Abstract
Posaconazole and voriconazole are commonly used for preventing invasive fungal disease (IFD), but few studies compared posaconazole with voriconazole for primary antifungal prophylaxis (PAP) in pediatric acute leukemia. To compare posaconazole with voriconazole for PAP in pediatric acute leukemia. This retrospective observational study enrolled pediatric patients with non-M3 acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL) between December 2017 and November 2019 in the Second Affiliated Hospital of Anhui Medical University. The patients received voriconazole or posaconazole for PAP. The primary outcome was the breakthrough of IFD. The secondary outcome was the overall survival (OS) and IFD-free survival of patients. A total of the 275 patients were enrolled, of which 120 patients taking voriconazole (43.6%) and 155 patients taking posaconazole (56.4%). The breakthrough of IFD occurred in 19 (15.8%) patients taking voriconazole and in 12 (7.7%) patients taking posaconazole (P = 0.035). There was no significant differences in IFD-free survival (P = 0.336) or OS (P = 0.069) between the patients taking voriconazole and posaconazole. In the subgroup of AML patients, the OS of patients taking posaconazole was better than those receiving voriconazole (P = 0.017). Posaconazole and voriconazole were comparable for PAP in patients with pediatric acute leukemia regarding the OS and IFD-free survival, but posaconazole might achieve a lower IFD breakthrough rate.
Similar content being viewed by others
Invasive fungal disease (IFD) can be a major complication of acute leukemia treatment, and is associated with significant morbidity and mortality1,2. The most common pathogens of IFD in childhood leukemia are Aspergillus and Candida3,4. The annual incidence of invasive candidiasis in children is 0.92 per 100,0005, but the risk increases in children with acute leukemia6,7. Increased intensity of therapy, such as chemotherapy and organ transplantation, have coincided with a substantial increase in the incidence of invasive fungal infections8.
Systemic antifungal prophylaxis can be an effective approach to reducing IFD, and primary antifungal prophylaxis (PAP) was recommended by the guidelines9. Posaconazole is approved for the prophylaxis of Aspergillus and Candida infections. In addition, posaconazole is used to treat oropharyngeal candidiasis, typically for patients refractory to treatment with fluconazole and itraconazole10,11,12. Voriconazole is approved for invasive aspergillosis, candidemia in non-neutropenic patients, esophageal candidiasis, and disseminated candidiasis10,11,12.
A systematic review and network meta-analysis showed that voriconazole might be the best choice for patients undergoing HSCT, and posaconazole might be the best prophylactic option for patients with AML or MDS13. Another study showed that posaconazole and voriconazole are effective in preventing IFDs in adult patients with hematological malignancy, but symptomatic adverse events were more common with voriconazole14. A cost-effectiveness analysis suggested a better cost-effectiveness of posaconazole compared to voriconazole for IFD prophylaxis in AML15. Overall, posaconazole and voriconazole are recommended as the most reasonable options for the prevention of IFD. However, there were few studies on posaconazole and voriconazole for PAP in pediatric acute leukemia.
Therefore, this study aimed to compare the efficacy of using posaconazole and voriconazole for PAP in pediatric acute leukemia.
Methods
Study design and participants
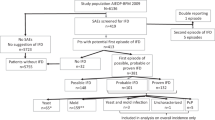
This retrospective, observational study included pediatric patients with non-M3 AML and acute lymphoblastic leukemia (ALL) , treated with PAP between December 2017 and November 2019 in the Department of Pediatric Hematology and Oncology of the Second Affiliated Hospital of Anhui Medical University (Hefei, China).
The inclusion criteria were (1) patients diagnosed with non-M3 AML or ALL with ≤ 14 years of age, (2) underwent chemotherapy, including induction therapy and consolidation therapy regimens, (3) received oral voriconazole or posaconazole for PAP, and (4) None received any antibacterial prophylaxis. The exclusion criteria were (1) received other drugs or intravenous preparations of voriconazole or posaconazole for PAP or (2) with a previous history of invasive fungal infection.
Treatment details
All children received only chemotherapy for acute leukemia. And they received voriconazole or posaconazole orally for PAP, which was started when neutropenia occurred (defined as neutrophil counts < 0.5 × 109/L). Prophylaxis dosing method of the voriconazole group: weight < 50 kg, 9 mg/kg/dose every 12 h, single dose not to exceed 200 mg; weight ≥ 50 kg, 200 mg/dose every 12 h, and of the posaconazole group: 4 mg/kg/dose, three times a day, with the highest dose not exceeding 200 mg/dose, three times a day. The PAP was administered until the neutrophil count increased to ≥ 0.5 × 109/L or a breakthrough IFD occurred. Medical notes were reviewed by the chief physician and attending physician. Drug concentration monitoring was not performed during administration. All treatments were provided on conventional wards without laminar flow.
Basic demographic and clinical characteristics were collected, including age, sex, subtype of leukemia, PAP strategy, the time of IFD breakthrough, and infection site.
Outcomes
The primary outcome was breakthrough of IFD (included proven IFD and probable IFD only). The secondary outcome was the patient prognosis, including IFD-free survival and overall survival (OS).
The breakthrough of IFD was diagnosed according to the definition of the consensus group of the European Organization for Research and Treatment of Cancer Invasive Fungal Infections Cooperative Group (EORTC)16 between at least 7 days after the start of PAP and 7 days after the end of PAP. IFD-free survival was defined as the time between treatment initiation and breakthrough of IFD. The OS was defined as the time between treatment initiation and death from any cause. The follow-up period ranged from the start of treatment to the last follow-up time.
Statistical analysis
Data were analyzed using SPSS 22.0 (IBM Corp., Armonk, NY, USA). Quantitative variables were presented as medians (ranges) and analyzed using the Mann–Whitney U-test. Categorical variables were presented as percentages (%) and analyzed using the chi-square test or Fisher's exact test. IFD-free survival and OS were determined using the Kaplan–Meier method, and the log-rank test was used to compare the curves. A two-sided P < 0.05 was considered statistical significant.
Results
This study included 275 pediatric patients with acute leukemia and aged 1 to 14 years. Among them, 219 (79.6%) diagnosed with ALL, and 56(20.4%) with non-M3 AML. There were 155 patients taking posaconazole (56.4%), and 120 patients taking voriconazole (43.6%). Age, sex, and diagnosis were comparable between the patients taking voriconazole and posaconazole (all P > 0.05) (Table 1).
Breakthrough of IFD occurred in 19 (15.8%) patients receiving voriconazole and in 12 (7.7%) patients receiving posaconazole (P = 0.035). There were 16 patients that developed IFD breakthrough during the induction stage, and 11 (9.2%) of them were treated with voriconazole, the other 5 (3.2%) were treated with posaconazole (P = 0.473). Most IFDs were in the lungs, with 17 (14.2%) in patients receiving voriconazole, and 8 (5.2%) in patients receiving posaconazole (P = 0.147) (Table 2). There were no significant differences in IFD-free survival (P = 0.336) or OS (P = 0.069) between the patients receiving voriconazole and posaconazole (Fig. 1A,B).
Kaplan–Meier plots for invasive fungal disease (IFD)-free survival and overall survival (OS). (A) IFD-free survival in all patients (P = 0.336). (B) OS in all patients (P = 0.069). (C) OS in acute lymphoblastic leukemia (ALL) (P = 0.251). (D) OS in acute myeloid leukemia (AML) (P = 0.017). (E) IFD-free survival in ALL (P = 0.267). (F) IFD-free survival in AML (P = 0.851). (G) Cumulative incidence of IFD in ALL. (H) Cumulative incidence of IFD in AML.
In the subgroup of AML patients, the OS of patients taking posaconazole was better than those taking voriconazole (P = 0.017), but there were no significant differences in the IFD-free survival (P = 0.851) (Fig. 1D,F). In the subgroup of ALL patients, the IFD-free survival (P = 0.267) and OS (P = 0.251) were not significantly different between the patients taking posaconazole or voriconazole (Fig. 1E,C).
Discussion
This study compared voriconazole with posaconazole for PAP in pediatric acute leukemia. The rate of breakthrough IFD during PAP was lower in the posaconazole group compared with (7.7% vs. 15.8%), which was higher than that in a previous study of adult patients with AML, ALL, and MDS (2.5% vs. 4.8%)17. Hachem et al.14 observed breakthrough IFDs in 3% and 0% of acute leukemia patients treated with posaconazole and voriconazole. It might be related to the environment in which the children were treated, and the definition of a breakout IFD may also be an influencing factor. This study used a standard definition of breakthrough IFD, which occurring between at least 7 days after the start of PAP and 7 days after the end of PAP16. In addition, children might be more susceptible to IFDs than adults because of a more immature immune system18,19.
The results suggested that the IFD-free survival or OS was not significantly different between the two treatments, but posaconazole might achieve a lower IFD breakthrough rate for PAP in pediatric patients with acute leukemia than voriconazole. The results might help the selection of drugs for the prophylactic management of IFDs in patients with pediatric acute leukemia.
Remission induction chemotherapy has been suggested to be the highest-risk phase for the development of IFD in patients undergoing initial treatments20,21. Several studies suggested that most IFDs in ALL and AML occurred during the induction stage and with stronger chemotherapy22,23,24. Similarly, in this study, 16 cases (16/31, 51.6%) of breakthrough IFDs occurred during induction. It can be related to severe neutropenia and high dosages of steroids25.
Some studies showed that proven and probable invasive aspergillosis is the most common infection in AML patients receiving active triazole PAP after intensive chemotherapy26,27,28. For patients with suspected IFD, computed tomography (CT) of the lungs and other investigations are recommended29. CT plays an important role in diagnosing and managing patients with fungal infections due to its ability to reveal early predictive signs of fungal infection30. This study showed that in 25 cases (25/31, 80.6%), the location of breakthrough IFD was in the lungs.
Patients with IFD often have to interrupt, delay, or change the chemotherapy regimens, which can undermine their long-term survival31. Kobayashi et al.32 found significantly lower survival in patients with IFD than those without IFD in children and adolescents with hematological malignancies, other malignant diseases, and aplastic anemia. Still, the effect of PAP on OS is unclear. Dahlen et al.26 found that posaconazole prophylaxis decreased the incidence of IFD but did not improve short-term OS. Another study concluded that PAP could reduce long-term mortality in salvaged patients needing successive treatment, such as allogeneic HSCT33. In this study, the choice of either posaconazole or voriconazole for primary prophylaxis had no significant effect on OS or IFD-free survival, which might related to the fact that the number of patients experiencing endpoint events was limited and the median survival time had not been reached. On the other hand, in the subgroup analysis of AML patients, the OS of patients in posaconazole group was better than those in voriconazole group. These results are supported by a cost-effectiveness analysis suggested a better cost-effectiveness of posaconazole vs. voriconazole for IFD prophylaxis in patients with AML, including a lower death rate, suggesting better patient outcomes with posaconazole and a smaller use of healthcare resources15.
This study had several limitations. First, it was a single-center retrospective study, and the number of patients was relatively small. The data that could be analyzed were limited to those in the patient charts. Second, microbiological identification of patients with breakthrough, as well as the plasma concentrations of posaconazole and voriconazole were not tested. Hence, the possibility of low concentrations in children with breakthrough IFD could not be confirmed. Third, the median survival time had not been reached, which might because the follow-up period was not long enough, and long-term follow-up was needed in future studies.
In conclusion, posaconazole and voriconazole were comparable for PAP in patients with pediatric acute leukemia regarding the OS and IFD-free survival, but posaconazole might achieve a lower IFD breakthrough rate. Multicenter clinical trial with large samples were needed in the future to confirm the results.
Ethical approval
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Anhui Medical University (PJ-YX201501). This article is a retrospective study. Therefore, the Institutional waived the requirement to obtain distinct written informed consent from the patients.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Supatharawanich, S. et al. Invasive fungal diseases in children with acute leukemia and severe aplastic anemia. Mediterr. J. Hematol. Infect. Dis. 13(1), e2021039. https://doi.org/10.4084/mjhid.2021.039 (2021).
Yeoh, D. K. et al. Invasive fungal disease in children with acute myeloid leukaemia: An Australian multicentre 10-year review. Pediatr. Blood Cancer 68(11), e29275. https://doi.org/10.1002/pbc.29275 (2021).
Sezgin Evim, M. et al. Invasive fungal infections in children with leukemia: Clinical features and prognosis. Turk. J. Haematol. 39(2), 94–102. https://doi.org/10.4274/tjh.galenos.2021.2021.0203 (2022).
Pana, Z. D., Roilides, E., Warris, A., Groll, A. H. & Zaoutis, T. Epidemiology of invasive fungal disease in children. J. Pediatr. Infect. Dis. Soc. 6(suppl_1), S3–S11 (2017).
Blyth, C. C. et al. Not just little adults: Candidemia epidemiology, molecular characterization, and antifungal susceptibility in neonatal and pediatric patients. Pediatrics 123(5), 1360–1368. https://doi.org/10.1542/peds.2008-2055 (2009).
Onciu, M. Acute lymphoblastic leukemia. Hematol. Oncol. Clin. North Am. 23(4), 655–674. https://doi.org/10.1016/j.hoc.2009.04.009 (2009).
Nazemi, K. J. & Malempati, S. Emergency department presentation of childhood cancer. Emerg. Med. Clin. North Am. 27(3), 477–495. https://doi.org/10.1016/j.emc.2009.04.008 (2009).
Groll, A. H. et al. European Conference on Infections in Leukaemia (ECIL-4): Fourth diagnosis, prevention, and treatment of invasive fungal diseases in pediatric patients with cancer or allogeneic haemopoietic stem-cell transplantation. Lancet Oncol. 15(8), e327–e340. https://doi.org/10.1016/S1470-2045(14)70017-8 (2014).
Lehrnbecher, T. et al. Clinical practice guideline for systemic antifungal prophylaxis in pediatric patients with cancer and hematopoietic stem-cell transplantation recipients. J. Clin. Oncol. 38(27), 3205–3216. https://doi.org/10.1200/jco.20.00158 (2020).
McKeny, P. T., Nessel, T. A. & Zito, P. M. Antifungal antibiotics (StatPearls, Treasure Island, 2022).
Shafiei, M., Peyton, L., Hashemzadeh, M. & Foroumadi, A. History of the development of antifungal azoles: A review on structures, SAR, and mechanism of action. Bioorg. Chem. 104, 104240. https://doi.org/10.1016/j.bioorg.2020.104240 (2020).
Allen, D., Wilson, D., Drew, R. & Perfect, J. Azole antifungals: 35 years of invasive fungal infection management. Expert Rev. Anti-Infect. Ther. 13(6), 787–798. https://doi.org/10.1586/14787210.2015.1032939 (2015).
Wang, J. et al. Comparison of antifungal prophylaxis drugs in patients with hematological disease or undergoing hematopoietic stem cell transplantation: A systematic review and network meta-analysis. JAMA Netw. Open 3(10), e2017652. https://doi.org/10.1001/jamanetworkopen.2020.17652 (2020).
Hachem, R. et al. Comparing the safety and efficacy of voriconazole versus posaconazole in the prevention of invasive fungal infections in high-risk patients with hematological malignancies. Int. J. Antimicrob. Agents 50(3), 384–388. https://doi.org/10.1016/j.ijantimicag.2017.03.021 (2017).
Al-Badriyeh, D. et al. Pharmacoeconomic evaluation of voriconazole versus posaconazole for antifungal prophylaxis in acute myeloid leukaemia. J. Antimicrob. Chemother. 65(5), 1052–1061. https://doi.org/10.1093/jac/dkq076 (2010).
Donnelly, J. P. et al. Revision and update of the consensus definitions of invasive fungal disease from the european organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin. Infect. Dis. 71(6), 1367–1376. https://doi.org/10.1093/cid/ciz1008 (2020).
Tang, L. et al. Posaconazole vs. voriconazole in the prevention of invasive fungal diseases in patients with haematological malignancies: A retrospective study. J. Mycol. Med. 28(2), 379–383. https://doi.org/10.1016/j.mycmed.2017.11.003 (2018).
Hope, W. W. et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin. Microbiol. Infect. 18(Suppl 7), 38–52. https://doi.org/10.1111/1469-0691.12040 (2012).
Steinbach, W. J. Epidemiology of invasive fungal infections in neonates and children. Clin. Microbiol. Infect. 16(9), 1321–1327. https://doi.org/10.1111/j.1469-0691.2010.03288.x (2010).
Tang, J. L. et al. High incidences of invasive fungal infections in acute myeloid leukemia patients receiving induction chemotherapy without systemic antifungal prophylaxis: A prospective observational study in Taiwan. PLoS One 10(6), e0128410. https://doi.org/10.1371/journal.pone.0128410 (2015).
Sun, Y. et al. Invasive fungal infection in patients receiving chemotherapy for hematological malignancy: A multicenter, prospective, observational study in China. Tumour Biol. 36(2), 757–767. https://doi.org/10.1007/s13277-014-2649-7 (2015).
Wang, S. S. et al. Invasive fungal infections in children with acute lymphoblastic leukaemia: Results from four Australian centres, 2003–2013. Pediatr. Blood Cancer 66(10), e27915. https://doi.org/10.1002/pbc.27915 (2019).
Inaba, H. et al. Infection-related complications during treatment for childhood acute lymphoblastic leukemia. Ann. Oncol. 28(2), 386–392. https://doi.org/10.1093/annonc/mdw557 (2017).
Korula, A. et al. Invasive fungal infection following chemotherapy for acute myeloid leukaemia-Experience from a developing country. Mycoses 60(10), 686–691. https://doi.org/10.1111/myc.12646 (2017).
Ruijters, V. J., Oosterom, N., Wolfs, T. F. W., van den Heuvel-Eibrink, M. M. & van Grotel, M. Frequency and determinants of invasive fungal infections in children with solid and hematologic malignancies in a nonallogeneic stem cell transplantation setting: A narrative review. J. Pediatr. Hematol. Oncol. 41(5), 345–354. https://doi.org/10.1097/mph.0000000000001468 (2019).
Dahlén, T. et al. Decreased invasive fungal disease but no impact on overall survival by posaconazole compared to fluconazole prophylaxis: A retrospective cohort study in patients receiving induction therapy for acute myeloid leukaemia/myelodysplastic syndromes. Eur. J. Haematol. 96(2), 175–180. https://doi.org/10.1111/ejh.12565 (2016).
Tormo, M. et al. Primary prophylaxis of invasive fungal infections with posaconazole or itraconazole in patients with acute myeloid leukaemia or high-risk myelodysplastic syndromes undergoing intensive cytotoxic chemotherapy: A real-world comparison. Mycoses 61(3), 206–212. https://doi.org/10.1111/myc.12728 (2018).
Keighley, C. L., Manii, P., Larsen, S. R. & van Hal, S. Clinical effectiveness of itraconazole as antifungal prophylaxis in AML patients undergoing intensive chemotherapy in the modern era. Eur. J. Clin. Microbiol. Infect. Dis. 36(2), 213–217. https://doi.org/10.1007/s10096-016-2780-z (2017).
Girmenia, C. et al. Breakthrough invasive fungal diseases in acute myeloid leukemia patients receiving mould active triazole primary prophylaxis after intensive chemotherapy: An Italian consensus agreement on definitions and management. Med. Mycol. 57(Supplement_2), S127–S137. https://doi.org/10.1093/mmy/myy091 (2019).
Chen, W. et al. Pulmonary invasive fungal disease and bacterial pneumonia: A comparative study with high-resolution CT. Am. J. Transl. Res. 11(7), 4542–4551 (2019).
Even, C. et al. Impact of invasive fungal disease on the chemotherapy schedule and event-free survival in acute leukemia patients who survived fungal disease: A case-control study. Haematologica 96(2), 337–341. https://doi.org/10.3324/haematol.2010.030825 (2011).
Kobayashi, R. et al. Risk factors for invasive fungal infection in children and adolescents with hematologic and malignant diseases: A 10-year analysis in a single institute in Japan. Pediatr. Infect. Dis. J. 37(12), 1282–1285. https://doi.org/10.1097/inf.0000000000002010 (2018).
Xu, X. H. et al. Evaluation of the implementation rate of primary antifungal prophylaxis and the prognosis of invasive fungal disease in acute leukemia patients in China. J. Infect. Chemother. 23(6), 360–367. https://doi.org/10.1016/j.jiac.2017.02.011 (2017).
Funding
This study was funded by Scientific Research Foundation of Anhui Medical University (No. 2021xkj168) and (No. 2022xkj049).
Author information
Authors and Affiliations
Contributions
N.W. and S.T. designed the research. S.T. and K.Z. wrote the manuscript and analyzed the data. J.C., L.Y., and Z.X. collected the clinical information and samples.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tu, S., Zhang, K., Wang, N. et al. Comparative study of posaconazole and voriconazole for primary antifungal prophylaxis in patients with pediatric acute leukemia. Sci Rep 13, 18789 (2023). https://doi.org/10.1038/s41598-023-46328-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46328-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.