2009).
Elabbas, S. et al. Treatment of highly concentrated tannery wastewater using electrocoagulation: Influence of the quality of aluminium used for the electrode. J. Hazard. Mater. 319, 69–77 (2016).
Li, G., Yang, C., Yao, Y. & Zeng, M. Electrocoagulation of chromium in tannery wastewater by a composite anode modified with titanium: parametric and kinetic study. Desalin. Water Treat. 171, 294–301 (2019).
Taheri, M., Moghaddam, M. R. A. & Arami, M. Techno-economical optimization of Reactive Blue 19 removal by combined electrocoagulation/coagulation process through MOPSO using RSM and ANFIS models. J. Environ. Manage. 128, 798–806 (2013).
Patel, S. R. & Parikh, S. P. Chromium removal from industrial effluent by electrocoagulation: Operating cost and kinetic analysis. J. Environ. Treat. Tech. 9, 621–628 (2021).
Dermentzis, K., Christoforidis, A., Valsamidou, E., Lazaridou, A. & Kokkinos, N. Removal of hexavalent chromium from electroplating wastewater by electrocoagulation with iron electrodes. Glob. Nest J 13, 412–418 (2011).
Deveci, E. Ü., Akarsu, C., Gönen, Ç. & Özay, Y. Enhancing treatability of tannery wastewater by integrated process of electrocoagulation and fungal via using RSM in an economic perspective. Process Biochem. 84, 124–133 (2019).
Hasan, M. A., Hashem, M. A., Arman, M. N. & Momen, M. A. Batch electrocoagulation process for removal of chromium from tannery wastewater. J. Eng. Sci. 29–34 (2021).
Moradi, M., Vasseghian, Y., Arabzade, H. & Khaneghah, A. M. Various wastewaters treatment by sono-electrocoagulation process: a comprehensive review of operational parameters and future outlook. Chemosphere 263, 128314 (2021).
Noubactep, C. Characterizing the reactivity of metallic iron in Fe0/UVI/H2O systems by long-term column experiments. Chem. Eng. J. 171, 393–399 (2011).
El-Taweel, Y. A., Nassef, E. M., Elkheriany, I. & Sayed, D. Removal of Cr (VI) ions from waste water by electrocoagulation using iron electrode. Egypt. J. Pet. 24, 183–192 (2015).
Das, D. & Nandi, B. K. Removal of hexavalent chromium from wastewater by electrocoagulation (EC): Parametric evaluation, kinetic study and operating cost. Trans. Indian Inst. Met. 73, 2053–2060 (2020).
Zhou, R. et al. Comparison of Cr (VI) removal by direct and pulse current electrocoagulation: Implications for energy consumption optimization, sludge reduction and floc magnetism. J. Water Process Eng. 37, 101387 (2020).
Aber, S., Amani-Ghadim, A. R. & Mirzajani, V. Removal of Cr (VI) from polluted solutions by electrocoagulation: Modeling of experimental results using artificial neural network. J. Hazard. Mater. 171, 484–490 (2009).
Mousazadeh, M. et al. Reclamation of forward osmosis reject water containing hexavalent chromium via coupled electrochemical-physical processes. Environ. Technol. 1–14 (2022).
Peng, H., Leng, Y. & Guo, J. Electrochemical removal of chromium (VI) from wastewater. Appl. Sci. 9, 1156 (2019).
Verma, S. K., Khandegar, V. & Saroha, A. K. Removal of chromium from electroplating industry effluent using electrocoagulation. J. Hazardous Toxic Radioact. Waste 17, 146–152 (2013).
Lu, J., Wang, Z.-R., Liu, Y.-L. & Tang, Q. Removal of Cr ions from aqueous solution using batch electrocoagulation: Cr removal mechanism and utilization rate of in situ generated metal ions. Process Saf. Environ. Prot. 104, 436–443 (2016).
Şengil, İA. Treatment of dairy wastewaters by electrocoagulation using mild steel electrodes. J. Hazard. Mater. 137, 1197–1205 (2006).
Al Aji, B. Electrocoagulation with bipolar iron electrodes for trivalent chromium removal from synthetic wastewater.
Moussa, D. T., El-Naas, M. H., Nasser, M. & Al-Marri, M. J. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J. Environ. Manage. 186, 24–41 (2017).
Benaissa, F., Kermet-Said, H. & Moulai-Mostefa, N. Optimization and kinetic modeling of electrocoagulation treatment of dairy wastewater. Desalin. Water Treat. 57, 5988–5994 (2016).
Shahriari, T., Bidhendi, G. N., Mehrdadi, N. & Torabian, A. Removal of chromium (III) from wastewater by electrocoagulation method. KSCE J. Civ. Eng. 18, 949–955 (2014).
Aryanti, P. T. P., Nugroho, F. A., Prabowo, B. H., Adriaan, M. R. & Aziz, M. A. The Influence of Applied Current Density and Agitation Speed During Electrocoagulation of Textile Wastewater. in 2nd International Seminar of Science and Applied Technology (ISSAT 2021) 242–245 (Atlantis Press, 2021).
Nouri, J., Mahvi, A. H. & Bazrafshan, E. Application of electrocoagulation process in removal of zinc and copper from aqueous solutions by aluminum electrodes. Int. J. Environ. Res. 4, 201–208 (2010).
Bingül, Z., Irdemez, Ş., Kul, S., Ekmekyapar Torun, F. & Demircioğlu, N. Investigation of organic and inorganic matters removal from tannery wastewater using iron plate electrode by electrocoagulation process. Int. J. Environ. Anal. Chem. 1–14 (2021).
Vasudevan, S., Lakshmi, J. & Vanathi, R. Electrochemical coagulation for chromium removal: process optimization, kinetics, isotherms and sludge characterization. Clean-Soil, Air, Water 38, 9–16 (2010).
Tawfk, A. et al. Electron donor addition for stimulating the microbial degradation of 1,4 dioxane by sequential batch membrane bioreactor: A techno-economic approach. Chemosphere 306, 135580 (2022).
Elmaadawy, K. et al. Microalgae-assisted fxed-flm activated sludge MFC for landfll leachate treatment and energy recovery. Process Saf. Environ. Prot. 160, 221–231 (2022).
Al-Sayed, A. et al. Effect of organic loading rates on the performance of membrane bioreactor for wastewater treatment behaviours, fouling, and economic cost. Sci Rep 13, 15601 (2023).
Ulambayar, R., Oyuntsetseg, J., Tsiiregzen, A. & Bayaraa, D. Removal of Cr 3+ by electrocoagulation from simulated wastewater. Mong. J. Chem. 15, 89–93 (2014).
Sharma, D., Chaudhari, P. K. & Prajapati, A. K. Removal of chromium (VI) and lead from electroplating effluent using electrocoagulation. Sep. Sci. Technol. 55, 321–331 (2020).
Acknowledgements
All authors would acknowledge the El-Shrouk Academy; Faculty of Science—Cairo University; National Research Centre and Canal Higher Institute of Engineering and Technology for their financial support.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Dr. H.A.E.G.: conceptualization, designing-outline, figures etc., writing-original draft. Dr. K.M.A.: writing, reviewing and editing. Dr. W.H.M.: writing, reviewing and editing. Dr. G.K.H.: conceptualization, writing original draft, writing, reviewing, and editing; Dr. R.M.M.: writing, reviewing and editing; dr. A.A.A. and Dr. H.A.E.G.: conceptualization, writing original draft, writing, reviewing, and editing. All of the authors consented to publish this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Gawad, H.A., Hassan, G.K., Aboelghait, K.M. et al. Removal of chromium from tannery industry wastewater using iron-based electrocoagulation process: experimental; kinetics; isotherm and economical studies. Sci Rep 13, 19597 (2023). https://doi.org/10.1038/s41598-023-46848-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46848-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.