Abstract
The Fritillaria species ranked as a well-known traditional medicine in China and has become rare due to excessive harvesting. To find reasonable strategy for conservation and cultivation, identification of new ecological distribution of Fritillaria species together with prediction of those responses to climate change are necessary. In terms of current occurrence records and bioclimatic variables, the suitable habitats for Fritillaria delavayi, Fritillaria taipaiensis, and Fritillaria wabuensis were predicted. In comparison with Maxent and GARP, Biomod2 obtained the best AUC, KAPPA and TSS values of larger than 0.926 and was chosen to construct model. Temperature seasonality was indicated to put the greatest influence on Fritillaria taipaiensis and Fritillaria wabuensis, while isothermality was of most importance for Fritillaria delavayi. The current suitable areas for three Fritillaria species were distributed in south-west China, accounting for approximately 17.72%, 23.06% and 20.60% of China's total area, respectively. During 2021–2100 period, the suitable habitats of F. delavayi and F. wabuensis reached the maximum under SSP585 scenario, while that of F. taipaiensis reached the maximum under SSP126 scenario. The high niche overlap among three Fritillaria species showed correlation with the chemical composition (P ≤ 0.05), while no correlation was observed between niche overlap and DNA barcodes, indicating that spatial distribution had a major influence on chemical composition in the Fritillaria species. Finally, the acquisition of species-specific habitats would contribute to decrease in habitat competition, and future conservation and cultivation of Fritillaria species.
Similar content being viewed by others
Introduction
Plants regulate balance between oxygen and carbon dioxide in atmosphere, reduce contents of air pollutants (SO2, NO2 and VOC), produce pharmaceutical and commercial ingredients for both humans and animals, and are therefore an essential component of ecosystems1. Recently, many studies reported that environmental factors (temperature, light, water, etc.) play roles in numerous physiological and metabolic processes in plants, thus substantially affecting the distribution and biodiversity of many plants2. On the other hand, the accumulation of plant secondary metabolites, which are regulated by the climate change, plays a role in plant-rhizospheric microbe interactions, thereby making significant contributions to the environmental properties3. According to the IPCC record, the global average temperature has continuously risen by 0.78 °C compared to that from 1885 to 1990 due to increased energy consumption and greenhouse gas emission4,5. Accordingly, warmer temperatures would impact plant growth and yield6,7, leading a rise in the extinction rate of species. However, many plants may not be able to make shift to suitable areas with sufficient rate at the current speed of climate change. Hence, detecting the degree of environmental change in the coming future and assessing its impact on plant distribution may be helpful in designing future conservation and cultivation methods8.
Fritillaria species are regarded as a well-known symbol of thoughts, which were initially introduced in Shijing, the most ancient form of poetry in China 3000 years ago. In addition to aesthetic value, bulbus Fritillaria cirrhosa (BFC), also called as the Chinese name “Chuan-Bei-mu”, has been used in traditional Chinese medicine to relieve cough and phlegm for more than 2000 years9. In terms of China Pharmacopoeia 2020, the bulbs of F. cirrhosa, F. unibracteata, F. przewalskii, F. delavayi, F. wabuensis, and F. taipaiensis are sources of BFC. Among the six species, F. cirrhosa, F. unibracteata, F. przewalskii, F. delavayi and F. wabuensis are grouped as high-altitude species distributed in the alpine areas of the Himalayan-Hengduan Mountains, while middle-altitude species (F. taipaiensis) is distributed mainly in Shanxi and Chongqing provinces. Recently, the imbalance between supply and requirement of BFC became severe partly due to the unsustainable harvesting of Fritillaria species. As per the Information System of Chinese Rare and Endangered Plants (ISCREP) (http://www.iplant.cn/rep/protlist), most wild Fritillaria species are endangered as their wild resources are rarely found in China. The beneficial ingredients of Fritillaria species are mainly composed of steroidal alkaloids10, whose biosynthesis mainly originates from the mevalonate (MVA) pathway11. The genes involved in the MVA pathway have been shown to be regulated by environmental factors, with those in the distribution area perhaps affecting their chemical composition and potential applications12. In addition, altitude serve as an important aspect affecting the biological growth and morphological plasticity of Fritillaria species13. Meanwhile, the high-altitude Fritillaria species may be a commercially important genetic pool as these plants are highly adapted to cold and dry climates14. All of these results have confirmed the important and active role of environment in plant physiology, productivity, and other processes. Therefore, it is vital to explore the correlation between the environmental variables and the distribution of Fritillaria species, and determine the key factors influencing its distribution.
Two kind of species distribution models (SDMs), such as maximum entropy (MaxEnt) and genetic algorithm for rule-set production (GARP), have been singly used to identify important environmental variables, ecological responses, and distribution areas15,16. Recently, ensemble models have been proposed as they may improve the accuracy compared to single models17. Regardless of modeling approach, area under the curve (AUC) of the receiver operating characteristic (ROC) curve, true skill statistics (TSS), and Kappa values, are usually used as evaluating criteria for confirmation of final model5. In our previous study, we predicted the potential distribution of F. unibracteata, F. cirrhosa and F. przewlkii by Maxent and GARP5; however, little is known regarding the other three Fritillaria species. In this study, the potential distribution of F. taipaiensis, F. delavayi and F. wabuensis were analyzed, which may provide more scientific support for the cultivation and conservation of Fritillaria resources. First, the occurrence records of F. delavayi, F. taipaiensis and F. wabuensis are collected. After evaluating the accuracy of various models, an ensemble model using Biomod2 package of R software is finally selected to assess what degree of environmental variables affecting the potential distribution range of the three Fritillaria species17,18,19. Moreover, in order to avoid the habitat competition of various Fritillaria species, the overlapping distribution and species-specific habitats of various original species is then tested. Second, the potential change in ecological distribution under four climate scenarios (SSP126, SSP245, SSP370, and SSP585) is predicted, by which sustainable strategy might be developed prior to the future trend happens. Third, it is hypothesized that the environmental distribution shows influence on the genetic information and chemical composition of plants, and therefore the correlation between spatial distribution and DNA sequences or chemical composition was evaluated, respectively.
Materials and methods
Occurrence records
The occurrence records of three Fritillaria species across their whole ranges in China were collected through the following sources: field surveys since 2017, related literature searching, and websites (Global Biodiversity Information Facility, GBIF, https://www.gbif.org/; Chinese Virtual Herbarium databases, CVH, https://www.cvh.ac.cn/; Plant Science of China, http://www.iplant.cn/frps). In total, we used records (temporal range of records: 1884–2020). When distribution points did not contain the exact geo-coordinates, the Chinese Satellite Map (https://map.bmcx.com/) was used to pick up the latitude and longitude. The repeated records were removed with only one point within each grid cell (20 km × 20 km). ArcGIS (v10.2, ESRI, Redlands, CA, USA) was utilized to analyze the actual distribution, richness, and diversity of three Fritillaria species. By using ArcGIS, all the present points of F. delavayi, F. taipaiensis, and F. wabuensis, were recorded and mapped a decimal degree format with 1° × 1° resolution, respectively.
Environmental parameters
In this study, 19 bioclimatic variables (bio01-bio19) with 2.5 arc-min spatial resolution from the WorldClim database version 2.020, and three topographic variables (slope, altitude, and aspect extracted in ArcMap, v10.2, https://www.arcgis.com/index.html) were initially selected. The 22 environmental variables (Supplementary Table 1) have been broadly used to explore environmental influences on alphine plants in the Himalayan-Hengduan Mountains21,22.
Prior to model construction, Spearman correlation analysis among the 22 environmental variables was undergone in order to avoid model overfitting. Specifically, using the aforementioned distribution points of Fritillaria, a correlation matrix using SPSS 2.0 was performed so as to extract the associated environmental variables. Then, in line with the methods outlined by Jiang et al., if paired environmental variables showed a coeffiecient of correlation > 0.8, the one with a higher contribution rate was subsequently remained (Supplementary Table 2) and used for further prediction23.
In order to analyze the influence of climate change on future species distribution, four shared socioeconomic pathways (SSP126, SSP245, SSP370, and SSP585) released by the CMIP6 and IPCC Assessment Report 6 (AR6) were applied, which represent the pathways of global sustainable, medium sustainable, local sustainable, and routine development, respectively. Based on the World Climate Database24, global projection was presented at a spatial resolution of 2.5 arc-min from 2021 to 2100 (20-year intervals). All environmental variables in raster format were aligned with the geographic space using WGS84 datum as the default parameters.
Evaluation of model and potential distribution of Fritillaria species
MaxEnt (v3.3.1, https://biodiversityinformatics.amnh.org/open_source/maxent/), GARP (v1.1.6, http://www.nhm.ku.edu/desktopgarp/) and Biomod2 (v4.3.1, https://cran.r-project.org/bin/windows/base/) models were initially used. Biomod2 is an ensemble SDM method capitalizing on ten singly used modeling techniques: artificial neural networks (ANN), classification tree analysis (CTA), flexible discriminant analysis (FDA), generalized additive models (GAM), generalized boosted models (GBM), generalized linear models (GLM), multivariate adaptive regression splines (MARS), MaxEnt, random forest (RF), and surface range envelope (SRE)25. In this study, surface range envelope strategy was used, and as a result, pseudo-absence points were randomly generated with the same number as true-presence records26. Then, eight commonly used algorithms were applied: GLM, RF, MaxEnt, ANN, MARS, FDA, CTA and SRE27. A model option in Biomod 2, each algorithm was run 15 times, for a total of 120 runs for the eight algorithms. In the data sets, various training/testing sets (85/15, 80/20 and 75/25) were conducted using 500 iterations in order to validate the models. Following initial tests, only modeling techniques with a ROC value > 0.9 were left to construct the final ensemble model. The test samples were selected using the bootstrap method.
The accuracy of the MaxEnt, GARP and Biomod2 models was evaluated by AUC, TSS, and Kappa values. For AUC analysis, the Jackknife method was selected to produce the response curve of the environmental variables. Then, after implementing 10 times repetition for each test, the AUC value was counted as the area enclosed by the ROC curve. AUC < 0.6 indicated failing performance, 0.6 ≤ AUC < 0.7 indicated poor performance, 0.7 ≤ AUC < 0.8 indicated moderate performance, 0.8 ≤ AUC < 0.9 indicated good performance, and 0.9 ≤ AUC < 1 indicated excellent performance28. TSS scores ranged from − 1 to 1, where values above 0.75 indicated excellent model performance29. Kappa coefficients were measures of correlation between the model predictions and truth. Kappa values ranged from − 1 to 1, where 1 indicated that 100% of prediction agrees with the truth, − 1 indicated that 100% of prediction disagreed with the truth30.
The prediction results under the optimal model were then imported into ArcGIS after which the current suitable habitats for three Fritillaria species were drawn. During ArcGIS mapping, four grades of suitable habitats were classified: highly (0.75–1), moderately (0.5–0.75), low (0.25–0.5), and not suitable habitat (0–0.25)31. Since the suitable habitats with probability ≥ 0.5 indicated that species lived comfortably in these areas32, the suitable ranges of the environmental variables were defined with a threshold of ≥ 0.533. The corresponding values of suitable range were acquired from the response curve of the Biomod2 model result.
Niche overlap of Fritillaria species
First, the mean Levins’ B1 (inverse concentration) and B2 (uncertainty) values34 were calculated as the niche breadth of each species in geographical and environmental space using ENMTools v1.335. The ranges of Levins’ B1 and B2 values distributed from 0 to 1, where higher values represented wide niche breadth, while lower values represented narrow niche breadth. Then, the niche overlap was evaluated using ENMTools package based on two indexes: Schoener’s D36 and Hellinger’s I35, whose values generally ranged from 0 (representing a small extent of niche overlap) to 1 (representing a high extent of niche overlap).
Finally, to strengthen the speculation, the overlapping regions including highly and moderately suitable habitats of paired species were visualized in ArcGIS, whose overlapping extent was counted according to the following equation5.
Aoverlap and Boverlap represent the records of species A and B in the overlapping regions respectively, while Atotal and Btotal are the total existing records related to species A and B.
Clustering analysis of Fritillaria species based on chemical metabolites
The cluster analysis based on the chemical composition of six Fritillaria species was performed in term of the method reported by An et al.37. Specifically, the chemical metabolites of Fritillaria species were obtained from the Traditional Chinese Medicine Integrated Database (TCMID, http://119.3.41.228:8000/tcmid/), which is currently the largest database, containing 25,210 secondary metabolites from 8159 herbs38. 40 chemical metabolites were extracted from five Fritillaria species in TCMID, of which 31 non-repetitive metabolites had SMILES information. Since the chemical metabolites information of F. taipaiensis was not contained in TCMID, four alkaloids of F. taipaiensis with potential medical values were collected from academic literature and were introduced into this study (Supplementary Table 3). Using the PaDel-descriptor software, the SMILES information for each metabolite was transformed into a PubChem binary fingerprint (Supplementary Table 4). PubChem contains 881 bits and has been widely used in chemo-informatics systems39. Next, a metabolites network was constructed in light of chemical structure similarity40, in which paired metabolites with Tanimoto coefficient value greater than 0.85 were considered to be highly similar in structure41. Third, metabolites with similar structures were clustered to produce metabolite-groups based on IPCA algorithm42. Fourth, a binary matrix between the metabolite-groups and plants was built, and accordingly the plants were clustered by Simpson coefficient43 and Jaccard coefficient44, respectively. Finally, the plant-plant dissimilarity in Jaccard clustering was used for the correlation analysis.
Phylogenetic analysis
In order to reveal the evolutionary relationship among the Fritillaria species, the ITS1, ITS2, ITS1 + ITS2 and complete chloroplast genomes of the six original species of BFC were collected from GenBank, some of which were deposited by our team (Zhang et al.). Phylogenetic trees based on Maximum likelihood were constructed using MEGA 7.0 with 1000 bootstrap repetitions45. The evolutionary distance, derived from nucleotide substitution rates based on the Tamura-Nei model46,47, was used for the further correlation analysis48.
Statistical methods
A linear trend line representing the correlation between evolutionary distances from DNA markers or plant-plant dissimilarity based on chemical composition and niche overlap was drawn. The R2 value indicates how well the data fit the line, where 1 signifies a strong correlation, while 0 refers to poor correlation. A Pearson correlation test was applied to check the statistical significance with a P-value of ≤ 0.05 to be statically significant49.
Results
The occurrence records of three Fritillaria species
Initially, a total of 147 distribution points were collected on three Fritillaria species by field survey, literature and websites search. Following refinement, 29 distribution points were removed and the remaining 118 individual sample points were recorded, of which 56, 45, and 17 records of F. delavayi, F. taipaiensis, and F. wabuensis were collected, respectively (Supplementary Table 5). In accordance to the sampling point map (Fig. 1), the ecological factor data of each sampling point was extracted. These three Fritillaria species were found to be distributed in the middle-latitude region (25.23°N–36.84°N). Meanwhile, the average latitudes of F. taipaiensis, and F. wabuensis were noted to be above 31°N, while that of F. delavayi was below 31°N. The distribution spans of three species in longitude were observed to be narrow, and hence their resource distribution was relatively concentrated.
Distribution points of F. delavayi (red), F. taipaiensis (yellow) and F. wabuensis (blue), respectively. MaxEnt v3.3.1: https://biodiversityinformatics.amnh.org/open_source/maxent/, ArcGIS v10.2: https://www.arcgis.com/.
Three species were distributed with an average altitude of more than 1000 m, in which F. delavayi showed the highest average altitude of 3395.29 m. The average aspect of F. delavayi and F. wabuensis was noted to be above 200, while that of F. taipaiensis was below 200. The slopes of three Fritillaria species were in the range of 88°–90°.
Model performance and importance of environmental factors
With the given training/testing sets (85/15, 80/20, and 75/25), compared with the MaxEnt and GARP models, the ensemble model Biomod2 was found to have the best performance according to AUC, KAPPA, and TSS values (Supplementary Table 6). When the training/testing set was 85/15, F. delavayi (AUC = 0.999, KAPPA = 0.964, and TSS = 0.970) was shown to possess the best performance, when the training/testing set was 80/20, F. taipaiensis (AUC = 0.997, KAPPA = 0.956, and TSS = 0.963) and F. wabuensis (AUC = 0.999, KAPPA = 0.962, and TSS = 0.980) had the best performances. Therefore, Biomod2 was confirmed to be the optimum model for three Fritillaria species, in which F. taipaiensis and F. wabuensis utilized 80/20 set, while F. delavayi utilized 85/15 set in the following analysis.
The internal Jackknife test determined the important environmental variables (Fig. 2). In case of independent application, Bio3 (31.2%), Elev (24%), Bio18 (17.8%), and Bio9 (12%) were highlighting parameters in the distribution model for F. delavayi, accounting for 85% of the variables. Bio4 (28.6%), Bio6 (17.2%), Bio12 (14.4%), Bio15 (12%), Elev (8%), and Bio3 (6.9%) contributed the most to the distribution model of F. taipaiensis, accounting for 87.1% of the variables. Moreover, the environmental variables with the highest influence on F. wabuensis were Bio4 (27.6%), Elev (24.6%), Bio12 (16.2%), Bio19 (9.1%) and Bio10 (7.3%), accounting for 84.8% of the variables.
The response curve demonstrated the quantitative relationship between the logistic probability of the presence and suitable ranges of environmental variables (Fig. 3). In terms of the survival areas with moderate and high suitability, the threshold (≥ 0.5) was chosen for the main bioclimatic parameters50, since plants live comfortably in such conditions. The existence probability of three Fritillaria species was shown to initially increase, after which it decreased with a rise in environmental factors. Specifically, the suitable ranges of Bio3, Bio9, Bio18, and Elev (Table 1) for F. delavayi were 40.89–48.59, − 6.49–3.77, 279.07–404.65 mm, and 2554.27–4284.06 m, respectively. Optimal isothermality (Bio3) reflects the regional temperature change and is calculated by the ratio between Bio2 and Bio7. When Bio3 reached 45.47, F. delavayi attained the highest existence probability of 0.74. When Bio9 reached the maximum at − 2.16, the existence for F.delavayi met the highest probability of 0.63. F. delavayi achieved the highest probability of existence of 0.598 as Bio18 reached 334.88 mm. Finally, the top probability of existence (0.68) was achieved once Elev reached 3782.91 m (Fig. 3A), by which confirmed that F. delavayi is high-altitude species.
In F. taipaiensis, the suitable range of Bio4, Bio6, Bio12 and Bio15 ranged from 715.51 to 888.61, from − 9.46 to 0.78 °C, from 633.38 to 1196.7 mm, and from 59.52 to 79.53, respectively (Fig. 3B). Moreover, the optimum values of Bio4, Elev, Bio19 and Bio12 were 279.08, 3828.33 m, 3.51 mm and 850.0 mm for F. wabuensis, respectively (Fig. 3C), in which the relative high altitude also demonstrated that F. wabuensis is high-altitude plant. In respect to F. taipaiensis, F. wabuensis showed the relative smaller temperature seasonality (Bio4), suggesting that the suitable presence of F. wabuensis was associated with lower variability in temperature. Meanwhile, F. taipaiensis and F. wabuensis shared similar value of annual precipitation (Bio 12), suggesting that both species shared similar water requirement. The suitable ranges and optimum values of environmental variables were listed in Table 1.
Current habitats of three Fritillaria species
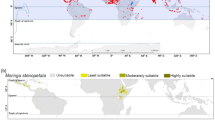
The predicting current habitats for F. delavayi demonstrated that highly suitable habitats were in Qinghai, Sichuan, Tibet and Yunnan provinces, accounting for 9.48% of China's total area. Moreover, the moderately suitable habitats were found to be distributed in the Qinghai, Tibet, and Yunnan provinces. The total suitable habitats for F. delavayi reached 1.70 × 106 km2, accounting for 17.72% of China's total area (Fig. 4A). For F. taipaiensis, the highly suitable habitat was shown to be distributed in the Gansu, Guizhou, Henan, Hubei, Shanxi, and Sichuan provinces, accounting for 7.03% of China's total area. Meanwhile, moderately suitable habitats included the Anhui, Fujian, Guizhou, Guangxi, Hubei, Jiangsu, Sichuan, Tibet, and Yunnan provinces. The total suitable habitat for F. taipaiensis comprised 2.21 × 106 km2, accounting for 23.06% of China's total area (Fig. 4B). As for F. wabuensis, the highly suitable habitat was shown to be in Gansu, Guizhou, Qinghai, Sichuan, Tibet, and Yunnan, while the moderately suitable habitat was almost in Chongqing, Gansu, Guizhou, Hubei, Shanxi, and Tibet provinces. The current suitable habitat for F. wabuensis was 1.98 × 106 km2, accounting for 20.60% of China's total area (Fig. 4C). Compared with other two Fritillaria species, F. delavayi showed the smallest suitable habitat, which was consistent with its endangered state.
Distribution map of suitable areas for F. delavayi, F. taipaiensis and F. wabuensis, respectively. MaxEnt v3.3.1: https://biodiversityinformatics.amnh.org/open_source/maxent/, ArcGIS v10.2: https://www.arcgis.com/.
Future changes in suitable habitat area
As listed in Supplementary Table 7, in F. delavayi (Fig. 5A), the maximum area of highly and moderately suitable habitats reached the maximum (12.14%, 11.77%, 11.90% and 12.49%) under SSP126, SSP245, SSP370 and SSP585 scenarios, respectively. For SSP585 scenario, the maximum in highly and moderately suitable habitat appeared at 2061–2080 period (Supplementary Fig. 2A). Accordingly, SSP585 was confirmed to be the optimum scenario for F. delavayi, as its highly and moderately suitable habitat reached the maximum during 2021–2100 at 1199,653.339 km2.
In F. taipaiensisi (Fig. 5B), the maximum area of the highly and moderately suitable habitat occurred with 12.24%, 11.79%, 11.27% and 11.08% under SSP126, SSP245, SSP370 and SSP585 scenarios, respectively. For SSP126 scenario, the maximum took place during 2021–2040 (Supplementary Fig. 2B). Overall, SSP126 was found to be the optimum scenario for F. taipaiensis with the maximum area (1174,666.794 km2) in the highly and moderately suitable habitats during 2021–2100.
In F. wabuensis (Fig. 5C), the areas of highly, moderately and total suitable habitats increased in a wave-like mode under the four scenarios, respectively. Under the SSP126, SSP245, SSP370 and SSP585 scenarios, the maximum area of highly and moderately suitable habitats was shown to be 9.05%, 4.54%, 10.57% and 27.42%, respectively. The highly and moderately suitable habitats reached the maximum during 2081–2100 under the SSP585 scenario (Supplementary Fig. 2C). SSP585 was shown to be the optimum scenario for F. wabuensis since the highly and moderately suitable habitats reached their maximum areas during 2021–2100 at 2632,258.065 km2, respectively.
Ecological niche overlapping of three Fritillaria species
As indicated by the highest B1 (0.295) and B2 (0.927), F. taipaiensis exhibited the broadest niche, while F. wabuensis showed a narrower niche (B1 and B2: 0.260 and 0.916, respectively) and F. delavayi had the narrowest niche (B1 and B2: 0.228 and 0.914, respectively). F. wabuensis and F. delavayi shared the more similarity due to the highest niche overlap (I: 0.938, D: 0.789), while F. taipaiensis showed lower similarity with F. delavayi (I: 0.756, D: 0.463) and F. wabuensis (I: 0.700, D: 0.432).
Then, the overlapping regions for three Fritillaria species were counted and drawn in Fig. 6, and hence the species-specific habitat of F. delavayi was found to be distributed in the central region of Yunnan province, while that of F. wabuensis was mainly distributed in Qinghai and Tibet provinces. Additionally, the species-specific habitat of F. taipaiensis was mainly concentrated in Chongqing, Guizhou, Henan, Hubei, Shanxi and Sichuan provinces as shown in Fig. 6A. The overlapping degree between F. wabuensis and F. delavayi was found to be 58.96%, while that of F. wabuensis and F. taipaiensis was 15.90% and that of F. taipaiensis and F. delavayi was 9.98% (Fig. 6B). The results were noted to be consistent with the results reported by ENMTools, in which the highest niche overlap was observed between F. wabuensis and F. delavayi.
Distribution patterns of three Fritillaria species. (A) Species-specific highly and moderately suitable habitats of Fritillaria delavayi, Fritillaria taipaiensis, and Fritillaria wabuensis. (B) Overlapping region of highly and moderately suitable habitats between paired species. MaxEnt v3.3.1: https://biodiversityinformatics.amnh.org/open_source/maxent/, ArcGIS v10.2: https://www.arcgis.com/.
Correlation analyses on DNA Phylogenies, clustering tree based on chemical metabolites with niche overlap of three Fritillaria species
Compared with ML trees based on ITS1, ITS2, and ITS1 + ITS2 that were weakly supported (Supplementary Fig. 1), that based on the whole CP genome exhibited higher support since three branches got bootstrap values of 100 BP (Fig. 7). Therefore, the CP genome was used to to evaluate correlation, though no correlation between evolutionary distance and niche overlap was observed (P > 0.05). This may be because genetic evolution is slower than changing speed in species distribution, or because fritillary distribution is influenced by artificial planting preferences.
Phylogenetic trees based on CP genomes and the Jaccard clustering tree based on dissimilarity of chemical composition. The pairwise distance (above the branches) and bootstrap value (under the branches) were listed. (A) Phylogenetic tree based on CP genomes; (B) Jaccard clustering tree based on dissimilarity of chemical composition.
On the other hand, two clustering trees based on the chemical composition were separately constructed. Compared with the Simpson tree, the Jaccard tree received higher support as three branches got bootstrap values of more than 70 BP, while branches in Simpson tree had bootstrap value of lower than 70 BP. In the Jaccard tree, six original species of Chuan-Bei-mu were grouped into two clades, in which clade 1 was composed of F. unibracteata and F. cirrhosa, and clade 2 was composed of other four Fritillaria species. In clade 2, F. taipaiensis was the sister to the other three species and showed a higher dissimilarity with F. delavayi and F. wabuensis. Using Jaccard result, the correlation between nichel overlap and chemical composition was evaluated, and hence a strong correlation was observed (R2 = 0.994, P = 0.050) (Table 2). The corresponding findings suggested that the chemical composition of various species was influenced by different spatial distributions.
Discussion
Although BFC has been used for healthy treatment for more than two thousand years, the insufficient amount of research conducted on BFC has hindered its sustainable conservation and application. In this study, the suitable habitats of three Fritillaria species were analyzed in detail under current and future climate scenarios. Accordingly, the spatial distribution of the Fritillaria species was found to strongly show influence on its chemical composition. The corresponding results could serve as an important step in making a sustainable strategy for future conservation.
Prior to the final model’s construction, the effectiveness of various models was significant in obtaining accurate prediction results. With respect to a single model, including Maxent and GARP, the ensemble model, Biomod2 was found to have the best performance due to its highest AUC, KAP and TSS values for each Fritillaria species, respectively. Previous analyses51,52,53 have shown that the prediction relied on a single forecast model, which severely compromised its usefulness. Even if the same dataset were used, the different single model achieved various predicting results. A potentially useful pathway may be the development of hybrid approaches, such as Biomod2, which combines a suite of algorithms using the same set of initial data and parameterization17,52. Biomod2 has been successfully applied in the distribution prediction of cocoa54, Fagus sylvatica55, lemurs56, and Orientocoluber spinalis18.
The distribution of three Fritillaria species represented obvious species-divergent distribution areas since F. delavayi, F. taipaiensis and F. wabuensis were arranged from west to east. F. delavayi was found to have a wide distribution with high altitude of 3395.29 m, including central and east Tibet, west Sichuan, north Yunnan, and south Qinghai provinces. The distribution area of F. delavayi was also in accord with a study that found that this species is confined to high-altitude medicinal plants21. The highly and moderately suitable habitat areas was shown to encompass 1.21 × 106 km2 for F. delavayi under current climate conditions. Intriguingly, certain moderately suitable habitats in Hubei and Shanxi provinces were also proposed to be introduced of F. delavayi in the future. Furthermore, F. wabuensis was observed to have the smallest number of distribution records mainly in northwest Sichuan and southwest Qinghai, with sporadic distribution in the Tibet and Yunnan provinces, and exhibited a similar geographic distribution as that of F. delavayi. The highly and moderately suitable habitats of F. wabuensis encompassed 1.19 × 106 km2, ranging mainly from Gansu, Guizhou, Qinghai, Sichuan, Tibet, and Yunnan provinces. The sporadic areas of the Guizhou and Yunnan provinces as moderately suitable habitats may also introduce this species in the future. In comparison with those of F. delavayi and F. wabuensis, F. taipaiensis moved east and was more intense in Chongqing, central and south Shanxi, northwest Hubei, southeast Gansu, and west Henan provinces, and exhibited the smallest highly and moderately suitable areas (1.16 × 106 km2) under current climate conditions.
Among various environmental factors, temperature plays the most important role in controlling the distribution of three Fritillaria species, accounting for 50.9%, 59.3% and 41.8% overall contribution towards F. delavayi, F. taipaiensis and F. wabuensis, respectively. Specifically, Bio3 in F. delavayi (31.2%), Bio4 in F. taipaiensis (28.6%) and F. wabuensis (27.6%) provided the biggest contribution to the distribution. Moreover, Bio6 provided the second biggest contribution to the distribution of F. taipaiensis (17.2%). Temperature was reported to affect bulb dormancy, ovule fertilization, photosynthesis, bulb yielding and total alkaloid contents of the Fritillaria species10,42,57,58. Supporting that optimum temperature seasonality ranged from 785.18 and 279.08 for F. taipaiensis and F. wabuensis, respectively, both species showed high levels of probability of presence. This fact confirmed that climatic characteristics shaped population and regeneration of species59. Additionally, the relatively wide Bio4 implied that F. wabuensis might be adaptive to various areas with broad temperature fluctuation, which aligned with Qin et al.14, who indicated that alpine plants showed tolerance to environmental stresses. Meanwhile, low temperature seasonality was found to be consistent since most of the distribution areas of both Fritillaria species are located in the Hengduan Mountains and its neighboring regions, most of where have mild to warm winters and temperate summers60. In addition to thermal seasonality, isothermality also played a role in the distribution of F. delavayi, having an optimum value of 45.47. The large isothermality ensured that F. delavayi can not only improve photosynthesis during the daytime due to the relatively high temperature, but reduce energy consumption of respiration due to the relatively low night temperature, which is beneficial for metabolite accumulation and growth42. A similar result was recently reported by Wang et al.61 who proved that isothermality made the highest contribution to Fritillaria cirrhosa. The optimum Bio6 of − 4.87 °C for F. taipaiensis suggested a physiological limit to its distribution. In the seeds of F. taipaiensis, cold stratification (0–10 °C) was found to break dormancy, while a temperature > 15 °C was unfavorable for decreasing degradation of the substance with germination-inhibiting activities and inhibited germination. Similar results were also obtained by Luo et al.62 and Zhang et al.63 who observed that cryogenic treatment was necessary to completely break the dormancy of F. taipaiensis and F. yuzhongensis seeds. On the contrary, relatively high temperature is suitable for seedling growth in F. taipaiensis62. In addition, suitable temperature plays role in sprout development, morphological maintaining and reproductive process of Fritillaria species64.
In respect to temperature, precipitation played a relatively minor role in distribution, accounting for 19.9%, 26.4% and 28.9% overall contribution to F. delavayi, F. taipaiensis and F. wabuensis, respectively. Similar result was also reported by Jiang et al.5 who observed less contribution of precipitation to the distribution of F. unibracteata, F. cirrhosa and F. przewalskii, indicating the similar water requirement of six original plants of Chuan-Bei-mu. Precipitation implements a considerable influence on the above- and underground organs of plants. Insufficient water has previously been reported to induce a reduction in biomass of plants65, longer germination period66, lower leaf mass65 and lower root density67, whereas sufficient water would increase root length and leaf area45 and promote germination68. In the case of low annual precipitation (< 633.38) for F. taipaiensis, low annual precipitation (< 521.88 mm) for F. wabuensis, and low Bio18 (< 279.07 mm) for F. delavayi, the presenting probability of Fritillaria species greatly reduced, indicating that precipitation was an important constraint to both species. Specifically, the annual growth period of Fritillaria species was divided into two stages that exhibited different precipitation demands. Generally, precipitation in the first stage (October to next April) was noted to be relatively low and accounted for 20% of the annual demand. Excessive water supplement was found to be unfavorable for cold stratification, which would aggravate Fritillaria rust and root rot. Furthermore, freezing rain could cause cellular oxidative damage, dehydration and destabilization to plants69,70. These evidences were consistent with our result that F. wabuensis got a low value of optimal Bio19 (3.51). Accordingly, low precipitation in the autumn was shown to be beneficial in preventing frost damage to the Fritillaria species. Next, abundant precipitation (80% of annual precipitation) in the second stage (May to September) implied that it was necessary to provide sufficient water during the bulb filling period. The balanced water supplement in this stage could improve metabolism turnover, organ formation as well as nutrient accumulation in plants. In general, the relatively low contribution of precipitation was supported by the fact that most of the distribution area, including the center area, of three Fritillaria species received the influence of monsoon from the Indian and Pacific oceans and has sufficient precipitation5.
Besides, altitude was the third important environmental factor controlling the distribution of three Fritillaria species, accounting for 24%, 8% and 24.6% overall contribution to F. delavayi, F. taipaiensis and F. wabuensis, respectively. The accumulation of metabolites and nutrients in underground and subaerial organs are influenced by altitude usually interacting with the availability of nutrients, water content71, temperature, ultraviolet intensity, CO2 concentration and UV-B33. For instance, the reproductive success of F. delavayi was critically affected by the temperature and ultraviolet intensity, and as a result, F. delavayi was distributed in a narrow altitude area57. Ma et al. reported that altitude influenced the growth of leaf and bulb traits, and the biomass of F. przewalskii bulb increased significantly with increasing altitude. In this study, the altitude of F. delavayi was found to range from 2554.27 to 4284.06 m, which was consistent with21, in which F. delavayi was confined to be an alpine plant. The similarly high altitude of F. delavayi (3782.91 m) and F. wabuensis (3828.33 m) ensured that both species could be adaptive to similar alpine environments. As a result, altitude was considered to be one significant factor affecting morphological change and distribution of the Fritillaria species13,50.
With increasing concentrations of greenhouse gas emissions, the suitable habitat range of F. taipaiensis and F. wabuensis increased despite varying sensitiveness. Under the low emission (SSP126) scenario, the highly and moderately suitable area of F. taipaiensis reached the maximum of 101.1% with respect to those in the current situation (Supplementary Fig. 2B), and F. wabuensis reached the maximum of 221.4% under the high emission (SSP580) scenario (Supplementary Fig. 2C). The corresponding findings confirmed that temperature positively influenced plants by accelerating growth rate, increasing size, implicating host resistance to pests, and improving soil nutrients72,73. On the other hand, the highly and moderately suitable area of F. delavayi was reduced by 1% under the high emission (SSP585) scenario, with respect to those in the current situation (Supplementary Fig. 2A), implying that continuous rise in temperature may confer a negative influence on plants, which may occur by hindering the regeneration of seeds74. Accordingly, temperature was determined to be the most significant factor shaping the distribution of the three Fritillaria species, which was consistent with Bio4, Bio3 and Bio6 having the most contribution to various species.
Ecological niche was another factor showing influence on the distribution of species. As determined by high Schoener’s D and Hellinger’s I, the high niche overlap among three Fritillaria species demonstrated that these species shared similar temperature, precipitation and elevation. Moreover, overlapping areas among three Fritillaria species were observed in Fig. 6, and approximately 58.96% of the distributions of F. delavayi were located in the overlapping area, confirming the high ecological niche among the three species. Taking that plants with close relationship usually share similar adaptive mechanism to living environments during evolution into consideration75, genetic relationship might correlate with ecological niche overlap. However, the spatial distribution was found to be poorly related to phylogeny with weak significance (Table 2). In this regard, diversification and speciation process were proposed to be caused by multiple driving factors, i.e., spatial distribution, reproductive isolation, sexual reproduction and geographical barrier76,77,78. Hopefully, if more factors that are involved with species evolution are applied into the correlation analysis, the statistical result would be more reliable. Additionally, the accumulation of chemical metabolites was influenced by environmental factors; hence, plants sharing common environmental factors may contain a similar chemical composition. In this study, the clustering result that was derived according to the chemical composition from various Fritillaria species were found to be correlated with their ecological niche overlap (P ≤ 0.050), suggesting that spatial distribution was an important factor in controlling chemical composition. However, unbalanced distribution of metabolites in various plants was observed, i.e., F. wabuensis was associated with only one metabolite. Meanwhile, F. delavayi was associated with seven metabolites with potential medical values. One reasons for this was that wild medicinal plants (such as F. delavayi) contain more compounds compared to cultivated plants (such as F. wabuensis), which have been selected artificially. Besides, more systematical studies were possibly carried out on the chemical information of important medicinal plants. Therefore, the relationship between spatial distribution and chemical composition might hopefully be more accurate in light of sufficient chemical metabolite samples.
However, although the suitable planting areas represented regions showing similar environmental conditions as those of current distribution region, economic or other environmental factor variations were not considered in this study. Specifically, the salt in soil was noted to be correlated with soil microbial communities that interacted with the Fritillaria species, thereby, influencing its healthy growth79. Moreover, excessive consumption of certain medicinal species could lead to extinction in spite of the current and (or) future available suitable habitats. Land use alteration has also resulted in decreasing availability of suitable habitats due to human use for economic purposes33. Declined forest cover and increased fragmentation in highly forested reserves may result in the disappearance of large amounts of currently suitable habitats because of ecological distribution80 Fortunately, most suitable areas of the three Fritillaria species were located in the newly established Giant Panda National Park and Sanjiangyuan National Park, China, which would be able to provide funds and actively manage the conservation of wild species, including Fritillaria, in both areas.
Conclusion
F. delavayi, F. taipaiensis and F. wabuensis, ranked as traditional Chinese Medicine, are widely used and have potentially major market value. However, their resources have rapidly decreased in light of excessive harvesting and insufficient conservation management, and hence information pertaining to suitable areas of the three Fritillaria species are urgently required. In this study, ecologically suitable habitats of three Fritillaria species were successfully predicted, which were mainly distributed in south-west Asia. Temperature was identified as the most important factor shaping the distribution of the three Fritillaria species, however, continuous rise in temperature due to greenhouse gas emissions would cause negative effect on Fritillaria species. The ecological niche overlap and species-specific distribution areas among the three Fritillaria species may serve as a useful reference in improving future plant introduction and cultivation.
Data availability
The data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Wei, X. et al. Phylloremediation of air pollutants: Exploiting the potential of plant leaves and leaf-associated microbes. Front. Plant Sci. 8, 1318 (2017).
Shen, L. et al. Prediction of the globally ecological suitability of Panax quinquefolius by the geographic information system for global medicinal plants (GMPGIS). Chin. J. Nat. Med. 17(7), 481–489 (2019).
Rieusset, L. et al. Secondary metabolites from plant-associated Pseudomonas are overproduced in biofilm. Microb. Biotechnol. 13(5), 1562–1580 (2020).
Stocker, T. F. Climate change the closing door of climate targets. Science 18, 280–282 (2013).
Jiang, R. et al. Modeling of the potential geographical distribution of three Fritillaria species under climate change. Front. Plant Sci. 12, 749838 (2022).
Dutta, M. et al. The interplay of DNA methyltransferases and demethylases with tuberization genes in potato (Solanum tuberosum L.) genotypes under high temperature. Front Plant Sci. 13, 933740 (2022).
Fu, G. & Sun, W. Temperature sensitivities of vegetation indices and aboveground biomass are primarily linked with warming magnitude in high-cold grasslands. Sci. Total Environ. 843, 157002 (2022).
Buonincontri, M. P. et al. Shedding light on the effects of climate and anthropogenic pressures on the disappearance of Fagus sylvatica in the Italian lowlands: Evidence from archaeo-anthracology and spatial analyses. Sci. Total Environ. 877, 162893 (2023).
Zhang, T. et al. Identification of evolutionary relationships and DNA markers in the medicinally important genus Fritillaria based on chloroplast genomics. PeerJ 9, e12612 (2021).
Hajihashemi, S., Brestic, M., Landi, M. & Skalicky, M. Resistance of Fritillaria imperialis to freezing stress through gene expression, osmotic adjustment and antioxidants. Sci. Rep. 10(1), 10427 (2020).
Liao, H. et al. Integrated transcriptomic and metabolomic analysis reveals the molecular basis of tissue-specific accumulation of bioactive steroidal alkaloids in Fritillaria unibracteata. Phytochemistry 214, 113831 (2023).
Qiao, Y. et al. Integrated RNA-seq and sRNA-seq analysis reveals miRNA effects on secondary metabolism in Solanum tuberosum L. Mol. Genet. Genom. 292(1), 37–52 (2017).
Ma, R., Xu, S., Chen, Y., Guo, F. & Wu, R. Allometric relationships between leaf and bulb traits of Fritillaria przewalskii Maxim grown at different altitudes. PLoS ONE 15(10), e0239427 (2020).
Qin, Y. et al. A phenylalanine ammonia lyase from Fritillaria unibracteata promotes drought tolerance by regulating lignin biosynthesis and SA signaling pathway. Int. J. Biol. Macromol. 213, 574–588 (2022).
Carvalho, B. M., Rangel, E. F., Ready, P. D. & Vale, M. M. Ecological niche modelling predicts southward expansion of Lutzomyia (Nyssomyia) flaviscutellata (Diptera: Psychodidae: Phlebotominae), Vector of Leishmania (Leishmania) amazonensis in south America, under climate change. PLoS ONE 10(11), e0143282 (2015).
Araújo, M. B. & New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 22(1), 42–7 (2007).
Di Febbraro, M. et al. Different facets of the same niche: Integrating citizen science and scientific survey data to predict biological invasion risk under multiple global change drivers. Glob. Change Biol. 29(19), 5509–5523 (2023).
Park, D., Jeong, H., Park, J. & Park, I. K. Distribution and habitat assessments of the Slenderracer, Orientocoluber spinalis, for the registration of nationally endangered species in the Republic of Korea. Sci. Rep. 13(1), 12025 (2023).
Gao, M. et al. Priority conservation area of Larix gmelinii under climate change: Application of an ensemble modeling. Front. Plant Sci. 14, 1177307 (2023).
Hradilová, I. et al. Variation in wild pea (Pisum sativum subsp. elatius) seed dormancy and its relationship to the environment and seed coat traits. PeerJ 7, e6263 (2019).
Lakey, D. K. Ecological status of high altitude medicinal plants and their sustainability: Lingshi, Bhutan. BMC Ecol. 16(1), 45 (2016).
Ni, L., Li, W., Zhao, Z., Gaawe, D. & Liu, T. Migration patterns of Gentiana crassicaulis, an alpine gentian endemic to the Himalaya-Hengduan Mountains. Ecol. Evol. 12(3), e8703 (2022).
Feng, X., Park, D. S., Liang, Y., Pandey, R. & Papeş, M. Collinearity in ecological niche modeling: Confusions and challenges. Ecol. Evol. 9, 10365–10376 (2019).
Abou-Shaara, H. et al. Modeling the invasion of the large hive beetle, Oplostomus fuligineus, into North Africa and South Europe under a changing climate. Insects 12, 275 (2021).
Gregorietti, M. et al. Cetacean presence and distribution in the central Mediterranean Sea and potential risks deriving from plastic pollution. Mar. Pollut. Bull. 173(Pt B), 112943 (2021).
Leta, S. et al. Modeling the global distribution of Culicoides imicola: an Ensemble approach. Sci. Rep. 9(1), 14187 (2019).
Ray, D., Marchi, M., Rattey, A. & Broome, A. A multi-data ensemble approach for predicting woodland type distribution: Oak woodland in Britain. Ecol. Evol. 11(14), 9423–9434 (2021).
Bebber, D. P. et al. Many unreported crop pests and pathogens are probably already present. Glob. Change Biol. 25(8), 2703–2713 (2019).
Changjun, G. et al. Predicting the potential global distribution of Ageratina adenophora under current and future climate change scenarios. Ecol. Evol. 11(17), 12092–12113 (2021).
Fernández, I. C. & Morales, N. S. One-class land-cover classification using MaxEnt: the effect of modelling parameterization on classification accuracy. PeerJ 7, e7016 (2019).
Ren, Z., Zagortchev, L., Ma, J., Yan, M. & Li, J. Predicting the potential distribution of the parasitic Cuscuta chinensis under global warming. BMC Ecol. 20, 28 (2020).
Liu, T. et al. Research of potential geograpical distribution of Picea purpurea based on GIS and MaxEnt under different climate conditions. Acta Bot. Boreal-Occident Sin. 42(3), 0481–0491 (2022).
Zhang, K., Yao, L., Meng, J. & Tao, J. MaxEnt modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 634, 1326–1334 (2018).
Levins, R. Evolution in Changing Environments: Some Theoretical Explorations (Princeton University Press, 1968).
Warren, D. L., Glor, R. E. & Turelli, M. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62, 2868–2883 (2008).
Schoener, T. W. The Anolis lizards of Bimini: Resource partitioning in a complex fauna. Ecology. 49, 704–726 (1968).
An, Q. et al. Predicting medicinal resources in Ranunculaceae family by a combined approach using DNA barcodes and chemical metabolites. Phytochem. Lett. 50, 67–76 (2022).
Chen, J. et al. Prediction of the taxonomical classification of the Ranunculaceae family using a machine learning method. New J. Chem. 46(11), 5150–5161 (2022).
Kim, S. et al. PubChem substance and compound databases. Nucleic Acids Res. 44(D1), D1202–D1213 (2016).
Willett, P. The calculation of molecular structural similarity: Principles and practice. Mol. Inform. 33(6–7), 403–413 (2014).
Chen, B. et al. Relating chemical structure to cellular response: an integrative analysis of gene expression, bioactivity, and structural data across 11,000 compounds. CPT Pharmacomet. Syst Pharm. 4(10), 576–584 (2015).
Li, K. Q. et al. Effect of temperature on physiologic indexes, bulb yielding and total alkaloids contents of Fritillaria cirrhosa. Zhongguo Zhong Yao Za Zhi 33(16), 1948–1951 (2008).
Banla, E. M. et al. Molecular and phenotypic diversity of groundnut (Arachis hypogaea L.) cultivars in Togo. Physiol. Mol. Biol. Plants 26(7), 1489–1504 (2020).
Teka, A., Asfaw, Z., Demissew, S. & Van Damme, P. Medicinal plant use practice in four ethnic communities (Gurage, Mareqo, Qebena, and Silti), south central Ethiopia. J. Ethnobiol. Ethnomed. 16(1), 27 (2020).
Song, S. et al. Structure basis of the caffeic acid O-methyltransferase from Ligusiticum chuanxiong to understand its selective mechanism. Int. J. Biol. Macromol. 194, 317–330 (2022).
Tamura, K. & Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 10, 512–526 (1993).
Mushineni, A. et al. First report of pearl millet bacterial leaf blight caused by Pantoea stewartii subspecies indologenes in India. Plant Dis. 105, 3736 (2021).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549 (2018).
Hassan Almukhattin, M. S. et al. Prevalence and predictors of Albuminuria in type 2 diabetes mellitus patients: A cross-sectional study from the United Arab Emirates. Curr. Diabetes Rev. 20, 22. https://doi.org/10.2174/1573399819666221014093352 (2022).
Liu, L. et al. Simulation of potential suitable distribution of original species of Fritillariae Cirrhosae Bulbus in China under climate change scenarios. Environ. Sci. Pollut. Res. 29, 22237–22250 (2022).
Hülber, K. et al. Uncertainty in predicting range dynamics of endemic alpine plants under climate warming. Glob. Change Biol. 22(7), 2608–2619 (2016).
Thuiller, W. Editorial commentary on “BIOMOD—optimizing predictions of species distributions and projecting potential future shifts under global change”. Glob. Change Biol. 20(12), 3591–2 (2014).
Zhou, X. et al. Regionalization of habitat suitability of Masson’s pine based on geographic information system and Fuzzy Matter-Element Model. Sci. Rep. 6, 34716 (2016).
Igawa, T. K., Toledo, P. M. & Anjos, L. J. S. Climate change could reduce and spatially reconfigure cocoa cultivation in the Brazilian Amazon by 2050. PLoS ONE 17(1), e0262729 (2022).
Pavlović, L., Stojanović, D., Mladenović, E., Lakićević, M. & Orlović, S. Potential elevation shift of the European beech stands (Fagus sylvatica L.) in Serbia. Front. Plant Sci. 10, 849 (2019).
Brown, J. L. & Yoder, A. D. Shifting ranges and conservation challenges for lemurs in the face of climate change. Ecol. Evol. 5(6), 1131–42 (2015).
Gao, Y., Wang, C., Song, B. & Du, F. Corolla retention after pollination facilitates the development of fertilized ovules in Fritillaria delavayi (Liliaceae). Sci. Rep. 9(1), 729 (2019).
Marković, M., Trifunović Momčilov, M., Uzelac, B., Jevremović, S. & Subotić, A. Bulb dormancy In vitro-Fritillaria meleagris: Initiation, release and physiological parameters. Plants 10(5), 902 (2021).
Hsiung, H. Y. et al. Local climate heterogeneity shapes population genetic structure of two undifferentiated insular Scutellaria species. Front. Plant Sci. 8, 159 (2017).
Jia, Y. et al. Evolutionary legacy of a forest plantation tree species (Pinus armandii): Implications for widespread afforestation. Evol. Appl. 13(10), 2646–2662 (2020).
Wang, J. J., Cao, B., Bai, C. K., Zhang, L. L. & Che, L. Potential distribution prediction and suitability evaluation of Fritillaria cirrhosa D. Don based on Maxent modeling and GIS. Bull. Bot. Res. 34, 642–649 (2014) (in Chinese).
Luo, M., Gao, J., Liu, R., Wang, S. & Wang, G. Morphological and anatomical changes during dormancy break of the seeds of Fritillaria taipaiensis. Plant Signal Behav. 18(1), 2194748 (2023).
Zhang, Y. J., Wang, H. M. & Zhou, R. L. A preliminary study on the ecological characteristics and germinating law of the seeds of Fritillaria yuzhongensis in the maxian mountain region of Gansup province. Acta Ecol. Sin. 12(2), 155–160 (1992) (In Chinese).
Marković, M. et al. Breaking the dormancy of snake’s head Fritillary (Fritillaria meleagris L.) in vitro bulbs-part 2: Effect of GA3 soaking and chilling on sugar status in sprouted bulbs. Plants 9(11), 1573 (2020).
Wan, C. et al. How does the environment affect wheat yield and protein content response to drought? A meta-analysis. Front. Plant Sci. 13, 896985 (2022).
Thabet, S. G., Moursi, Y. S., Karam, M. A., Graner, A. & Alqudah, A. M. Genetic basis of drought tolerance during seed germination in barley. PLoS ONE 13(11), e0206682 (2018).
Funk, J. L., Larson, J. E. & Ricks-Oddie, J. Plant traits are differentially linked to performance in a semiarid ecosystem. Ecology 102(5), e03318 (2021).
Płażek, A. et al. Seed hydropriming and smoke water significantly improve low-temperature germination of Lupinus angustifolius L. Int. J. Mol. Sci. 19(4), 992 (2018).
Jaikumar, N. S. et al. Nucleic acid damage and DNA repair are affected by freezing stress in annual wheat (Triticum aestivum) and by plant age and freezing in its perennial relative (Thinopyrum intermedium). Am. J. Bot. 107(12), 1693–1709 (2020).
Takahashi, D., Uemura, M. & Kawamura, Y. Freezing tolerance of plant cells: from the aspect of plasma membrane and microdomain. Adv. Exp. Med. Biol. 1081, 61–79. https://doi.org/10.1007/978-981-13-1244-1_4 (2018).
Zuo, Y. W. et al. Contrasting responses of Rhizosphere fungi of Scutellaria tsinyunensis, an endangered plant in southwestern China. Microbiol. Spectr. 10(4), e0022522 (2022).
Nechols, J. R. et al. Effect of temperature on plant resistance to arthropod pests. Environ. Entomol. 49(3), 537–545 (2020).
Zettlemoyer, M. A., Schultheis, E. H. & Lau, J. A. Phenology in a warming world: differences between native and non-native plant species. Ecol. Lett. 22, 1253–1263 (2019).
Kondo, T., Mikubo, M., Yamada, K., Walck, J. L. & Hidayati, S. N. Seed dormancy in Trillium camschatcense (Melanthiaceae) and the possible roles of light and temperature requirements for seed germination in forests. Am. J. Bot. 98(2), 215–226 (2011).
Polic, D. et al. Linking large-scale genetic structure of three Argynnini butterfly species to geography and environment. Mol. Ecol. 31(16), 4381–4401 (2022).
Calixto-Rojas, M., Lira-Noriega, A., Rubio-Godoy, M., Pérez-PoncedeLeón, G. & Pinacho-Pinacho, C. D. Phylogenetic relationships and ecological niche conservatism in killifish (Profundulidae) in Mesoamerica. J. Fish Biol. 99(2), 396–410 (2021).
Chozas, S., Chefaoui, R. M., Correia, O., Bonal, R. & Hortal, J. Environmental niche divergence among three dune shrub sister species with parapatric distributions. Ann. Bot. 119(7), 1157–1167 (2017).
Mallet, J. & Mullen, S. P. Reproductive isolation is a heuristic, not a measure: A commentary on Westram. J. Evol. Biol. 35(9), 1175–1182 (2022).
Jiao, N., Song, X., Song, R., Yin, D. & Deng, X. Diversity and structure of the microbial community in rhizosphere soil of Fritillaria ussuriensis at different health levels. PeerJ 10, e12778 (2022).
Xu, Y. et al. Have China’s national forest reserves designated since 1990 conserved forests effectively?. J. Environ. Manage. 306, 114485 (2022).
Funding
This study was partially funded by the Sichuan Science and Technology Program (No. 2018SZ0061), the Sichuan Administration of TCM program (No. 2020JC0128 and 2021MS116), and the Fundamental Research Funds for the Central Universities (No. 2682021ZTPY017 and 2682022ZTPY053).
Author information
Authors and Affiliations
Contributions
D.H., Q.A. and S.H. performed the experiments. D.H., Q.A., S.H., G.T., H.Q., and Y.C., analyzed the data. J.Z. and H.L. contributed to the materials and analysis tool, wrote and were responsible for the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, D., An, Q., Huang, S. et al. Biomod2 modeling for predicting the potential ecological distribution of three Fritillaria species under climate change. Sci Rep 13, 18801 (2023). https://doi.org/10.1038/s41598-023-45887-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-45887-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.