Abstract
A new clown beetle species, Bacanius neoponerae, is described from Mexican nests of the arboreal ponerine ant Neoponera villosa found in the tank bromeliad Aechmea bracteata. Adult beetles were found in brood chambers or inner refuse piles, but also outside the ant nests, in decaying organic matter between the bromeliad leaves. No direct interactions between ants and microhisterid beetles could be observed. Several lines of evidence suggest a close relationship either with the ants, specific microhabitats within the ant nests or the bromeliads. Sample site elevation, colony size, monthly rainfall and collecting site were the main variables predicting the association. Almost half of the N. villosa colonies were associated with the microhisterids, and larger colonies favored their presence, especially during the driest months of the year. Two specimens were found in a nest of another ant species, Camponotus atriceps, also inhabiting A. bracteata. The new species is the seventh of the genus Bacanius reported from Mexico. This is the second time a species of this genus is associated with ants, and the fourth record of a histerid beetle cohabiting with ponerine ants. The small size of these beetles and their very protective body structure may facilitate their cohabitation with such aggressive hosts.
Similar content being viewed by others
Introduction
Ant colonies and the resources stored in their nests are targeted by a myriad of organisms that primarily parasitize ants and their brood or their social structure, benefiting from suitable environmental conditions and enemy-free space1,2,3. Symbionts in ant nests establish obligate or facultative relationships with ants and range from highly integrated species, adapted in many ways to live with ants, to poorly integrated species that attempt to escape from ants and rely on fleetness, or species that are very small relative to their host and go unnoticed3,4,5,6,7.
Histeridae (Coleoptera) is a very diverse family, both ecologically and morphologically, with over 4700 extant valid species and subspecies distributed worldwide8,9. There are ten or eleven subfamilies currently recognized, depending on author10. Histerids, commonly known as clown beetles, are primarily generalist predators of immature and adult insects. They are associated with a wide range of habitats and with a variety of substrates including dung, carrion, leaf litter, or other decaying plant and organic matter where their prey are present11,12. Multiple histerid lineages have evolved to establish obligate symbioses with various animals, for example, as obligate inhabitants of bird and mammal nests and burrows13,14. One species has been found to be a parasite of the cocoons of large Neotropical tarantulas15 and many species are frequently found inhabiting social insect nests and hives13,16,17. These obligate inquilines often exhibit distinctive defensive morphological modifications as well as behavioral and chemical adaptations that facilitate their symbioses2,12,14,18. Clown beetles are characterized by their body shape and structures that protect themselves from attack, such as trichomes and specialized cuticular depressions (fossae) where their appendages can be folded. Obligate myrmecophilous and termitophilous histerids from the subfamily Haeteriinae have long attracted attention18,19,20,21, but myrmecophilous representatives of other subfamilies have remained understudied. Details of the natural history of most species are scarce. Most described species are known only from the original description and were collected through flight interception, and in some cases with carrion, fruit, or dung baited traps which can provide an indication of their food preferences.
Specifically, there is a lack of information about histerid distribution and ecology in the Neotropics22. Here, we describe a new species of Bacanius (Dendrophilinae: Bacaniini) and provide information on its ecology and association with ants in southeastern Mexico. Currently, the Bacaniini tribe includes 170 species, most of which are from tropical regions23 (N. Degallier, unpubl.). According to Kovarik and Caterino13, Bacanius is a worldwide distributed genus of microhisterid found in rotten wood, under bark, and in forest litter. Six subgenera of Bacanius are recognized and 75 extant species are catalogued8,9. Of the 63 genera and 240 species of histerids reported from Mexico8,24,25,26,27,28, only six species of Bacanius have been reliably cited from this country: B. (Gomyister) gomyi Yélamos from Xalapa, Veracruz29; B. (Gomyister) subcarinatus Wenzel & Dybas from Cordoba, Veracruz22; B. (Gomyister) montanus Mazur & Sawoniewicz and B. (Gomyister) irlanda Mazur & Sawoniewicz from Lagunas de Montebello and Finca Irlanda, Chiapas30; B. (s. str.) pusillus Wenzel (this species is no more considered to be a Degallierister: Degallier, unpublished) from Penuela, Tierra Blanca and Tezonapa, Veracruz, and Tamazunchale, San Luis Potosi31; and B. (s. str.) scalptus Lewis from Cordoba, Veracruz22. Additionally, an unidentified necrophilous species of Bacanius was reported from Gómez Farías, Jalisco32 but may correspond to one of the species cited above. Biological data concerning Bacanius species remain scarce; however, specimens have been collected within superficial soil and decaying rotten logs where, according to Kovarik and Caterino13, they probably feed on fungal spores, and at least one ‘necroxenous’ species (species accidentally occurring on carrion) was attracted to carrion-baited traps32. To date, association with ants has been reported on a single occasion, involving a single male specimen of an undetermined species of Bacanius found in a sample of soil and workers from a refuse deposit of a bivouac of Eciton dulcium crassinode Borgmeier in Barro Colorado Island, Panama33.
Material and methods
Sampling and collection sites
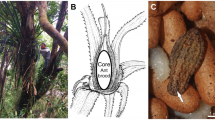
The beetles were found in association with the nests of the Neotropical arboreal ant Neoponera villosa (Fabricius) (Hymenoptera, Formicidae, Ponerinae) (Fig. 1A) which, in the southern part of the Yucatan Peninsula, Mexico, nests almost exclusively in the epiphytic tank bromeliad Aechmea bracteata (Swartz) Grisebach (Poales, Bromeliaceae)34,35 (Fig. 1B). Individuals of the new microhisterid species were collected along with the ants as part of a larger project focused on the invertebrate fauna associated with N. villosa35. A total of 82 N. villosa colonies were collected between January 2016 and January 2019, from 3 zones (Nuevo Becal and Ejido Blasillo in the state of Campeche and Sian Ka’an Biosphere Reserve in Quintana Roo), in the southern part of the Yucatan Peninsula (Fig. 1C). The climate of the region is of the “Aw” type according to García36, i.e., warm, sub-humid, with rainfall during the summer months and drought in March–April. The arrival of cold fronts from mid-November to February (‘cold’ season) are locally known as “Nortes” (associated with northerly winds) and provide occasional winter precipitation with daily minimum temperatures of 11 °C. Vegetation type of the sampling zones corresponds to tropical forest in different successional stages37. Different numbers of colonies were collected per zone and per season due to differences in access facilities and obtaining collection permits. Twenty-five N. villosa colonies were collected in the Sian Ka’an Biosphere Reserve (10, 9, and 6 colonies during the dry, the rainy and the cold season, respectively); 24 in Nuevo Becal (10, 4 and 10) and 29 in Ejido Blasillo (12, 6 and 11), accounting for a total of 32 colonies sampled during the dry season, 19 during the rainy season, and 27 during the cold season.
Ant material identification
According to the keys and descriptions available for the complex of species of the Neoponera foetida group38,39,40, the Neoponera ants inhabiting most of the epiphytes collected in our study (82 of 84) run to N. villosa: anterior face of petiole almost straight, vertical, posterior face broadly convex, base of legs reddish, and anterior margin of clypeus concave medially (see Fig. S1 in41). Our initial identification was confirmed by J.H.C. Delabie (Laboratorio de Mirmecologia CEPEC/CEPLAC, Itabuna-BA, Brazil) in an earlier work42 and further DNA extraction and barcoding35,43 (GenBank accession numbers MK779595, MK779597, MK779600, MK779602 and MK779604; see more specifically Fig. S1 in43) also showed that the DNA sequences of ants used in our study clustered with all the other publicly available molecular data for N. villosa. For the two epiphytes inhabited by Camponotus, ant identification was carried out using taxonomic keys or descriptions44 and online databases such as AntWeb45 and specimens run to C. atriceps (Smith) (Formicinae: Camponotini): large size (about 10 mm or more), propodeum narrow and elongate, clypeus with median longitudinal carina, head relatively short and broad, scapes and legs with abundant long, brown or golden, erect setae, mesosoma generally densely hairy.
Neoponera villosa is an opportunistic cavity breeder; colonies are established in dead wood, live trees and in the central portion of A. bracteata bromeliads where ants build chambers separated by septa made of small debris to house the brood. Colonies were essentially polygynous and contained a mean number of 3 dealate queens, 98 workers, 42 pupae and 41 larvae35. Workers measure 12–13 mm46 and are very aggressive, with a painful sting. All invertebrates present both in the core of the bromeliads (the ant nests and their immediate border) and those located in the periphery of the nests, including the ants and their brood, were preserved in 96% ethanol, and identified to the lowest possible taxon with available keys. An additional colony of N. villosa nesting in a dead branch of a live tree was collected on October 11th, 2022, within the campus of El Colegio de la Frontera Sur, Chetumal, Quintana Roo, and inspected for the presence of any inquiline, particularly for Coleoptera. Two colonies of another ant species (C. atriceps), also nesting in A. bracteata bromeliads were collected on March 19th, 2018, at Ejido Blasillo and were also inspected for myrmecophiles. Field sampling complied with the current laws of Mexico (collection permit FAUT-0277 from Secretaría del Medio Ambiente y Recursos Naturales—Dirección General de Vida Silvestre granted to GP-L).
Morphological and molecular studies
Clown beetles were examined using a Leica MZ8 dissecting stereomicroscope (6.3–100×) and a JEOL-JSM6010 scanning electron microscope (SEM). For SEM analysis, specimens were dehydrated in a graded ethanol series from 70 to 100% and let to dry at room temperature or soaked twice in hexamethyldisilazane (© Sigma Aldrich, St Luis, MO, USA) and dry evaporated; they were then fixed onto stubs, and sputter coated with gold before observation. Approximate measurements and ratios of measurements were obtained through a 0.01 mm graduated object micrometer or using SEM images. Photos of the specimens were taken on the imaging station of the Entomology laboratory of the Muséum National d'Histoire Naturelle, Paris (MNHN). Photos were stacked with Zerene Stacker 1.04 (Rik Littlefield, Zerene Systems LLC) and processed in Photoshop®. Methods for specimen dissection, illustration preparation, terminology, body part measurement conventions and abbreviations follow Degallier and Tishechkin47.
Five specimens were used for DNA extraction. Due to the small size of the histerids, extraction was performed on the whole specimens using a standard glass fiber method48. Polymerase chain reactions were performed to amplify the mitochondrial cytochrome c oxidase subunit I (COI) gene, using the ZplankF1_t1 and ZplankR1_t1 primers originally developed for zooplankton49 that work with good success in most groups of invertebrates50. PCR protocols follow Montes-Ortiz and Elías-Gutiérrez51. The PCR products were visualized on a 2% agarose gel (E-Gel 96 Invitrogen), and positive PCR products were sent for sequencing to Eurofins Genomics, LLC, Kentucky, USA. Sequences were edited using CodonCode v. 3.0.1 (CodonCode Corporation, Dedham, MA, USA) and uploaded to the Barcode of Life Database (BOLD, boldsystems.org, dataset DS-HISTERPY) and to GenBank (www.ncbi.nlm.nih.gov/genbank/; Accession Numbers: OQ706395-98). Specimens were recovered after the lysis step from the glass fiber filter plate and preserved in 96% ethanol.
We added the obtained sequences to a database of published COI barcodes. The resulting matrix included 136 sequences for a total of 22 putative species in five genera belonging to the Dendrophilinae (Table S1). For the ID-tree, the alignment was carried out using MUSCLE52 with default settings. This expanded matrix was used to obtain a consensus tree inferred by using the Maximum Likelihood method based on the Tamura-Nei model53. The model was selected after the lowest Bayesian Information Criterion (BIC) from the matrix. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood approach, and then selecting the topology with superior log likelihood value. Support was estimated using 500 bootstrap replications. All analyses were conducted using the Mega V7.0 software.
Ecology
We further gathered and analyzed data for some environmental and host colony predictor variables that may influence the presence or absence of the histerid in N. villosa nests. A classification tree was built with the “repart” library54, with bagging as the ensemble method. A total of 78 colonies were considered for statistical analyses (Nuevo Becal: 24 colonies, Ejido Blasillo: 29 colonies, and Sian Ka’an Biosphere Reserve: 25 colonies); four records were not considered as they were from incomplete colonies (only a few workers and no brood). The data set (n = 78 records) considered the following predictors: (a) the sampling zone, altitude, mean monthly temperature, and total monthly precipitation; (b) the colony size and type of colony (queenright or queenless). The Random Forest algorithm (see55) establishes the outcome based on the predictions of the decision trees. It predicts by taking the average or mean of the output from various trees, therefore increasing the number of trees increases the precision of the outcome. Significant predictor variables were further explored, and levels were compared with either a parametric test after the Shapiro–Wilk test was used to check if data were normally distributed, or with a non-parametric test. All analyses were performed using R Statistical Software (v4. 1.256).
Vouchers and nomenclatural act
Extracted specimens of the new species were deposited as vouchers (Catalog Numbers C-2570–2574) along with N. villosa workers, at the Arthropoda Collection of El Colegio de la Frontera Sur (ECO-CH-AR) in Chetumal, Quintana Roo, Mexico. The collections where type-material of the new species was deposited are cited in square brackets by the following acronyms:
BMNH: British Museum (Natural History), London, UK.
CHND: Nicolas Degallier collection, Paris, France.
CHYG: Yves Gomy collection, Nevers, France, then Zoologische Staatssammlung München, Munich, Germany.
ECO-CH-AR: Artropoda Collection of El Colegio de la Frontera Sur, Chetumal, Quintana Roo, Mexico.
FMNH: Field Museum of Natural History, Chicago IL, USA.
MNHN: Muséum National d’Histoire Naturelle, Paris, France.
The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature, and hence the new name contained herein is available under that Code from the electronic edition of this article. This published work and the nomenclatural act it contains have been registered in ZooBank, the online registration system for the ICZN (https://zoobank.org/urn:lsid:zoobank.org:pub:653C11BD-A9BD-4580-95EF-AB096AA1250E).
Results
Description of the new species
Dendrophilinae Reitter, 1909
Bacaniini Kryzhanovskij & Reichardt, 1976
Bacanius neoponerae Degallier & Gomy, new species [https://zoobank.org/urn:lsid:zoobank.org:act:C618E6B3-C8A5-4CB9-B6D3-BB02674ED4F8]
(Figs. 2, 3, 4, 5 and 6, S1, S2)
SEM micrograph of the head (fronto-ventral view) of Bacanius neoponerae n. sp. showing the mouth parts, the medial part of prosternum, and the prothoracic legs. ac, antennal club; as, antennal scape; ce, compound eye; cl, clypeus; lbi, labium; lbp, labial palp; lbr, labrum; md, mandible; mxp, maxillary palp; pstl, prosternal lobe; ptl, prothoracic legs. Photos: M. Elías-Gutiérrez & G. Pérez-Lachaud.
SEM micrographs of Bacanius neoponerae n. sp.: left eye and antenna (A), mouth parts, prosternal lobe, and setigerous labrum (B), mentum and palpi (C), left mandible with bifid apex (D). ga, galea; mdba, mandible bifid apex; other abbreviations as in Fig. 4. Photos: M. Elías-Gutiérrez & G. Pérez-Lachaud.
Type locality. Ejido Blasillo, Campeche, Mexico
HOLOTYPE (sex undetermined, due to the difficulty of safely dissecting such a tiny specimen): Mexico, Campeche, Ejido Blasillo, [Nest 72] 18° 07′ 29.0100″ N, 89° 19′ 53.8121″ W, 261 m asl, 19-III-2018 [MNHN].
PARATYPES: Mexico, Campeche, Ejido Blasillo: 2 ex., [Nest 21] 18°07′14.6172″ N, 89° 19′ 20.4649″ W, 247 m asl, 11-III-2017 [MNHN]; 2 ex., idem [FMNH]; 2 ex., idem [CHYG]; 2 ex., idem, [Nest 26] 18° 07′ 13.6056″ N, 89° 19′ 47.7929″ W, 263 m asl, 10-VI-2017 [CHND]; 1 ex., idem [CHYG]; 1 ex., idem [Nest 27] 18° 07′ 13.6056″ N, 89° 19′ 47.7929″ W, 263 m asl [ECO-CH-AR, C-2571]; 1 ex., idem [CHYG]; 1 ex., idem, [Nest 71] 18° 07′ 27.6888″ N, 89° 19′ 52.7064″ W, 261 m asl, 19-III-2018 [CHND]; 1 ex., idem [ECO-CH-AR, C-2572]; 2 ex., idem, [Nest 72] 18° 07′ 29.0100″ N, 89° 19′ 53.8121″ W, 261 m asl [CHND]; 1 ex., idem [ECO-CH-AR, catalog number C-2574]; 1 ex., idem [Nest 87] 18° 07′ 15.8448″ N, 89° 19′ 50.8152″ W, 238 m asl, 16-I-2019 [CHYG]; 2 ex., idem, 18°07′14.6172″ N, 89° 19′ 20.4649″ W, 247 m asl, 11-III-2017, in material between the bromeliad leaves, outside the ant nests [CHND]; Mexico, Quintana Roo, Sian Ka'an: 1 ex., [Nest 39] 19° 42′ 21.6000″ N, 87° 49′ 46.1191″ W, 12 m asl, 16-VIII-2017 [MNHN]; 2 ex., idem, [Nest 43] 19° 42′ 28.3716″ N, 87° 49' 37.2919″ W, 15 m asl [CHND]; 1 ex., idem [ECO-CH-AR, C-2570]; 2 ex., idem [CHYG]; 2 ex., idem [Nest 50] 19° 43′ 10.5492″ N, 87° 48' 43.6449″ W, 24 m asl, 21-X-2017 [BMNH]; 1 ex., idem [Nest 52] 19° 42′ 50.8212″ N, 87° 49′ 08.7931″ W, 12 m asl, [CHND]; 3 ex., idem [Nest 78] 19° 40′ 41.5560″ N, 87° 51′ 52.9818″ W, 10 m asl, 12-IV-2018 [CHND]; 3 ex., idem, in material in the bromeliad leaves, outside the ant nests [CHND]; Mexico, Campeche, Nuevo Becal: 2 ex., [Nest 55] 18° 36′ 39.3552″ N, 89° 16′ 15.5372″ W, 239 m asl, 4-XII-2017 [CHYG]; 1 ex., idem [Nest 56] 18° 36′ 16.9956″ N, 89° 16′ 41.4997″ W, 233 m asl [ECO-CH-AR, C-2573]; 1 ex., idem [Nest 60] 18° 36′ 32.4864″ N, 89° 16′ 42.9678″ W, 238 m asl, 9-III-2018 [MNHN]; 1 ex., idem [Nest 66] 18° 36′ 38.3112″ N, 89° 16′ 34.6558″ W, 231 m asl [CHND]; 1 ex., idem [Nest 68] 18° 36′ 38.6676″ N, 89° 16′ 32.2050″ W, 228 m asl [CHND].
Habitus (Figs. 2, 3). Length (pronotum + elytra): (0.92–)0.99(–1.11) mm. Maximum width: (0.71–)0.77(–0.85) mm. Maximum thickness: (0.51–)0.58(–0.65) mm, (1.25–)1.29(–1.33) times as long as wide, (1.6–)1.7(–1.86) times as long as thick (N = 12). Body elongated oval, convex dorsally. Dorsal surface, at least on the pronotum, with short setigerous simple punctation.
Head (Figs. 2B, 3A, 4, 5A–D). Eyes present. Labrum setigerous. Frons smooth between the punctures. Punctuation of the clypeus equal to or smaller than that of the frons. Apex of mandibles bifid. Antennal scape not dilated. Antennal club without separate sutures. Margins of the forehead above the eyes diverging forward.
Pronotum (Figs. 2, S1). Punctuation (diameter about 0.01 mm) not joined by grooves, punctures well marked, 1.5 to 2 diameters apart, coarser on the disk than laterally. Anterior angles of the pronotum with a pore. Base without a row of distinct punctures. Scutellum invisible. Pronotal stria close to the anterior margin (less than 0.018 mm) and not crenulate.
Elytra (Figs. 2A, C, S1, S2). Punctuated, without a common prebasal zone punctuated differently on either side of the suture; presence of a pore to the outer third of the base. Punctures not connected by grooves, uniform and smaller than those on the pronotum. Disc without transverse stria. Outer half of the elytra with 2 striae (dorsal and subhumeral/marginal), of which the dorsal and subhumeral are at least twice as distant at the base as apically. Dorsal stria not curved along the base, being shortened basally, reaching the apex but not the sutural angle backwards. Subhumeral stria complete, not united to the dorsal stria apically; elytra without additional basal striae. Sutural stria absent. Punctuation of the apex non-strigate. Epipleura impunctate, epipleural stria present, sinuate and complete. A pore is present on the basal quarter and ventrally to the stria.
Pygidium (Figs. 3A, C, 6A, S2). Pygidium less strongly punctuated than elytra, the punctures rounded, 2–4 diameters apart.
Sterna (Figs. 3, 4, 5 B, 6A, S2). Prosternal lobe with an anterior marginal stria, slightly emarginated in the middle, punctuated only on its anterior half, without irregular longitudinal striation. Alae of the prosternum incised to receive the antennal funicle. Prosternal striae converging forward and divergent backwards. Prosternal carina punctuated. Mesosternum without striae or sulci, with a transverse line of larger points forward, with the marginal stria laterally in continuity with the lateral metasternal stria. Meso-metasternal disc smooth or finely punctuated (diameter of punctures ≤ 0.009 mm). Meso-metasternal stria absent, the darker trace of the suture visible and curved backwards. Disc of the metasternum completely smooth, without fine apical median keel, sometimes with a low preapical tubercle, without lateral longitudinal rows of punctures, lateral metasternum punctuated, with only a lateral stria and an "S"-shaped short mesopostcoxal stria on each side. Mesopostcoxal area punctuated and mesepimeron punctuated, the latter with a stria forming a very open angle. Metepimeron impunctate. First abdominal ventrite with rounded punctuation, as large or larger than that of the lateral metasternum, without a row of distinct punctures along its anterior margin, with one lateral stria. Postmetacoxal stria absent.
Legs (Figs. 2B, 3A, B, 4, S2). Protibiae with 1–2 denticles along the outer edge, punctuated on their ventral side. Protarsi without modified setae in addition to simple ones. Profemorae ventrally with a microstriate area.
Male genitalia (Figs. 6B, C). Parameres of the aedeagus curved ventrally at the apex, with two preapical setae ventrally but without apical ones.
Distribution. Known only from Mexico.
Biological information. Specimens of the new species were found associated with N. villosa ants nesting in A. bracteata bromeliads. In addition, two specimens were found in a colony of another ant species, C. atriceps, also inhabiting A. bracteata.
Etymology. The name relates to the host of the new species, belonging to the ponerine ant genus Neoponera.
Remarks. To our knowledge, B. neoponerae is part of the Neotropical Bacaniini (Dendrophilinae) characterized by a length (pronotum + elytra) greater than 0.9 mm, with invisible scutellum, no prescutellar stria at the base of the pronotum, the anterior stria of the pronotum close to the margin and not crenulate, the first dorsal stria of the elytra shortened forward and reaching the sutural angle apically, no sutural or transverse elytral striae, and the pronotal punctuation not joined by furrows. According to Wenzel's key31, the Neotropical species that most resemble B. neoponerae are B. convergens Schmidt, described from southeastern Brazil, and B. subcarinatus from Veracruz, Mexico. Bacanius neoponerae is distinguished from these species by the following combination of characters: prosternal lobe punctuated, without longitudinal striation (with irregular longitudinal striation in B. convergens); short setigerous punctuation (glabrous in B. convergens); punctuation of the clypeus equal to or smaller than that of the forehead (larger in B. convergens); visible meso-metasternal suture (invisible in B. convergens); elytra with a pore situated at the outer third of the base (at the middle of the elytral base in B. subcarinatus); elytral punctuation uniform (stronger along the suture in B. subcarinatus); dorsal stria of elytra shortened at the base (complete or barely shortened in B. subcarinatus); pygidium less strongly punctuated than the elytra (more strongly punctuated than the elytra in B. subcarinatus).
Molecular analyses
Out of the five individuals extracted, four yielded CO1 fragment sequences with a length of 600 bp. The maximum likelihood phylogenetic analysis placed B. neoponerae in a clade containing the only other Bacanius species for which COI sequences have been published (B. punctiformis) but clearly separated from this species and from others in the same subfamily (Fig. 7) confirming its status based on morphology. DNA barcoding supported the generic placement of the new species. Pairwise sequence distances between the new species and B. punctiformis ranged from 2.43 to 2.49%. No genetic variability was found in COI sequences. The four sequences obtained here were grouped in a separate clade and they are similar, with pairwise differences from 0.02 to 0.1%.
Condensed ID-Tree. The evolutionary history was inferred by using the Maximum Likelihood method based on the Tamura-Nei53 model (n = number of sequences in each branch).
Ecology
A total of 173 specimens of B. neoponerae, all adults, were retrieved from the epiphytic bromeliad A. bracteata sheltering N. villosa colonies. Globally, histerids were associated with 43.6% (34/78) of the colonies: 10 of the colonies sampled in Sian Ka’an, 8 from Nuevo Becal, and 16 from Ejido Blasillo. Ninety-seven specimens (56.1%), observed in more than two thirds of these colonies (24/34) (range: 1–18 individuals per colony), were found in the central part of the bromeliads where the ant nests were established, mainly in the detritus inside the nest chambers. Seventy-six additional specimens (43.9%) were also found in the bromeliads inhabited by N. villosa, but outside the ant nest, among the detritus accumulated between the peripheral leaves of A. bracteata. Wherever the location of the histerid beetles within the bromeliad, no direct interaction between them and the ants or their brood could be observed.
The Random Forest classification tree showed that the most important variables predicting the association of B. neoponerae with the N. villosa colonies were altitude of the sampling site, followed by colony size, monthly rainfall and collecting zone in decreasing order of importance (Fig. S3). Histerids were associated with ant colonies year-round. Thirty-eight percent of the colonies collected during the dry season, 42% of those collected during the rainy season, and 52% of those collected during the cold season harbored histerids. However, quantitatively, half the histerids were recovered during the dry season. During this season, colonies associated with histerids contained a higher mean number of specimens (7.73 ± 2.5 (n = 12) than during the rainy and cold seasons (5.2 ± 1.8 (n = 8) and 3.3 ± 0.5 (n = 14), respectively) (Fig. S4); however, figures varied widely and differences in abundance were non-significant (Kruskal–Wallis test, H = 0.04, n.s.). At the spatial scale, colonies collected at Ejido Blasillo (i.e., at a relatively higher altitude) were more frequently encountered in association with histerids and the beetles were more numerous. A total of 90 individuals were collected at Ejido Blasillo, 64 in Sian Ka’an and 19 at Nuevo Becal from 29, 25, and 24 colonies, respectively. In addition, larger colonies favored the presence of B. neoponerae: the mean colony size of the colonies that were associated with histerids was significantly higher (227 ± 27 adult ants), than that of the colonies without histerids (173 ± 15 adult ants) (Student t test, p = 0.034). The colony size frequency distribution of both colonies sheltering histerids and those without beetles followed a normal distribution (Wwith = 0.902, p < 0.05; Wwithout = 0.468, p < 0.05).
Discussion
Histeridae are robust beetles characterized, in general, by a broadly globular body shape and protective morphological structures complemented by the ability to retract the head and appendages into anatomical cavities, which further protect against attack5,16,57,58,59. According to Parker and Kronauer60, such a protective anatomical ground plan of histerids and some other beetles such as aleocharine staphylinids, predisposed these beetles to myrmecophily. Many clown beetle species, primarily in the subfamilies Haeteriinae and Chlamydopsinae, are known to be associated with ants in diverse ways and several are entirely ant-dependent, displaying complex strategies to integrate the host nests and cope with worker aggressiveness16,59,61. Less is known, however, about myrmecophilous species belonging to other subfamilies, although associations with ants have been reported on various occasions13,62. Species of the tribe Bacaniini (Dendrophilinae) have rarely been suspected of being associated with ants. A species of the genus Australanius, A. verrucosus Gomy, was described from material preserved along with two ant workers63 of the dolichoderine genus Iridomyrmex (determination Georg Fischer, pers. comm., and J-PL), and there is only one previous report of an unidentified species of Bacanius recorded with the army ant Eciton dulcium crassinode (Dorylinae)33. However, the nature of the latter association was not clearly determined and may be facultative since only one specimen (a male) was collected from a refuse deposit in a bivouac and the species may casually exploit resources there, as has been reported for species in six histerid subfamilies (Abraeinae, Dendrophilinae, Haeteriinae, Histerinae, Saprininae, and Tribalinae) collected in the refuse piles of various ant genera such as Atta, Acromyrmex, Eciton, Pheidole, and Solenopsis24,25,33,62.
Here we describe a new species of the tribe Bacaniini and provide ecological information on its association with the arboreal ant N. villosa. Ecological information on histerid beetles is rather scarce and biological information on Bacanius species almost absent. Current records include subcortical tree samples and leaf litter samples, including for the type species B. tantillus LeConte, which was described from material collected from under bark and in fungi64. According to Kovarik65, the ancestral feeding habits of most adult histerids is predation; however, adults of several lineages feed on fungal spores or microbiota coatings, and Pražák21 mentions members of the genus Bacanius as spore-feeding species, but without giving any precise reference. Most spore-feeding taxa are also capable of some predation, and their larvae are predatory as in other histerid beetles65, but specialized species have modified maxillary galea with setae that serve as combs for gathering spores65. Interestingly, the reproductive rate of these species is highest under conditions that are also ideal for fungal fructification (heat, humidity)21 such as those found within the bromeliads. Histerids also appear to be preadapted to exploiting ephemeral systems: there are only two larval instars, and their large eggs produce precocious larvae capable of capturing prey shortly after hatching65. Future examination of the mouth parts and gut contents of B. neoponerae adults may provide information on the feeding habits of the new species.
At regional and local scales, we found that B. neoponerae was associated with 43.6% of the colonies of N. villosa collected in A. bracteata and, in more than two-thirds of these, specimens were located within the brood chambers conditioned by the ants in the core of the bromeliads, strongly suggesting a myrmecophilic syndrome. In the Yucatan Peninsula, the new species was also found in one of the two A. bracteata bromeliads inhabited by another ant species belonging to a different ant subfamily (C. atriceps, Formicinae), although in very small numbers (only two specimens), suggesting a close but not exclusive association with N. villosa ants and, presumably, a strong preference for microhabitats created by litter accumulation and ant activities in the tank bromeliad.
In addition to its small size (about 1 mm), B. neoponerae is protected from attack by its globular body shape, thick cuticle, and ability to fully withdraw the first pair of legs and the head. Unspecialized, unwelcome ant guests are known to use simple behavioral strategies to escape host detection, such as prudent behavior66. Besides slow movement, small size seems to contribute to evade ant aggression: myrmecophiles much smaller than their host are, in general, mostly ignored while those that match the host’s size are attacked59. As shown in a previous study, most arthropod species associated with N. villosa established in A. bracteata are small body size arthropods relative to their host (workers are 12–13 mm), mainly Coleoptera (various species of Staphylinidae, Ptiliidae, Nitidulidae) and Hymenoptera (other small ant species or small parasitoid wasps)35. Small body size and prudent behavior in addition to a protective body shape, may favor cohabitation of B. neoponerae with this aggressive ant within bromeliads. The fact that the beetles were found inside the nests suggests that ants tolerate or ignore these small beetles. Their aggressive hosts may indirectly provide the beetles a degree of protection from predators as well as many resources and the benefit of a warm and humid environment.
Based on our analyses, a combination of factors determined the presence of B. neoponerae in our samples: they were more frequently encountered in colonies at relatively higher altitude and during the driest months of the year and were particularly abundant in association with larger colonies of N. villosa, the last two drivers being determinant for beetle presence. Tank bromeliads are unique canopy microhabitats where species-specific aquatic and terrestrial biota develop. Like other tank bromeliads, A. bracteata provides refuge for a diverse array of invertebrates and vertebrates, particularly during the dry season when they account for a substantial portion of the water available in the canopy35,67,68,69,70. Tank bromeliads can store from a few milliliters to several liters of water, depending on the size and number of reservoirs per plant71. In A. bracteata, water reservoirs are formed by the tightly interlocked leaves and contain 34 ml on average71, and groups of shoots at different ontogenetic stages are commonly present on a given tree. In experimental settings, N. villosa colonies tend to select the larger plant when offered two A. bracteata plants of different sizes43, therefore populous colonies in the field are likely to occupy and defend larger plants or groups of plants with the largest water reservoirs thereby exerting greater attraction to other arthropods. Furthermore, the larger the ant colonies are, the more substantially they can alter their nesting sites by creating different microhabitats, thus increasing the availability of resources for potential guests. In fact, the most populous ant species are thought to support a highly diverse symbiont community due to the different microhabitats present in their nests and the longevity of the colonies, that allow to sustain numerous associates over longer periods2,72. The correlation we found in our study between B. neoponerae abundance and N. villosa colony size supports this hypothesis.
Our report is the second case of a Bacanius associated with ants and the first case of a Dendrophilinae associated with ponerine ants. This is also only the fourth time a histerid species has been found cohabiting with ants of the subfamily Ponerinae. An adult of Acritus megaponerae Bickhardt (Abraeinae) has previously been described from Bulawayo, Zimbabwe, in a nest of Megaponera analis (Latreille) (= M. foetens)73, and a single specimen of Mecistostethus loretoensis (Bruch) (= Tarsilister loretoensis) (Histerinae) has been reported in Argentina in the brood chamber of Pachycondyla striata Smith74; finally, unidentified adult histerids, probably scavengers, have been found alive near the refuse chambers, inside several nests of the giant ant Dinoponera grandis (Guérin-Méneville) (= D. australis) in Brazil75. Notably, in all these cases the host is distinguished by its high aggressiveness and corresponds to a large ant species relative to the histerid guest (12–13 mm vs. about 1 mm in the association with N. villosa; about 13 mm76 vs. about 3 mm74 in the association with P. striata; 5–18 mm77 vs. about 0.8 mm73 in the association with M. analis; and D. grandis, with a body length of 22–27 mm78, is known as one of the largest ant species). Much more research needs to be carried out in the future to reveal whether this new species is restricted to ants nesting in A. bracteata and to determine whether B. neoponerae is an obligate or facultative guest of N. villosa or a generalist commensal of hosts sharing the same nesting habitat. Specifically, a close examination of other ant colonies found in A. bracteata, including the dolichoderine ant Dolichoderus bispinosus Olivier, the main competitor of N. villosa in the Yucatan Peninsula in using this tank bromeliad as nesting site34,79, would clarify whether B. neoponerae is primarily dependent on N. villosa or on specific microhabitats within this tank bromeliad.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Atsatt, P. R. Lycaenid butterflies and ants: Selection for enemy-free space. Am. Nat. 118, 638–654 (1981).
Hölldobler, B. & Wilson, E. O. The Ants (Springer, 1990).
Hölldobler, B. & Kwapich, C. L. The Guests of Ants: How Myrmecophiles Interact with Their Hosts (Harvard University Press, 2022).
Wasmann, E. Neue Beiträge zur Kenntniss der Paussiden, mit biologischen und phylogenetischen Bemerkungen. Notes Leyd. Mus. 25, 1–82 (1904).
Kistner, D. H. The social insects’ bestiary. In Social Insects Vol. 3 (ed. Hermann, H. R.) 1–244 (Academic Press, 1982).
Lachaud, J.-P., Lenoir, A. & Witte, V. (eds) Ants and Their Parasites 2012. Psyche Special Issue (Hindawi Publishing Corporation, 2012).
Lachaud, J.-P., Lenoir, A. & Hughes, D. P. (eds) Ants and Their Parasites 2013. Psyche Special Issue (Hindawi Publishing Corporation, 2013).
Mazur, S. A concise catalogue of the Histeridae (Insecta: Coleoptera) (Warsaw University of Life Sciences, 2011).
Integrated Taxonomic Information System (ITIS) https://www.itis.gov/servlet/SingleRpt/SingleRpt
Zhou, Y.-L., Caterino, M. S., Ren, D. & Ślipiński, A. Phylogeny and evolution of Mesozoic and extant lineages of Histeridae (Coleoptera), with discovery of a new subfamily Antigracilinae from the lower Cretaceous. Cladistics 36, 521–539. https://doi.org/10.1111/cla.12418 (2020).
Dégallier, N. & Gomy, Y. Caractères généraux et techniques de récolte des Coléoptères Histeridae. L’Entomologiste 39, 9–17 (1983).
Caterino, M. S. & Vogler, A. P. The phylogeny of the Histeroidea (Coleoptera: Staphyliniformia). Cladistics 18, 394–415. https://doi.org/10.1111/j.1096-0031.2002.tb00158.x (2002).
Kovarik, P.W. & Caterino, M.S. Histeridae Gyllenhal, 1808. 2nd Ed, In: Handbook of Zoology Part 38, Coleoptera, vol. 1: Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim), (Eds. Beutel, R. G. & Leschen, R. A. B.) 281–314 (W. de Gruyter, Berlin, 2016).
Caterino, M. S. & Maddison, D. R. An early and mysterious histerid inquiline from Cretaceous Burmese amber (Coleoptera, Histeridae). ZooKeys 733, 119–129. https://doi.org/10.3897/zookeys.733.23126 (2018).
Dégallier, N., Kovarik, P. W. & Tishechkin, A. K. Description d’une nouvelle espèce de Scapicoelis Marseul, 1862, parasite probable de cocoons de Mygales, et redescription de S. tibialis Marseul, 1862 (Coleoptera, Histeridae, Hetaeriinae). Bull. Soc. Entomol. Fr. 125, 243–256. https://doi.org/10.32475/bsef_2135 (2020).
Parker, J. Myrmecophily in beetles (Coleoptera): Evolutionary patterns and biochemical mechanisms. Myrmecol. News 22, 65–108 (2016).
Krüger, E., Kahlow, C., Leivas, F. W. T. & Schühli, G. S. Scientific note: the histerid beetle Omalodes foveola (Coleoptera: Histeridae) found as a Melittophile, co-inhabiting Africanized honeybee hives in Brazil. Apidologie 48, 572–574. https://doi.org/10.1007/s13592-017-0492-8 (2017).
Helava, J. V. T., Howden, H. F. & Ritchie, A. J. A review of the New World genera of the myrmecophilous and termitophilous subfamily Hetaeriinae (Coleoptera: Histeridae). Sociobiology 10, 127–386 (1985).
Wheeler, W. M. Studies on myrmecophiles. II. Hetærius. J. N.Y Entomol. Soc. 16, 135–143 (1908).
Lea, A. M. On Australian Histeridae (Coleoptera). Trans. R. Entomol. Soc. Lond. 1924, 239–263 (1925).
Pražák, J. S. Evolution of histeroid beetles (Coleoptera: Histeroidea): phylogenetics, fossil record and life histories. Bachelor's Thesis. Charles University, Faculty of Science, Prague, Czech Republic (2021).
Wenzel, R. L. & Dybas, H. New and little known Neotropical Histeridae (Coleoptera). Fieldiana, Zool. 22, 433–472 (1941).
Sokolov, A. V. & Perkovsky, E. E. The first Eocene species of Bacanius (Coleoptera: Histeridae: Dendrophilinae) from Rovno amber. Rus. Entomol. J. 29, 157–160. https://doi.org/10.15298/rusentj.29.2.06 (2020).
Caterino, M. S. & Tishechkin, A. K. A systematic revision of Baconia Lewis (Coleoptera, Histeridae, Exosternini). ZooKeys 343, 1–297. https://doi.org/10.3897/zookeys.343.5744 (2013).
Caterino, M. S. & Tishechkin, A. K. A systematic revision of Operclipygus Marseul (Coleoptera, Histeridae, Exosternini). ZooKeys 271, 1–401. https://doi.org/10.3897/zookeys.271.4062 (2013).
Caterino, M. S. & Tishechkin, A. K. A revision of the Phelister haemorrhous species group (Coleoptera, Histeridae, Exosternini). ZooKeys 854, 41–88. https://doi.org/10.3897/zookeys.854.35133 (2019).
Caterino, M. S. & Tishechkin, A. K. Recognition and revision of the Phelister blairi group (Coleoptera, Histeridae, Exosternini). ZooKeys 1001, 1–154. https://doi.org/10.3897/zookeys.1001.58447 (2020).
Caterino, M. S. & Tishechkin, A. K. A new genus and species of Histerid beetle from western Mexico showing a remarkable sexual mesotibial dimorphism (Coleoptera: Histeridae: Histerinae: Exosternini). Coleopt. Bull. 76, 357–363. https://doi.org/10.1649/0010-065X-76.3.357 (2022).
Yélamos, T. Descripción de una nueva especie de Bacanius (Coleoptera: Histeridae) endogeo de México. Nouv. Rev. Entomol. (Nouv. Sér.) 12, 255–258 (1996).
Mazur, S. & Sawoniewicz, M. Descripción de dos especies nuevas del género Bacanius (Coleoptera, Histeridae) de México. Dugesiana 15, 73–75. https://doi.org/10.32870/dugesiana.v15i2.3852 (2008).
Wenzel, R. L. On the classification of the histerid beetles. Fieldiana, Zool. 28, 51–151 (1944).
Naranjo-López, A. G. & Navarrete-Heredia, J. L. Coleópteros necrócolos (Histeridae, Silphidae y Scarabaeidae) en dos localidades de Gómez Farías, Jalisco, México. Rev. Colomb. Entomol. 37, 103–110 (2011).
Rettenmeyer, C. W. Arthropods associated with neotropical army ants with a review of the behavior of these ants (Arthropoda; Formicidae: Dorylinae). PhD Thesis. University of Kansas (1961). https://biodiversity.uconn.edu/wpcontent/uploads/sites/556/2017/10/Rettenmeyer_dissertation_OCR.pdf
Dejean, A., Olmsted, I. & Snelling, R. R. Tree-epiphyte-ant relationships in the low inundated forest of Sian Ka’an Biosphere Reserve, Quintana Roo, Mexico. Biotropica 27, 57–70. https://doi.org/10.2307/2388903 (1995).
Rocha, F. H., Lachaud, J.-P. & Pérez-Lachaud, G. Myrmecophilous organisms associated with colonies of the ponerine ant Neoponera villosa (Hymenoptera: Formicidae) nesting in Aechmea bracteata bromeliads: a biodiversity hotspot. Myrmecol. News 30, 73–92. https://doi.org/10.25849/myrmecol.news_030:073 (2020).
García, E. Modificaciones al sistema de clasificación climática de Köppen (para adaptarlo a las condiciones de la República Mexicana) (Segunda edición). Universidad Autónoma de México, 1973).
Islebe, G. A., Sánchez-Sánchez, O., Valdéz-Hernández, M. & Weissenberger, H. Distribution of vegetation types. In Biodiversity and Conservation of the Yucatán Peninsula (eds Islebe, G. A. et al.) 39–53 (Springer, 2015).
Lucas, C. et al. A multidisciplinary approach to discriminating different taxa in the species complex Pachycondyla villosa (Formicidae). Biol. J. Linn. Soc. 75, 249–259. https://doi.org/10.1046/j.1095-8312.2002.00017.x (2002).
Mackay, W. P. & Mackay, E. E. The systematics and biology of the New World ants of the genus Pachycondyla (Hymenoptera: Formicidae) (Edwin Mellon Press, Lewiston, 2010).
Fernandes, I. O., De Oliveira, M. L. & Delabie, J. H. C. Description of two new species in the Neotropical Pachycondyla foetida complex (Hymenoptera: Formicidae: Ponerinae) and taxonomic notes on the genus. Myrmecol. News 19, 133–163. https://doi.org/10.25849/myrmecol.news_019:133 (2014).
Pérez-Lachaud, G. & Lachaud, J.-P. Hidden biodiversity in entomological collections: The overlooked co-occurrence of dipteran and hymenopteran ant parasitoids in stored biological material. PLoS ONE 12, e0184614. https://doi.org/10.1371/journal.pone.0184614 (2017).
Pérez-Lachaud, G., Jervis, M. A., Reemer, M. & Lachaud, J.-P. An unusual, but not unexpected, evolutionary step taken by syrphid flies: the first record of true primary parasitoidism of ants by Microdontinae. Biol. J. Linn. Soc. 111, 462–472. https://doi.org/10.1111/bij.12220 (2014).
Rocha, F. H., Lachaud, J.-P., Hénaut, Y., Pozo, C. & Pérez-Lachaud, G. Nest site selection during colony relocation in Yucatan Peninsula populations of the ponerine ants Neoponera villosa (Hymenoptera: Formicidae). Insects 11, 200. https://doi.org/10.3390/insects11030200 (2020).
Hashmi, A. A. A revision of the Neotropical ant subgenus Myrmothrix of genus Camponotus (Hymenoptera: Formicidae). Studia Ent. 16, 1–140 (1973).
AntWeb (2022) Version 8.8. California Academy of Science. https://www.antweb.org. Accessed 24 August 2022.
Wheeler, W. M. The ants of Texas, New Mexico and Arizona. Part I. Bull. Am. Mus. Nat. Hist. 24, 399–487 (1908).
Degallier, N. & Tishechkin, A. K. Révision du genre Scapicoelis Marseul, 1862, avec la description de 28 espèces nouvelles (Insecta, Coleoptera, Histeridae, Haeteriinae). Faunitaxys 10, 1–87 (2022).
Ivanova, N. V., Dewaard, J. R. & Hebert, P. D. N. An inexpensive, automation-friendly protocol for recovering high-quality DNA. Mol. Ecol. Notes 6, 998–1002. https://doi.org/10.1111/j.1471-8286.2006.01428.x (2006).
Prosser, S., Martínez-Arce, A. & Elías-Gutiérrez, M. A new set of primers for COI amplification from freshwater microcrustaceans. Mol. Ecol. Res. 13, 1151–1155. https://doi.org/10.1111/1755-0998.12132 (2013).
Elías-Gutiérrez, M., Valdez-Moreno, M., Topan, J., Young, M. R. & Cohuo-Colli, J. A. Improved protocols to accelerate the assembly of DNA barcode reference libraries for freshwater zooplankton. Ecol. Evol. 8, 3002–3018. https://doi.org/10.1002/ece3.3742 (2018).
Montes-Ortiz, L. & Elías-Gutiérrez, M. Faunistic survey of the zooplankton community in an oligotrophic sinkhole, Cenote Azul (Quintana Roo, Mexico), using different sampling methods, and documented with DNA barcodes. J. Limnol. 77, 428–440. https://doi.org/10.4081/jlimnol.2018.1746 (2018).
Edgar, R. C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucl. Acids Res. 32, 1792–1797 (2004).
Tamura, K. & Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 10, 512–526. https://doi.org/10.1093/oxfordjournals.molbev.a040023 (1993).
Therneau, T., Atkinson, B. & Ripley, B. Rpart: Recursive partitioning. R Package Version 4.1–3 (2013). http://CRAN.R-project.org/package=rpart
Cutler, D. R. et al. Random forest for classification in ecology. Ecology 88, 2783–2792. https://doi.org/10.1890/07-0539.1 (2007).
R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna (2021). https://www.R-project.org
Kistner, D. H. Social and evolutionary significance of social symbionts. In Social Insects Vol. 1 (ed. Hermann, H. R.) 339–413 (Academic Press, 1979).
Caterino, M. S. & Dégallier, N. A review of the biology and systematics of Chlamydopsinae (Coleoptera: Histeridae). Invertebr. Syst. 21, 1–28. https://doi.org/10.1071/IS06017 (2007).
von Beeren, C. et al. Multiple phenotypic traits as triggers of host attacks towards ant symbionts: body size, morphological gestalt, and chemical mimicry accuracy. Front. Zool. 18, 46. https://doi.org/10.1186/s12983-021-00427-8 (2021).
Parker, J. & Kronauer, D. J. C. How ants shape biodiversity. Curr. Biol. 31, R1208-1214. https://doi.org/10.1016/j.cub.2021.08.015 (2021).
von Beeren, C. & Tishechkin, A. Nymphister kronaueri von Beeren & Tishechkin sp. nov., an army ant-associated beetle species (Coleoptera: Histeridae: Haeteriinae) with an exceptional mechanism of phoresy. BMC Zool. 2, 3. https://doi.org/10.1186/s40850-016-0010-x (2017).
Navarrete-Heredia, J. L. Beetles associated with Atta and Acromyrmex ants (Hymenoptera: Formicidae: Attini). Trans. Am. Entomol. Soc. 127, 381–429 (2001).
Gomy, Y. Un nouveau genre et trois nouvelles espèces d’Histeridae Bacaniini d’Australie (Coleoptera). Bull. Soc. Entomol. Fr. 114, 189–193 (2009).
LeConte, J. L. Synopsis of the species of the Histeroid genus Abræus (Leach,) inhabiting the United States, with descriptions of two nearly allied new genera. Proc. Acad. Nat. Sci. Philadelphia 6, 287–292 (1853).
Kovarik, P.W. Phylogeny, chaetotaxy, and ecology of the Onthophilinae and Tribalinae (Coleoptera: Histeridae). PhD Thesis. The Ohio State University (1994).
Parmentier, T., De Laender, F., Wenseleers, T. & Bonte, D. Prudent behavior rather than chemical deception enables a parasite to exploit its ant host. Behav. Ecol. 29, 1225–1233. https://doi.org/10.1093/beheco/ary134 (2018).
Beutelspacher Baigts, C.R. Bromeliáceas como ecosistemas. Con especial referencia a Aechmea bracteata (Swartz) Griseb (Plaza y Valdés, 1999).
Armbruster, P., Hutchinson, R. A. & Cotgreave, P. Factors influencing community structure in a South American tank bromeliad fauna. Oikos 96, 225–234. https://doi.org/10.1034/j.1600-0706.2002.960204.x (2002).
Frank, J. H. & Lounibos, L. P. Insects and allies associated with bromeliads: a review. Terrest. Arthrop. Rev. 1, 125–153. https://doi.org/10.1163/187498308X414742 (2009).
Hénaut, Y. et al. A tank bromeliad favors spider presence in a neotropical inundated forest. PLoS ONE 9, e114592. https://doi.org/10.1371/journal.pone.0114592 (2014).
Zotz, G., Leja, M., Aguilar-Cruz, Y. & Einzmann, H. J. R. How much water is in the tank? An allometric analysis with 205 bromeliad species. Flora 264, 151557. https://doi.org/10.1016/j.flora.2020.151557 (2020).
Kronauer, D. J. C. & Pierce, N. E. Myrmecophiles. Curr. Biol. 21, R208–R209. https://doi.org/10.1016/j.cub.2011.01.050 (2011).
Bickhardt, H. Ein neuer myrmecophiler Acritus aus Südafrika. Entomol. Blätter 12, 1–2 (1916).
Bruch, C. Un género nuevo de histérido mirmecófilo (Coleoptera). Rev. Mus. La Plata 33, 277–281 (1932).
Paiva, R. V. S. & Brandão, C. R. F. Nests, worker population, and reproductive status of workers, in the giant queenless ponerine ant Dinoponera Roger (Hymenoptera Formicidae). Ethol. Ecol. Evol. 7, 297–312. https://doi.org/10.1080/08927014.1995.9522938 (1995).
Mackay, W. P. & Mackay, E. E. The Systematics and Biology of the New World Ants of the Genus Pachycondyla (Hymenoptera: Formicidae) (The Edwin Mellen Press, Lewiston, New York, 2010). https://doi.org/10.13140/2.1.4271.8726.
Rödel, M.-O. & Braun, U. Associations between anurans and ants in a West African Savanna (Anura: Microhylidae, Hyperoliidae and Hymenoptera: Formicidae). Biotropica 31, 178–183. https://doi.org/10.1111/j.1744-7429.1999.tb00128.x (1999).
Dias, A. M. & Lattke, J. E. Large ants are not easy—The taxonomy of Dinoponera Roger (Hymenoptera: Formicidae: Ponerinae). Eur. J. Taxon. 784, 1–66. https://doi.org/10.5852/ejt.2021.784.1603 (2021).
Dejean, A. & Olmsted, I. Ecological studies on Aechmea bracteata (Swartz) (Bromeliaceae). J. Nat. Hist. 31, 1313–1334. https://doi.org/10.1080/00222939700770741 (1997).
Acknowledgements
We are grateful to Holger Weissenberger, Javier Valle-Mora, Alma Estrella García Morales, and Humberto Bahena-Basave (Ecosur) for assistance with Figure 1, statistical analysis, DNA extraction, and photo edition, respectively. Tomáš Lackner (Bavarian State Collection of Zoology, Munich, Germany) helped with photographs of the ants associated with Australanius verrucosus for their identification. Antoine Mantilleri (MNHN), György Makranczy (Hungarian Natural History Museum, Budapest, Hungary), Jessica K. Wadleigh (FMNH), Matus Soltis (Ruzomberok, Slovaquie), Vincenzo Vomero (Museo Civico di Zoologia, Roma, Italia), and Tomáš Lackner kindly provided access to histerid material for comparison. We also thank Julian Flavell for improving the English, and Rodrigo M. Feitosa and another reviewer for their constructive comments on an earlier version of the manuscript. FRV acknowledges support from CONACyT, Mexico (scholarship grant no. 269825). SEM photos were generated at the SEM facility located at Ecosur-Chetumal. DNA sequencing was financed by GP-L and J-PL personal incomings.
Nicolas Degallier and Yves Gomy—Retired employees (no formal institutional affiliation).
Author information
Authors and Affiliations
Contributions
G.P.-L. and J.-P.L. designed the research; F.H.R collected and separated the material; G.P.-L., J.-P.L., F.H.R., and M.E.-G. analyzed the data; N.D. and Y.G. identified and described the new beetle species; J.-P.L. identified the ants; G.P.-L. and M.E.-G. performed the phylogenetic analyses and MEB observations; G.P.-L., J.-P.L. and N.D. wrote the first draft of the paper; all authors contributed substantially to the text and revised it.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pérez-Lachaud, G., Degallier, N., Gomy, Y. et al. Cohabitation with aggressive hosts: description of a new microhisterid species in nests of a ponerine ant with ecological notes. Sci Rep 13, 18484 (2023). https://doi.org/10.1038/s41598-023-45692-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-45692-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.