Abstract
We compared the efficacy and safety of autologous-serum (AS) and platelet-rich plasma (PRP) eye drops for dry eye (DE) treatment in primary Sjögren’s syndrome (SS). This prospective, randomized, double-blinded clinical study included patients diagnosed with primary SS DE. Thirty-eight participants were randomly assigned to the AS or PRP groups. Corneal and conjunctival staining scores, Schirmer I test, tear film break-up time (TBUT), and ocular surface disease index (OSDI) scores were evaluated at 4 and 12 weeks. Conjunctival impression cytology (CIC) metaplasia grade and goblet cell density grade at 12 weeks were compared with those at baseline. Corneal and conjunctival staining scores and TBUT significantly improved at 4 and 12 weeks in both groups (all p < 0.005). No significant difference between the AS and PRP groups was observed at 4 and 12 weeks. The Schirmer I values, OSDI scores, CIC metaplasia grade, and goblet cell density grade did not significantly change at 4 and 12 weeks in either group. Both AS and PRP eye drops are effective for primary SS DE without a significant difference. Considering that the preparation time of PRP is shorter than that of AS, PRP can be a good alternative treatment for primary SS DE.
Similar content being viewed by others
Introduction
Artificial tears, secretagogues, and anti-inflammatory eye drops have been widely used for dry eye (DE) treatment1. Fox et al.2 showed that autologous serum (AS) eye drops are useful for the treatment of intractable DE. The composition of AS is similar to that of tears in terms of pH; osmolarity; and levels of vitamin A, immunoglobulin A, and growth factor3, which have epitheliotropic potentials and help epithelial healing in ocular surface disorders4. Primary Sjögren’s syndrome (SS) is an autoimmune disease that causes the destruction of exocrine glands, such as the lacrimal glands, without any associated systemic disease5,6. SS can induce severe chronic DE due to infiltration of lymphocytes in the lacrimal glands and conjunctiva7. AS has been reported to have a beneficial effect on symptoms, ocular surface parameters, and confocal microscopic findings in primary SS DE8,9.
Platelet-derived eye drops, such as those containing platelet-rich plasma (PRP), plasma rich in growth factors, and platelet lysate, have recently been introduced for treating ocular surface disorders10,11,12,13. As platelets play a key role in wound healing owing to the high growth factor and cytokine levels14, various platelet-derived preparations have been used in regenerative medicine and orthopedic and maxillofacial surgeries15. Among them, PRP has been more frequently used than other platelet-derived preparations in eye drops for ocular surface disorders13,16,17,18,19. Kim et al.19 reported higher epidermal growth factor (EGF) levels in PRP than in AS and a higher success rate with PRP than with AS for persistent epithelial defects. Metheetrairut et al.20 reported significantly higher transforming growth factor (TGF)-β and fibronectin levels in PRP than in AS; however, AS and PRP showed similar effects on ocular surface signs in non-SS DE.
The clinical effect of AS and PRP in primary SS DE has not been extensively evaluated. This prospective randomized study aimed to compare the efficacy of AS and PRP eye drops on clinical DE parameters and goblet cells in primary SS DE.
Results
Patient enrolment
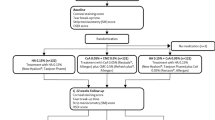
A total of 38 patients were screened for enrolment between March 2018 and March 2019, and 36 patients were ultimately included. Four patients who did not meet the primary efficacy endpoints and two patients who withdrew their consent were excluded from the full-analysis set (FAS) (Fig. 1). All patients were female, and there was no significant age difference between the two groups (AS group, 54.56 ± 11.939 years vs. PRP group, 54.07 ± 11.283 years; p = 0.739) (Table 1).
Primary efficacy endpoints
Corneal staining score
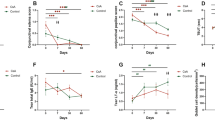
The baseline corneal staining score was not significantly different between the two groups (p = 0.518). Corneal staining scores improved at 12 weeks from baseline in both groups (AS group, from 3.09 ± 0.995 to 2.13 ± 1.185; p = 0.000 vs. PRP group, from 3.25 ± 0.844 to 2.23 ± 0.992; p = 0.000) (Table 2), and there was no significant difference between the two groups at 12 weeks (p = 0.952) (Fig. 2A).
Conjunctival staining score
The baseline conjunctival staining score was not significantly different between the two groups (p = 0.487). Conjunctival staining scores improved at 12 weeks from baseline in both groups (AS, from 3.28 ± 1.853 to 1.44 ± 1.39; p = 0.000 vs. PRP, from 2.93 ± 2.054 to 1.5 ± 1.478; p = 0.000) (Table 2), and there was no significant difference between the two groups at 12 weeks (p = 0.867) (Fig. 2B).
Secondary efficacy endpoints
Corneal and conjunctival staining scores at 4 weeks were compared with those at baseline. Significant reductions in the corneal staining score (p = 0.002 and p = 0.001 in the AS and PRP groups, respectively) and conjunctival staining score (p = 0.000 and p = 0.001 in the AS and PRP groups, respectively) were observed in both groups (Table 2 and Fig. 2). The Schirmer I value did not significantly change from baseline at any time point in either group (Table 2). Tear film break-up time (TBUT) improved in both groups at 4 and 12 weeks (p = 0.000 and p = 0.000 at 4 weeks; p = 0.000 and p = 0.001 at 12 weeks in the AS and PRP groups, respectively), and there was no significant difference between the two groups at 4 and 12 weeks (p = 0.533 and p = 0.983, respectively) (Table 2). The ocular surface disease index (OSDI) score did not improve from baseline at any time point in either group (Table 2). Corrected distance visual acuity did not significantly change throughout the 12 weeks in either group (data not shown). Additionally, conjunctival impression cytology (CIC) metaplasia and goblet cell density grades did not significantly change at 12 weeks from baseline in either group (Table 3).
Safety endpoints
Clinical laboratory examinations, vital signs assessment, and physical examination were performed in patients who received at least one drop of either drug. Eighteen and 16 patients from the AS and PRP groups, respectively, were included in the safety analysis (Fig. 1). No adverse event was reported in either group.
Discussion
This prospective study demonstrated that both AS and PRP eye drops are effective in improving corneal and conjunctival staining scores in primary SS DE with no significant difference at 4 and 12 weeks. In addition, TBUT improved in both groups, with no significant difference between them. Previous studies conducted on patients with non-SS DE20 and primary SS DE21 have also reported similar clinical outcomes in both groups.
The tear film has epitheliotropic factors, such as growth factors, vitamins, electrolytes, and neuropeptides that are important in the growth and migration of epithelial cells for epithelial homeostasis22,23. AS acts as a lubricant and supplies growth factors, cytokines, vitamins, and nutrients, similar to tears, that are crucial for ocular surface homeostasis24. EGF increases migration and proliferation of epithelial cells, and TGF-β decreases epithelial cell proliferation24,25,26. Fibronectin increases cell migration, and vitamin A is thought to be essential for normal epithelial cell growth27,28. Therefore, AS has been widely used for ocular surface disorders that do not respond to conventional commercial eye drops. Platelets are great reservoirs of growth factors, such as platelet-derived growth factor, TGF-β, EGF, and fibronectin, that are stored in α-granules and help in wound healing and tissue repair29,30. AS and PRP have a similar composition of growth factors and healing factors31; however, some studies suggested that PRP might be more advantageous than AS because the proinflammatory cytokine levels derived from leukocytes and monocytes, which are harmful to patients with immunologic disorders, are higher in AS21,32 and the epitheliotropic factor levels are higher in PRP than in AS19. TGF-β concentrations are five times higher in AS than in tears33. Excessively high TGF-β has been reported to suppress wound healing and promote stromal fibrosis and opacity in in-vitro studies34,35. Therefore, AS must be diluted to 20% to decrease the concentration of TGF-β10. In this study, we compared the effect of 20% AS and PRP eye drops on primary SS DE.
Some studies reported that PRP contains a 1.5 times higher level of platelets than AS36,37 and fewer leukocytes, which may result in an increased release of pro-inflammatory cytokines compared to AS38. Although growth factors do not directly correlate with platelet concentration39, higher levels of platelets and growth factors within the preparation might yield a better treatment outcome21. However, the concentrations of platelets and leukocytes in PRP and AS might vary according to the preparation method and percent concentration40, and no uniform preparation method exists for blood-derived eye drops; therefore, platelet and leukocyte concentrations might differ among the studies. A limitation of our study is that we did not perform a composition analysis, hence we could not elaborate on the relationship between the composition of eye drops and clinical outcomes. Further studies evaluating the exact composition of eye drops according to the selected preparation methods and percent concentrations may help improve the analyses of the clinical effect of the eye drops.
Compared with AS, PRP has a shorter preparation time using a commercial preparation kit because it does not require 2 h of clotting time. Although the preparation protocol of PRP varies according to the hospital, in our study, it was prepared with 3 min of centrifugation using a commercial preparation kit. Concerning the stability of PRP, Metheetrairut et al.20 reported that growth factors in PRP were stable for at least 3 months after storage at − 20 °C. They also reported an increase in some growth factors over time in PRP stored at 4 °C. They attributed the increase in growth factor concentrations to platelet activation.
The Schirmer I values did not improve in either group in our study. As the lacrimal gland is damaged in primary SS, we speculate that 12 weeks of AS or PRP treatment might not be sufficient to improve lacrimal gland function. The OSDI scores showed no improvement in both groups, but a previous study20 in patients with non-SS DE reported improved OSDI scores in both groups with no significant difference between the groups. Our study demonstrated that patients with SS DE had higher levels of inflammatory cytokines, such as tumor necrosis factor-α, interleukin (IL)-1β, IL-6, IL-17, and matrix metalloproteinase 9, on the ocular surface than in those with non-SS DE41.
This study used only AS or PRP without anti-inflammatory eye drops. Therefore, we speculate that no combined use of anti-inflammatory eye drops might be the cause of no improvement in Schirmer I and OSDI symptom scores. Further studies comparing AS and PRP with concurrent use of anti-inflammatory eye drops in primary SS DE in a real-world setting are warranted.
In this study, goblet cell metaplasia and cell density did not significantly change at 12 weeks from the baseline in the AS and PRP groups. Goblet cell metaplasia and a decrease in cell density are observed more frequently in SS DE than in non-SS DE42,43. Noble et al.44 demonstrated that treatment with 50% AS improved CIC parameters in both SS and non-SS DE, and Alio et al.18 demonstrated that 100% PRP improved goblet cell density in non-SS and SS DE. We speculate that a 20% concentration of AS or PRP might not be effective in goblet cell proliferation in primary SS DE.
This study had a limitation in that a population consisting of only females was included, which may have resulted in selection bias. Further studies including participants of both sexes are warranted.
In conclusion, both 20% AS and 20% PRP without anti-inflammatory eye drops are effective in improving ocular surface parameters in primary SS DE with no significant difference. Considering the shorter preparation time than that for AS, PRP eye drops might be a good alternative treatment for primary SS DE.
Methods
Ethics statement
This study adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review board of Seoul St. Mary’s Hospital (KC17CESV0562). Written informed consent was obtained from all participants. This trial was registered in the Clinical Research Information Service (CRiS), Republic of Korea (KCT0008103; 12/01/ 2023).
Study design and inclusion and exclusion criteria
This prospective, randomized, double-blinded clinical trial recruited patients diagnosed with primary SS with an ocular staining score of ≥ 5 points by a rheumatologist in accordance with the American College of Rheumatology and the European League Against Rheumatism (ACR-EULAR) criteria, 201645. Patients with secondary SS and those using disease-modifying medications were excluded. Considering the higher prevalence of DE in women than in men (F:M = 9:1), the study population only included women46. Patients with the following conditions that could affect clinical DE parameters were excluded: patients who had received systemic steroid or immunosuppressive treatment within the previous 3 months; those who were pregnant or lactating; those receiving hormone treatment for menopause; patients on medication for systemic diseases, such as diabetes mellitus; those with hypertension, allergic disease, thyroid disease, depressive disorder, allergic conjunctivitis, or eyelid abnormalities; those who had undergone ocular surgery within the previous 3 months; those with ocular complications, such as ocular inflammation, infection, or trauma; patients actively using contact lenses or punctal plugs; and those using topical treatments other than artificial tears, including topical cyclosporin or steroid eye drops, within 3 months before the study period.
Study protocol
After initial screening, a total of 36 women were enrolled and categorized into two groups using block randomization. The random number table was generated by an independent statistician using Excel (Microsoft Corp., Redmond, WA, USA). Eye drops were prepared by an independent pharmacist, and both participants and study investigators were blinded to the eye drops administered. AS and PRP eye drops were each administered to 18 patients (36 eyes). The sample size was calculated based on the rule of thumb for a pilot study suggested by Julious47. According to this rule, at least 12 patients were required per group; therefore, considering a dropout rate of 20%, a total of 36 patients (72 eyes) were included.
All patients used their allocated eye drops (AS or PRP) for 12 weeks at a dosage of one drop per eye six times daily in both eyes with an interval of 2 h between drops. Administration of 1–2 drops of artificial tears (sodium hyaluronate 0.1%) was allowed if needed. Participants were followed up at 4 and 12 weeks.
Preparation of autologous serum and platelet-rich plasma eye drops
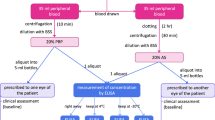
For the preparation of AS eye drops, 24 mL of peripheral venous blood was allowed to clot for 2 h at room temperature (20–25 °C) to separate the serum completely from the solid constituents. After centrifugation at 3500 g for 15 min, the serum was carefully isolated under sterile conditions in a laminar flow hood. Next, the serum was diluted to 20% (vol/vol) concentration with 0.1% (wt/vol) sodium hyaluronate preservative-free eye drops (Tearin Free; DHP Korea Co., Ltd, Seoul, Korea). Aliquots of diluted serum were stored in sterile 5 mL bottles with ultraviolet-light protection.
For the preparation of PRP eye drops, 22 mL of venous blood was withdrawn in a tube with 3 mL of 3.2% sodium citrate, and the tube was shaken slowly to prevent clotting. The blood was injected into a PRS Bio Kit (Prodizen, Seoul, Korea) through the blood inlet. The PRS Bio Kit and counter-balance kit (Prodizen, Seoul, Korea) were inserted into the centrifuge and centrifuged at 3000 g for 3 min. After centrifugation, the blood was divided into three layers (plasma, buffy coat, and red blood cells). The buffy coat was extracted using a 1-cc syringe, and plasma was extracted using a 5-cc syringe. Using a PRP connector, the buffy coat was mixed with the plasma to prepare PRP. PRP was diluted to 20% (vol/vol) concentration with 0.1% (wt/vol) sodium hyaluronate preservative-free eye drops (Tearin Free; DHP Korea Co., Ltd, Seoul, Korea).
Patients were instructed to store opened and unopened bottles of AS and PRP eye drops at 4 °C in a refrigerator and − 20 °C in a freezer, respectively.
Clinical assessments
Corneal and conjunctival staining scores at 12 weeks were defined as the primary efficacy endpoints, and those at 4 weeks were defined as the secondary efficacy endpoints. Additionally, the Schirmer I, TBUT, and OSDI scores at 4 and 12 weeks were considered secondary efficacy endpoints. The changes in CIC metaplasia and goblet cell density grades from baseline to 12 weeks were evaluated.
The OSDI score, TBUT, corneal staining, conjunctival staining, and Schirmer I test value were assessed in order by a single investigator (S-H.C.). For TBUT evaluation, fluorescein was mixed in 15–30 mL of saline, and one drop of the solution was applied to the superotemporal conjunctiva. The average of three TBUT values was evaluated with cobalt blue light8.
Ocular staining scores, including corneal and conjunctival staining scores, were determined following the Sjögren’s International Collaborative Clinical Alliance registry ocular examination protocol48. Corneal punctate epithelial erosions (PEEs) were enumerated and scored after staining with fluorescein. Corneal scores were assigned as follows: PEE absent, zero points; 1–5 PEEs, one point; 6–30 PEEs, two points; and > 30 PEEs, three points. An additional point was awarded for each of the following cases: patches of confluent staining, staining in the pupillary area, and the presence of filaments. For conjunctival staining assessment, 1% lissamine green dye was administered at the inferior conjunctiva, and temporal and nasal bulbar conjunctival staining scores were evaluated separately, as follows: 0–9 dots, 0 points; 10–32 dots, one point; 33–100 dots, two points; and > 100 dots, three points.
For the Schirmer I test, Schirmer strips (Eagle Vision, Memphis, TN, USA) were placed in the lateral one-third of the lower eyelid without anesthesia, and the wetted length was evaluated after 5 min.
Impression cytology
CIC was performed at least 15 min after all ocular examinations. Polyethersulfone filters (Supor 200 membrane; Pall Corporation, Port Washington, NY, USA) were applied to the superotemporal nonexposed bulbar conjunctiva after halving (13 mm × 6.5 mm). The samples were used for periodic acid–Schiff (PAS) staining.
Periodic acid–Schiff staining
The applied filter paper was fixed for approximately 10 min in a solution of glacial acetic acid, formaldehyde, and ethyl alcohol in a 1:1:20 volume ratio49. PAS stain and counterstain with hematoxylin were performed50, and squamous metaplasia degree and goblet cell density were evaluated under a microscope. Squamous metaplasia was graded 0–3 points in accordance with Nelson’s grading system51 as follows: Grade 0, normal cells with normal density; Grade 1, reduced nucleocytoplasmic ratio (1:3) with decreased density; Grade 2, a nucleocytoplasmic ratio of 1:4 to 1:5 with an absence of cells; and Grade 3, a large eosinophilic cytoplasm with folded edges and pyknotic nuclei (nucleocytoplasmic ratio > 1:6) with an absence of cells plus squamous metaplasia. Thus, a higher score indicated greater metaplasia of epithelial cells. The goblet cell density grade (1–4 points) was evaluated by counting the average number of goblet cells per 100 epithelial cells in four high-power fields (HPFs), as previously described, as follows: Grade 1, > 30 goblet cells/four HPFs; Grade 2, 15–30 goblet cells/four HPFs; Grade 3, 5–15 goblet cells/four HPFs; and Grade 4, < 5 goblet cells/four HPFs52.
Statistical analysis
The outcome was evaluated via a per-protocol set (PPS), FAS, and safety analyses. PPS analysis was completed in patients who finished the originally allocated treatment. FAS analysis was performed in patients who instilled at least one dose of the allocated drug with available primary efficacy endpoint data. In case of missing values, the last observation carried forward method was used for analysis. Efficacy endpoints were evaluated mainly using PPS, and FAS was also analyzed. Safety analysis was performed in all patients who received at least one dose of either drug.
Normality was tested using the Kolmogorov–Smirnov test. A paired t-test and the Wilcoxon signed-rank test were used to compare parametric and nonparametric data between the groups, respectively. Additionally, an independent t-test and the Mann–Whitney U test were used to compare parametric and nonparametric data in each group, respectively. The chi-square or Fisher’s exact test was used to evaluate the correlation of adverse events in the safety analysis. Statistical analysis was performed using Statistical Package for the Social Sciences software (version 22.0; IBM Corp., Armonk, NY, USA), and statistical significance was set at p < 0.05.
Data availability
The datasets generated and analyzed in the current study are available from the corresponding author on reasonable request.
References
Jones, L. et al. TFOS DEWS II management and therapy report. Ocul. Surf. 15, 575–628 (2017).
Fox, R. I., Chan, R., Michelson, J. B., Belmont, J. B. & Michelson, P. E. Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthrit. Rheum. 27, 459–461 (1984).
Quinto, G. G., Campos, M. & Behrens, A. Autologous serum for ocular surface diseases. Arq. Bras. Oftalmol. 71, 47–54 (2008).
Tsubota, K., Goto, E., Shimmura, S. & Shimazaki, J. Treatment of persistent corneal epithelial defect by autologous serum application. Ophthalmology 106, 1984–1989 (1999).
Moutsopoulos, H. M. et al. Sjogren’s syndrome (Sicca syndrome): Current issues. Ann. Intern. Med. 92, 212–226 (1980).
Lemp, M. A. & Foulks, G. N. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul. Surf. 5, 75–92 (2007).
Hikichi, T., Yoshida, A. & Tsubota, K. Lymphocytic infiltration of the conjunctiva and the salivary gland in Sjogren’s syndrome. Arch. Ophthalmol. 111, 21–22 (1993).
Hwang, J. et al. Comparison of clinical efficacies of autologous serum eye drops in patients with primary and secondary Sjogren syndrome. Cornea 33, 663–667 (2014).
Semeraro, F. et al. Effect of Autologous serum eye drops in patients with Sjogren syndrome-related dry eye: Clinical and in vivo confocal microscopy evaluation of the ocular surface. In Vivo 30, 931–938 (2016).
Giannaccare, G. et al. Blood derived eye drops for the treatment of cornea and ocular surface diseases. Transfus. Apher. Sci. 56, 595–604 (2017).
Alio, J. L., Pastor, S., Ruiz-Colecha, J., Rodriguez, A. & Artola, A. Treatment of ocular surface syndrome after LASIK with autologous platelet-rich plasma. J. Refract. Surg. 23, 617–619 (2007).
Fea, A. M. et al. The effect of autologous platelet lysate eye drops: An in vivo confocal microscopy study. BioMed. Res. Int. 2016, 8406832 (2016).
López-Plandolit, S., Morales, M. C., Freire, V., Etxebarría, J. & Durán, J. A. Plasma rich in growth factors as a therapeutic agent for persistent corneal epithelial defects. Cornea 29, 843–848 (2010).
Lubkowska, A., Dolegowska, B. & Banfi, G. Growth factor content in PRP and their applicability in medicine. J. Biol. Regul. Homeost. Agents. 26, 3S-22S (2012).
Nurden, A. T. The biology of the platelet with special reference to inflammation, wound healing and immunity. Front. Biosci. (Landmark Ed.) 23, 726–751 (2018).
Alio, J. L., Rodriguez, A. E. & WróbelDudzińska, D. Eye platelet-rich plasma in the treatment of ocular surface disorders. Curr. Opin. Ophthalmol. 26, 325–332 (2015).
Sanchez-Avila, R. M. et al. The effect of immunologically safe plasma rich in growth factor eye drops in patients with Sjogren syndrome. J. Ocul. Pharmacol. Ther. 33, 391–399 (2017).
Alio, J. L., Colecha, J. R., Pastor, S., Rodriguez, A. & Artola, A. Symptomatic dry eye treatment with autologous platelet-rich plasma. Ophthalmic Res. 39, 124–129 (2007).
Kim, K. M., Shin, Y. T. & Kim, H. K. Effect of autologous platelet-rich plasma on persistent corneal epithelial defect after infectious keratitis. Jpn. J. Ophthalmol. 56, 544–550 (2012).
Metheetrairut, C. et al. Comparison of epitheliotrophic factors in platelet-rich plasma versus autologous serum and their treatment efficacy in dry eye disease. Sci. Rep. 12, 8906 (2022).
Wróbel-Dudzińska, D. et al. The comparison between the composition of 100% autologous serum and 100% platelet-rich plasma eye drops and their impact on the treatment effectiveness of dry eye disease in primary Sjogren syndrome. J. Clin. Med. 25, 3126 (2023).
Rauz, S. & Saw, V. P. Serum eye drops, amniotic membrane and limbal epithelial stem cells—tools in the treatment of ocular surface disease. Cell Tissue Bank. 11, 13–27 (2010).
Geerling, G., Maclennan, S. & Hartwig, D. Autologous serum eye drops for ocular surface disorders. Br. J. Ophthalmol. 88, 1467–1474 (2004).
Yamada, C., King, K. E. & Ness, P. M. Autologous serum eyedrops: Literature review and implications for transfusion medicine specialists. Transfusion 48, 1245–1255 (2008).
Kitazawa, T. et al. The mechanism of accelerated corneal epithelial healing by human epidermal growth factor. Invest. Ophthalmol. Vis. Sci. 31, 1773–1778 (1990).
Soni, N. G. & Jeng, B. H. Blood-derived topical therapy for ocular surface diseases. Br. J. Ophthalmol. 100, 22–27 (2016).
Sommer, A. Xerophthalmia and vitamin A status. Prog. Retin. Eye Res. 17, 9–31 (1998).
Nishida, T. et al. Fibronectin promotes epithelial migration of cultured rabbit cornea in situ. J. Cell Biol. 97, 1653–1657 (1983).
Marx, R. E. Platelet-rich plasma (PRP): What is PRP and what is not PRP?. Implant Dent. 10, 225–228 (2001).
Strandberg, G. et al. Standardizing the freeze-thaw preparation of growth factors from platelet lysate. Transfusion 57, 1058–1065 (2017).
Anitua, E. et al. Autologous serum and plasma rich in growth factors in ophthalmology: Preclinical and clinical studies. Acta Ophthalmol. 93, e605–e614 (2015).
Freire, V., Andollo, N., Etxebarria, J., Durán, J. A. & Morales, M. C. In vitro effects of three blood derivatives on human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 53, 5571–5578 (2012).
Tsubota, K. et al. Treatment of dry eye by autologous serum application in Sjogren’s syndrome. Br. J. Ophthalmol. 83, 390–395 (1999).
Pancholi, S., Tullo, A., Khaliq, A., Foreman, D. & Boulton, M. The effects of growth factors and conditioned media on the proliferation of human corneal epithelial cells and keratocytes. Graefes. Arch. Clin. Exp. Ophthalmol. 236, 1–8 (1998).
Imanishi, J. et al. Growth factors: Importance in wound healing and maintenance of transparency of the cornea. Prog. Retin. Eye Res. 19, 113–129 (2009).
Wroblewski, A. P., Mejia, H. A. & Wright, V. J. Application of platelet-rich plasma to enhance tissue repair. Oper. Tech. Orthop. 20, 98–105 (2010).
Brass, L. Understanding and evaluating platelet function. Hematol. Am. Soc. Hematol. Educ. Program. 2010, 387–396 (2010).
O’Neil, E. C., Henderson, M., Massaro-Giordano, M. & Bunya, V. Y. Advances in dry eye disease treatment. Curr. Opin. Ophthalmol. 30, 166–178 (2019).
Pulcini, S. et al. Apheresis platelet rich-plasma for regenerative medicine: An in vitro study on osteogenic potential. Int. J. Mol. Sci. 22, 8764 (2021).
Radtke, A. V., Goodale, M. B. & Fortier, L. A. Platelet and leukocyte concentration in equine autologous conditioned plasma are inversely distributed by layer and are not affected by centrifugation rate. Front. Vet. Sci. 7, 173 (2020).
Yang, S. et al. The use of conjunctival staining to measure ocular surface inflammation in patients with dry eye. Cornea 38, 698–705 (2019).
Pflugfelder, S. C. et al. Conjunctival cytologic features of primary Sjogren’s syndrome. Ophthalmology 97, 985–991 (1990).
Haller-Schober, E. M. et al. Evaluating an impression cytology grading system (IC score) in patients with dry eye syndrome. Eye (Lond). 20, 927–933 (2006).
Noble, B. A. et al. Comparison of autologous serum eye drops with conventional therapy in a randomised controlled crossover trial for ocular surface disease. Br. J. Ophthalmol. 88, 647–652 (2004).
Shiboski, C. H. et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjogren’s Syndrome: A consensus and data-driven methodology involving three international patient cohorts. Arthrit. Rheumatol. 69, 35–45 (2017).
Delaleu, N., Jonsson, M. V., Appel, S. & Jonsson, R. New concepts in the pathogenesis of Sjogren’s syndrome. Rheum. Dis. Clin. North Am. 34, 833–845 (2008).
Julious, S. A. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceut. Statist. 4, 287–291 (2005).
Whitcher, J. P. et al. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjogren’s Syndrome International Registry. Am. J. Ophthalmol. 149, 405–415 (2010).
Tseng, S. C. Staging of conjunctival squamous metaplasia by impression cytology. Ophthalmology 92, 728–733 (1985).
Singh, R., Joseph, A., Umapathy, T., Tint, N. L. & Dua, H. S. Impression cytology of the ocular surface. Br. J. Ophthalmol. 89, 1655–1659 (2005).
Nelson, J. D., Havener, V. R. & Cameron, J. D. Cellulose acetate impressions of the ocular surface. Dry eye states. Arch. Ophthalmol. 101, 1869–1872 (1983).
Anshu-Munshi, M. M., Sathe, V. & Ganar, A. Conjunctival impression cytology in contact lens wearers. Cytopathology 12, 314–320 (2001).
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (grant number: 2020R1A2B5B01002407).
Author information
Authors and Affiliations
Contributions
M.-J.K.: conceptualization; data curation; formal analysis; validation; visualization; writing—original draft. S.-H.C.: conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; supervision; validation; visualization; writing—review & editing. J.H.L.: data curation; formal analysis. J.H.: data curation; formal analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, MJ., Lee, J.H., Hwang, J. et al. Efficacy and safety of platelet-rich plasma and autologous-serum eye drops for dry eye in primary Sjögren’s syndrome: a randomized trial. Sci Rep 13, 19279 (2023). https://doi.org/10.1038/s41598-023-46671-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46671-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.