Abstract
Purpose
To explore the efficacy and relevant mechanism of 0.05% cyclosporine A (CsA) eye drops (II) monotherapy in patients with allergic conjunctivitis-associated dry eye (ACDE).
Methods
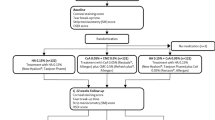
Prospective, randomized, controlled study. Fifty-three patients with mild-to-moderate ACDE were randomly assigned to two groups. The CsA group received 0.05% CsA eye drops (II) monotherapy four times daily. The control group received 0.1% olopatadine twice daily combined with 0.1% preservative-free artificial tears four times daily. Clinical symptoms and signs, tear total IgE, and lymphotoxin-α (LT-α) concentrations were assessed at pre- and post-treatment days 7, 30, and 60. And we further measured six tear cytokines levels using a microsphere-based immunoassay.
Results
The CsA group showed significant improvement in symptoms (Ocular Surface Disease Index and itching scores) and signs (conjunctival hyperaemia, conjunctival oedema, conjunctival papillae, tear break-up time (TBUT), corneal fluorescein staining, and goblet cell density) at each follow-up period compared to pre-treatment (all P < 0.050). And its improvement in itching scores (P7th < 0.001, P30th = 0.039, and P60th = 0.031) and TBUT (P7th = 0.009, P30th = 0.003, and P60th = 0.005) was more significant than the control group at all follow-up periods. The tear total IgE, interleukin (IL)-5, IL-6, periostin, eotaxin-3, and MMP-9 levels significantly decreased in the CsA group at day 60 after treatment (all P < 0.050). And the changed values in tear total IgE were positively correlated with the change in itching scores.
Conclusions
0.05% CsA eye drops (II) monotherapy can rapidly improve the symptoms and signs, especially in ocular itching and TBUT, in patients with ACDE. And its efficacy is superior to 0.1% olopatadine combined with artificial tears. Moreover, CsA downregulates the expression levels of tear inflammatory cytokines, including tear total IgE, IL-5, IL-6, periostin, eotaxin-3, and MMP-9. Among that, the reduction in tear total IgE levels may reflect the improvement of ocular itching.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data is available from the corresponding author upon reasonable request.
References
Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo C-K, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–83.
Villani E, Rabbiolo G, Nucci P. Ocular allergy as a risk factor for dry eye in adults and children. Curr Opin Allergy Clin Immunol. 2018;18:398–403.
Leonardi A, Modugno RL, Salami E. Allergy and dry eye disease. Ocul Immunol Inflamm. 2021;29:1168–76.
Dominick LO, Justin TK, Jennifer H, Eric B, Milton MH. Prevalence of allergic conjunctivitis, ocular surface disease subtypes, and mixed disease. Invest Ophthalmol Vis Sci. 2014;55:2751.
Akasaki Y, Inomata T, Sung J, Nakamura M, Kitazawa K, Shih KC, et al. Prevalence of comorbidity between dry eye and allergic conjunctivitis: a systematic review and meta-analysis. J Clin Med. 2022;11:3643.
Chen L, Pi L, Fang J, Chen X, Ke N, Liu Q. High incidence of dry eye in young children with allergic conjunctivitis in Southwest China. Acta Ophthalmol. 2016;94:e727–30.
Dupuis P, Prokopich CL, Hynes A, Kim H. A contemporary look at allergic conjunctivitis. Allergy Asthma Clin Immunol. 2020;16:5.
Nye M, Rudner S, Bielory L. Emerging therapies in allergic conjunctivitis and dry eye syndrome. Expert Opin Pharmacother. 2013;14:1449–65.
Nguyen E, Yanes D, Imadojemu S, Kroshinsky D. Evaluation of cyclosporine for the treatment of DRESS syndrome. JAMA Dermatol. 2020;156:704–6.
Fauquert J-L. Diagnosing and managing allergic conjunctivitis in childhood: the allergist’s perspective. Pediatr Allergy Immunol. 2019;30:405–14.
Raizman MB, Hamrah P, Holland EJ, Kim T, Mah FS, Rapuano CJ, et al. Drug-induced corneal epithelial changes. Surv Ophthalmol. 2017;62:286–301.
Gao M, Zhao L, Liang R, Zhu Q, Zhao Q, Kong X. Evaluation of the efficacy and safety of topical 0.05% cyclosporine eye drops (II) in the treatment of dry eye associated with Primary Sjögren’s Syndrome. Ocul Immunol Inflamm. 2023;31:1662–1668.
Labib BA, Chigbu DI. Therapeutic targets in allergic conjunctivitis. Pharm (Basel). 2022;15:547.
Bao J, Tian L, Meng Y, Wu B, Wang J, He J, et al. Total IgE in tears accurately reflects the severity and predicts the prognosis of seasonal allergic conjunctivitis. Clin Transl Allergy. 2022;12:e12139.
Lin X, Huang J-F, Liu Z. Large scale, prospective, multicenter, clinical evaluation of point-of-care lymphotoxin alpha (LTA) test in dry eye disease. Investig Ophthalmol Vis Sci. 2020;61:116.
Suárez-Cortés T, Merino-Inda N, Benitez-Del-Castillo JM. Tear and ocular surface disease biomarkers: a diagnostic and clinical perspective for ocular allergies and dry eye disease. Exp Eye Res. 2022;221:109121.
Rhim JW, Eom Y, Yoon EG, Park SY, Choi Y, Song JS, et al. Efficacy of a 0.05% cyclosporine a topical nanoemulsion in dry eyes with obstructive meibomian gland dysfunction. Jpn J Ophthalmol. 2022;66:254–63.
Asian Dry Eye Association China Chapter. Chinese expert consensus on dry eye: definition and classification (2020). Chin J Ophthalmol. 2020;56:418–22.
Nelson JD, Havener VR, Cameron JD. Cellulose acetate impressions of the ocular surface. Dry eye states. Arch Ophthalmol. 1983;101:1869–72.
Patel D, Wairkar S. Recent advances in cyclosporine drug delivery: challenges and opportunities. Drug Deliv Transl Res. 2019;9:1067–81.
Prabhasawat P, Tesavibul N, Mahawong W. A randomized double-masked study of 0.05% cyclosporine ophthalmic emulsion in the treatment of meibomian gland dysfunction. Cornea. 2012;31:1386–93.
Leong E, Pang Z, Stadnyk AW, Lin TJ. Calcineurin Aα contributes to IgE-dependent mast-cell mediator secretion in allergic inflammation. J Innate Immun. 2022;14:320–34.
Kam KW, Chen LJ, Wat N, Young AL. Topical olopatadine in the treatment of allergic conjunctivitis: a systematic review and meta-analysis. Ocul Immunol Inflamm. 2017;25:663–77.
Vichyanond P, Kosrirukvongs P. Use of cyclosporine A and tacrolimus in treatment of vernal keratoconjunctivitis. Curr Allergy Asthma Rep. 2013;13:308–14.
Villareal AL, Farley W, Pflugfelder SC. Effect of topical ophthalmic epinastine and olopatadine on tear volume in mice. Eye Contact Lens. 2006;32:272–6.
Wan KH-N, Chen LJ, Rong SS, Pang CP, Young AL. Topical cyclosporine in the treatment of allergic conjunctivitis: a meta-analysis. Ophthalmology. 2013;120:2197–203.
Leonardi A, Messmer EM, Labetoulle M, Amrane M, Garrigue J-S, Ismail D, et al. Efficacy and safety of 0.1% ciclosporin A cationic emulsion in dry eye disease: a pooled analysis of two double-masked, randomised, vehicle-controlled phase III clinical studies. Br J Ophthalmol. 2019;103:125–31.
Cui H, Liu F, Fang Y, Wang T, Yuan B, Ma C. Neuronal FcεRIα directly mediates ocular itch via IgE-immune complex in a mouse model of allergic conjunctivitis. J Neuroinflamm. 2022;19:55.
Ambroziak AM, Szaflik J, Szaflik JP, Ambroziak M, Witkiewicz J, Skopiński P. Immunomodulation on the ocular surface: a review. Cent Eur J Immunol. 2016;41:195–208.
Hashimoto T, Mishra SK, Olivry T, Yosipovitch G. Periostin, an emerging player in itch sensation. J Invest Dermatol. 2021;141:2338–43.
Izuhara K, Nunomura S, Nanri Y, Ono J, Takai M, Kawaguchi A. Periostin: an emerging biomarker for allergic diseases. Allergy. 2019;74:2116–28.
García-Posadas L, Hodges RR, Diebold Y, Dartt DA. Context-dependent regulation of conjunctival goblet cell function by allergic mediators. Sci Rep. 2018;8:12162.
Yoon K-C, Jeong I-Y, Park Y-G, Yang S-Y. Interleukin-6 and tumor necrosis factor-alpha levels in tears of patients with dry eye syndrome. Cornea. 2007;26:431–7.
Keller C, Hellsten Y, Steensberg A, Pedersen BK. Differential regulation of IL-6 and TNF-alpha via calcineurin in human skeletal muscle cells. Cytokine. 2006;36:141–7.
Cook EB, Stahl JL, Barney NP, Graziano FM. Olopatadine inhibits TNF-alpha release from human conjunctival mast cells. Ann Allergy Asthma Immunol. 2000;84:504–8.
Nakae S, Suto H, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc Natl Acad Sci USA. 2005;102:6467–72.
Kang MJ, Kim HS, Kim MS, Kim EC. The Correlation between matrix metalloproteinase-9 point-of-care immunoassay, tear film osmolarity, and ocular surface parameters. J Ophthalmol. 2022;2022:6132016.
Chen H, Chen H, Liang L, Zhong Y, Liang Y, Yu Y, et al. Evaluation of tear protein markers in dry eye disease with different lymphotoxin-alpha expression levels. Am J Ophthalmol. 2020;217:198–211.
Davies K, Mirza K, Tarn J, Howard-Tripp N, Bowman SJ, Lendrem D, et al. Fatigue in primary Sjögren’s syndrome (pSS) is associated with lower levels of proinflammatory cytokines: a validation study. Rheumatol Int. 2019;39:1867–73.
Ma J, Li C, Zhao Y, Shen Z, Hu B, Peng R, et al. Ophthalmic manifestations are associated with reduced tear lymphotoxin-α levels in chronic ocular graft-versus-host disease. BMC Ophthalmol. 2022;22:18.
Messmer EM, von Lindenfels V, Garbe A, Kampik A. Matrix metalloproteinase 9 testing in dry eye disease using a commercially available point-of-care immunoassay. Ophthalmology. 2016;123:2300–8.
Park JY, Kim BG, Kim JS, Hwang JH. Matrix metalloproteinase 9 point-of-care immunoassay result predicts response to topical cyclosporine treatment in dry eye disease. Transl Vis Sci Technol. 2018;7:31.
Kuprash DV, Boitchenko VE, Yarovinsky FO, Rice NR, Nordheim A, Rühlmann A, et al. Cyclosporin A blocks the expression of lymphotoxin alpha, but not lymphotoxin beta, in human peripheral blood mononuclear cells. Blood. 2002;100:1721–7.
Funding
This study was funded by the Beijing Bethune Charitable Foundation Project (BJ-GY2021008J), and Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-037A).
Author information
Authors and Affiliations
Contributions
XTJ and YYQ participated in the patient’s follow-up and evaluation, data analysis, and drafted the manuscript. NG was responsible for patient enrolment and follow-up. CZ was responsible for the revision of the article. SZZ and RBY participated in project supervision, data interpretation, and manuscript revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiao, X., Qi, Y., Gao, N. et al. Exploration of efficacy and mechanism of 0.05% cyclosporine eye drops (II) monotherapy in allergic conjunctivitis-associated dry eye. Eye 38, 937–944 (2024). https://doi.org/10.1038/s41433-023-02807-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02807-2