Abstract
Bupivacaine (BPV) can cause severe central nervous system toxicity when absorbed into the blood circulation system. Rapid intravenous administration of lipid emulsion (LE) could be used to treat local anaesthetic toxicity. This study aimed to investigate the mechanism by which the BDNF-TrkB/proBDNF-p75NTR pathway regulation by LE rescues BPV induced neurotoxicity in hippocampal neurons in rats. Seven- to nine-day-old primary cultured hippocampal neurons were randomly divided into 6 groups: the blank control group (Ctrl), the bupivacaine group (BPV), the lipid emulsion group (LE), the bupivacaine + lipid emulsion group (BPV + LE), the bupivacaine + lipid emulsion + tyrosine kinase receptor B (TrkB) inhibitor group (BPV + LE + K252a), the bupivacaine + lipid emulsion + p75 neurotrophic factor receptor (p75NTR) inhibitor group (BPV + LE + TAT-Pep5). All hippocampal neurons were incubated for 24 h, and their growth state was observed by light microscopy. The relative TrkB and p75NTR mRNA levels were detected by real-time PCR. The protein expression levels of brain-derived neurotrophic factor (BDNF), proBDNF, TrkB, p75NTR and cleaved caspase-3 were detected by western blotting. The results showed that primary hippocampal neuron activity was reduced by BPV. As administration of LE elevated hippocampal neuronal activity, morphology was also somewhat improved. The protein expression and mRNA levels of TrkB and p75NTR were decreased when BPV induced hippocampal neuronal toxicity, while the expression of BDNF was increased. At the same time, BPV increased the original generation of cleaved caspase-3 protein content by hippocampal neurons, while the content of cleaved caspase-3 protein in hippocampal neurons cotreated with LE and BPV was decreased. Thus, this study has revealed LE may reduce apoptosis and promote survival of hippocampal neurons by regulating the BDNF-TrkB pathway and the proBDNF-p75NTR pathway to rescue BPV induced central neurotoxicity in rats.
Similar content being viewed by others
Introduction
The advantages of local anaesthesia techniques throughout the perioperative period have been recognized1,2. Although many measures have been taken to improve the safety of local anaesthesia3,4, body absorption and the systemic toxicity of local anaesthetics remain concerns. Convulsion and cardiac arrest are the most harmful acute complications of the systemic toxicity of local anaesthetics. Central nervous system (CNS) toxicity, manifesting as convulsions, appears earlier than cardiocirculatory system toxicity, and the toxic dose for the CNS is lower than the dose needed to cause cardiac arrest5,6. Therefore, the prevention and treatment of CNS toxicity are particularly important when using local anaesthetics.
Clinically, when local anaesthetics caused CNS toxicity in the past, sedatives were mainly used to treat or relieve the toxic symptoms, but the incidence of respiratory inhibition was relatively high. Once anoxia occurs, it will aggravate CNS toxicity and increase its sequelae. In addition, sedatives themselves have adverse effects on neurons and potentially neurotoxic effects on the brain7. In recent years, there has been increasing evidence supporting the use of lipid emulsion (LE) in the treatment of local anaesthesia poisoning, especially bupivacaine poisoning. a commonly used local anaesthetic in clinical practice5,6,8,9,10,11. Cell experiments and isolated heart model experiments have shown that LE treatment can reduce BPV-induced cell death and promote the recovery of cardiac function12,13. This may be related to the fact that LE significantly improves the mitochondrial function of cardiomyocytes and reverses BPV-induced apoptosis14. However, existing studies have given more attention to the cardiotoxicity of BPV15,16,17, and less attention has been given to its CNS toxicity mechanism. The occurrence and development of CNS toxic convulsions are closely related to the hippocampus18,19,20. Convulsions caused by local anaesthesia can increase the apoptosis of hippocampal neurons in rats20. Although laboratory data and case reports support treating bupivacaine CNS toxicity with LE, and the positive effect is clear6,8, the exact mechanism has not been uncovered.
It is generally believed that the CNS toxicity of bupivacaine occurs because bupivacaine blocks the inhibitory pathway of the brain, resulting in the relative activation of the excitatory pathway. If the concentration of bupivacaine in the blood continues to rise, the inhibitory and excitatory pathways will be inhibited at the same time, thus inhibiting the entire CNS21. During this process, there is an imbalance between excitatory amino acids and inhibitory amino acids in the brain, causing disorders of brain nerve function. In the brain, the balance between excitatory amino acids and inhibitory amino acids is mainly due to the binding of BDNF to TrkB and/or proBDNF to p75NTR, which activate the regulation of downstream PI3K/Akt or Ras/MAPK signalling pathways. However, it has been reported that bupivacaine can inhibit the PI3K/Akt signalling pathway and lead to the occurrence of neuronal apoptosis22,23. Therefore, we speculate that bupivacaine can activate the downstream pathway through its interaction with BDNF, TrkB, and/or proBDNF-p75NTR, causing the excitatory amino acids and inhibitory amino acids in the brain to be out of balance, resulting in CNS toxicity, which eventually leads to cell apoptosis or death.
Based on previous studies and literature reports, it has been confirmed that the CNS toxicity of BPV is caused by an imbalance in the expression of excitatory amino acids, inhibitory amino acids, and their corresponding receptors in the brain. LE can improve the imbalance and play a role in treating CNS toxicity caused by bupivacaine24. Therefore, we sought to answer the following question: does LE reverse the CNS toxicity induced by BPV by regulating BDNF-TrkB/proBDNF-p75NTR?
This experiment utilized primary hippocampal neurons as the research object to study the relationship between the BDNF-TrkB/proBDNF-p75NTR pathway and BPV-induced hippocampal neuron toxicity, and to determine whether it participated in the process of LE treatment of BPV-induced CNS toxicity, to provide a more solid theoretical basis for its wide clinical applications.
Materials and methods
Chemicals and antibodies
Bupivacaine hydrochloride injection was purchased from Zhaohui Pharmaceutical (Shanghai, China). 20% lipid emulsion was purchased from the Kelun industry group (Sichuan, China). TAT-Pep5 was obtained from Merck Millipore (Germany). Poly-L-lysine solution and K252a were purchased from Sigma (USA). Trypsin–EDTA (0.25%), Dulbecco's modified Eagle's medium (DMEM), neurobasal medium, B27 supplement, and foetal bovine serum (FBS) were purchased from Gibco (USA). BDNF and TrkB antibodies were purchased from Abcam (USA). The proBDNF antibody was purchased from Novusbio (USA). Cleaved Caspase-3 (D175) was purchased from Signalway Antibody (SAB, USA). p75NTR was purchased from CST(USA). Biotinylated goat anti-mouse IgG whole antibody and β-actin were obtained from Santa Cruz (USA).
Primary hippocampal neuron culture
SD rats were sterilized with 75% ethanol within 24 h of birth. The pups were decapitated with fresh sterile scissors and the removed head was placed on sterile gauze. The hippocampal tissue from both sides was separated quickly. The hippocampal tissues were placed into 0.25% pancreatic enzyme and digested for 20 min at 37 ℃. After digestion, the tissue was washed and fixed. After collecting the supernatant, the cells were extracted from the supernatant. Next, cells were plated in 96-well plates and 6- well plates that were treated with polylysine, cleaned with inoculation fluid 4 h after inoculation, and replaced with neuronal complete medium. The next day, half the amount of liquid was changed, and the culture was replaced with cytarabine (complete medium and a final concentration of 2.5 μg/ml cytarabine) after 48 h. Then, the culture fluid was changed every 2–3 d, half the amount of liquid was changed each time, and the liquid was cultured for 7–9 days and reserved.
Experimental classification
Hippocampal neurons cultured for 7–9 days were divided into six groups: control, the bupivacaine group(BPV), the lipid emulsion group(LE), the bupivacaine + lipid emulsion group(BPV + LE), the bupivacaine + lipid emulsion + TrkB inhibitor group(BPV + LE + 200 nM K252a), and the bupivacaine + lipid emulsion + p75NTR inhibitor group(BPV + LE + 2 μM TAT-Pep5).
MTT assay for the activity of primary hippocampal neurons
Primary hippocampal neurons were divided into different groups and treated in 96-well plates. Next, 25 μM MTT was added to each well to a final concentration of 1 mg/ml. The wells were mixed and then cultured at 37 °C for 4 h. After that, 100 μM solution (25% SDS + 50% DMF) was added to each well and incubated overnight at 37 °C. The absorbance values of each well were read at a wavelength of 570 nm by a microplate reader, and the experiment was repeated three times.
Determining the half maximal inhibitory concentration (IC50) of BPV: Primary hippocampal neurons were treated with different concentrations of BPV for 24 h, cell viability was measured by the MTT method, and the IC50 of BPV was calculated. The concentration of the BPV stock solution was 0.75%, and the highest dosing concentration was 0.25%. It was diluted three times, and a total of 9 concentrations were set.
Real-time PCR assay
Total RNA from cultured hippocampal neurons in each group was extracted with TRIzol reagent. First-strand cDNAs were generated using the PrimeScriptTM RT reagent kit (TaKaRa, Japan). Finally, according to the manufacturer’s recommendations, quantitative real-time PCR was performed using the SYBR premix reaction system (TaKaRa, Japan). GAPDH was used as the endogenous control. The assay was performed using fluorescence quantitative PCR instrument (Applied Biosystems, USA) to analyse the relative expression levels of mRNAs. The relative gene expression was calculated using the 2−ΔΔCT method. ( The primers are listed in Table 1.)
Western blot analysis
The total protein of hippocampal neurons in each group was extracted, and the protein concentration was measured with the bicinchoninic acid (BCA) protein assay kit. Protein samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS‒PAGE), transferred to polyvinylidene fluoride (PVDF) membranes, blocked, and incubated with anti-BDNF (1:800), anti-proBDNF (1:200), anti- cleaved caspase-3 (1:500), anti-p75NTR (1:500), or anti-TrkB (1:1000) antibodies diluted with TBST containing 1% bovine serum albumin(BSA) overnight at 4 °C. On the next day, the membrane was washed three times with TBST for 10 min each time. The membrane was incubated with an HRP-labelled secondary antibody at a dilution of 1:5000 for approximately 1 h at room temperature. After rinsing, the membranes were exposed to an X-ray film. Quantification of bands was performed by scanning the films.
Statistical analyses
All data were analysed using SPSS 21.0 software (IBM, USA). All values are expressed as the means ± SDs. Data were analysed by one-way analysis of variance (ANOVA) to determine statistically significant differences. P value < 0.05 was considered significant.
Ethics approval and consent to participate
The study was approved by the Committee on the Ethics of Animal Experiments at Ningxia Medical University (Approval No. 2015–058). Animals were cared for in accordance with the University’s standards for care and use of laboratory animals, and all procedures were performed in accordance with the revised Animals (Scientific Procedures) Act 1986. Meanwhile, ARRIVE guidelines were followed during this study.
Results
Effect of bupivacaine on the activity of primary hippocampal neurons
The IC50 of BPV was 0.03362%, which was converted into a molar concentration of 980.4 μM (Fig. 1A). Therefore, BPV at concentrations of 800 μM, 1000 μM, and 1200 μM was selected to treat primary hippocampal neurons for 12 h, 24 h, and 36 h, respectively. The activity of hippocampal neurons was measured by MTT assay. As shown in Fig. 1B, the activity of hippocampal neurons decreased with increasing BPV concentration; with the same concentration of BPV, the activity of hippocampal neurons decreased with increasing treatment time. The activity of hippocampal neurons decreased by approximately 50% after 24 h with 1000 μM or 1 mM BPV. Therefore, this concentration and time were selected for subsequent experiments (Fig. 1).
Effects of different bupivacaine concentrations and treatment times on hippocampal neurons. (A) The IC50 of BPV was 0.03362%, which was converted into a molar concentration of 980.4 μM. (B) The activity of hippocampal neurons decreased with the increase of BPV concentration and treatment time. The activity of hippocampal neurons decreased by about 50% after 24 h with 1000 μM. (x̅ ± s, triplicate). Same BPV concentration, compared with the 12 h: aP < 0.05; Same BPV concentration, compared with 24 h: bP < 0.05; Same treatment time, compared with 800uM: cP < 0.05; Same treatment time, compared with 1000uM: dP < 0.05.
Effect of LE on hippocampal neuron activity
LE at three concentrations, 0.5%, 1%, and 2%, was selected to treat hippocampal neurons exposed to BPV and the activity of hippocampal neurons was observed. As shown in Fig. 2, compared with the control group, the BPV group’s hippocampal neuronal activity was reduced (P < 0.05). Compared with the BPV group, the activity of hippocampal neurons in the 0.5% LE group, 1% LE group, 2% LE group, BPV + 0.5% LE group, BPV + 1% LE group, and BPV + 2% LE group was increased (P < 0.05) (Fig. 2).
The activity of hippocampal neurons was studied using an MTT assay (1000 μM, 24 h). The BPV group’s hippocampal neuronal activity was reduced compared with the control group, (P < 0.05). Compared with the BPV group, the activity of hippocampal neurons in the 0.5% LE group, 1% LE group, 2% LE group, BPV + 0.5% LE group, BPV + 1% LE group, and BPV + 2% LE group was increased (P < 0.05). (x̅ ± s, triplicate). Compare with Ctrl: aP < 0.05; compared with group BPV: bP < 0.05.
Growth status of hippocampal neurons
As shown in Fig. 3, the hippocampal neurons of the Ctrl group and the LE group were plump, with strong stereoscopic sense and thick and long protrusions, and were interwoven into a dense network. In the BPV group, hippocampal neurons were wrinkled, with thin, short, or no protrusions, significantly reduced connections between neurons and no dense network formation. The hippocampal neurons in the BPV + LE group were round, with a small amount of wrinkling and long protrusions, which could interact with the surrounding neurites to form a network. The hippocampal neurons in the BPV + LE + K252a group and the BPV + LE + TAT-Pep5 group were arranged into clusters, the cell body was wrinkled, and protuberances were present, which could be interwoven into a network with the surrounding neuronal protuberances (Fig. 3).
Growth status of hippocampal neurons in six groups (200×) (1000 μM, 24 h, triplicate) Ctrl group (A) and LE group (C): The hippocampal neurons were plump, with strong stereoscopic sense, thick and long protrusions, and were interwoven into a dense network. BPV group (B): The hippocampal neurons were wrinkled, with thin, short, or no protrusions, significantly reduced connections between neurons and no dense network formation. BPV + LE group (D): The hippocampal neurons were round, with a small amount of wrinkling and long protrusions, which could interact with the surrounding neurites to form a network. BPV + LE + K252a group (E) and BPV + LE + TAT-Pep5 (F) group: The hippocampal neurons were arranged into clusters, the cell body was wrinkled, and protuberances were present, which could be interwoven into a network with the surrounding neuronal protuberances. (A) Control; (B) BPV; (C) LE; (D) BPV + LE; (E) BPV + LE + K252a; (F) BPV + LE + TAT-Pep5.
Expression of TrkB and p75NTR mRNA in six groups of hippocampal neurons
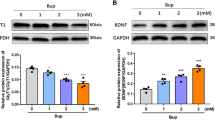
TrkB mRNA expression in the six groups of hippocampal neurons is shown in Fig. 4A. Compared with that in the Ctrl group, TrkB mRNA expression in the BPV group was decreased (P < 0.05). Compared with the BPV group, TrkB mRNA expression was increased in the BPV + LE group, BPV + LE + K252a group, and BPV + LE + TAT-Pep5 group (P < 0.05). Compared with that in the LE group, TrkB mRNA expression in the BPV + LE group, BPV + LE + K252a group, and BPV + LE + TAT-Pep5 group was decreased, but the difference was not statistically significant (P > 0.05).
Expression of TrkB and p75NTR mRNA in six groups of hippocampal neurons. (1000 μM, 24 h). The mRNA level of TrkB were decreased when BPV induced hippocampal neuronal toxicity, while the expression of BPV + LE group was increased. The mRNA level of p75NTR were decreased when BPV induced hippocampal neuronal toxicity, while the expression of BPV + LE group was decreased compared with the LE group. (A) Comparison of TrkB mRNA expression (x̅ ± s, triplicate); (B) Comparison of expression of p75NTR mRNA (x̅ ± s, triplicate). Compared with the control: aP < 0.05; compared with group BPV: bP < 0.05; compared with group LE: cP < 0.05; compared with group BPV + LE + K252a: eP < 0.05.
The expression of p75NTR mRNA in the six groups of hippocampal neurons is shown in Fig. 4B. Compared with the control group, p75NTR mRNA expression in the BPV group, LE group, BPV + LE group, BPV + LE + K252a group, and BPV + LE + TAT-Pep5 group was decreased (P < 0.05). Compared with the BPV group, the expression of p75NTR mRNA in the LE group and the BPV + LE + K252a group was increased (P < 0.05), the expression of p75NTR mRNA in the BPV + LE group was also increased, but the difference was not statistically significant (P > 0.05). The expression of p75NTR mRNA in the BPV + LE group, BPV + LE + K252a group, and BPV + LE + TAT-Pep5 group was decreased compared with the LE group (P < 0.05). The expression of p75NTR mRNA increased in the BPV + LE + K252a group and decreased in the BPV + LE + TAT-Pep5 group compared with the BPV + LE group, but the difference was not statistically significant (P > 0.05). Compared with the BPV + LE + K252a group, the expression of p75NTR mRNA in the BPV + LE + TAT-Pep5 group was decreased (P < 0.05) (Fig. 4).
BDNF, proBDNF, TrkB, p75NTR, and Cleaved caspase-3 protein expression
The expression of BDNF protein in the six groups of hippocampal neurons is shown in Fig. 5, A1–A2, and the difference between the groups was statistically significant (P < 0.05). Compared with the Ctrl group, BDNF protein expression in the BPV group, LE group, and BPV + LE group was increased, with a statistically significant difference (P < 0.05). Compared with that in the BPV group, the expression of BDNF protein in the LE group, BPV + LE group, BPV + LE + K252a group, and BPV + LE + TAT-Pep5 group was decreased (P < 0.05). Compared with the LE group, BDNF protein expression was increased in the BPV + LE group and decreased in the BPV + LE + K252a group and BPV + LE + TAT-Pep5 group, and the difference was not statistically significant (P > 0.05). Compared with that in the BPV + LE group, BDNF protein expression in the BPV + LE + K252a group and the BPV + LE + TAT-Pep5 group was decreased (P < 0.05).
Expression of BDNF, proBDNF, TrkB, p75NTR, and Cleaved caspase-3 proteins in six groups of hippocampal neurons(1000 μM, 24 h) (Electrophoretic gels and blots were cropped, the samples derive from the same experiment and that gels/blots were processed in parallel.). The protein expression of TrkB and p75NTR were decreased when bupivacaine induced hippocampal neuronal toxicity, while the expression of BDNF was increased and proBDNF showed no significant change. BPV increased the original generation of cleaved caspase-3 protein content by hippocampal neurons, and LE and BPV decreased the cleaved caspase-3 protein content of hippocampal neurons. A1-E1: Protein expression blot of BDNF, proBDNF, TrkB, p75NTR, and Cleaved caspase-3; A2-E2, The relative expression levels of BDNF, proBDNF, TrkB, p75NTR, and Cleaved caspase-3 proteins were standardized with internal reference to β-actin (x̅ ± s , triplicate). Compared with the control: aP < 0.05; compared with group BPV: bP < 0.05; compared with group LE: cP < 0.05; compared with group BPV + LE: dP < 0.05; compared with group BPV + LE + K252a: eP < 0.05.
There was no significant change in the expression of proBDNF protein in the six groups of hippocampal neurons (P > 0.05), as shown in Fig. 5, B1 and B2.
TrkB protein expression in six groups of hippocampal neurons is shown in Fig. 5, C1–C2. Compared with the Ctrl group, TrkB protein expression in the BPV group, BPV + LE + K252a group, and BPV + LE + TAT-Pep5 group was decreased (P < 0.05). Compared with the BPV group, TrkB protein expression in the BPV + LE group was increased (P < 0.05), TrkB protein expression in the BPV + LE + K252a group was not significantly changed (P > 0.05), and TrkB protein expression in the BPV + LE + TAT-Pep5 group was decreased (P < 0.05). Compared with that in the BPV + LE group, TrkB protein expression in the BPV + LE + K252a group and BPV + LE + TAT-Pep5 group was decreased (P < 0.05). Compared with the BPV + LE + K252a group, TrkB protein expression in the BPV + LE + TAT-Pep5 group decreased (P < 0.05).
The expression of p75NTR protein in the six groups of hippocampal neurons is shown in Fig. 5, D1–D2. Compared with the control group, p75NTR protein expression was decreased in the BPV group, BPV + LE group, BPV + LE + K252a group and BPV + LE + TAT-Pep5 group (P < 0.05). Compared with the BPV group, both the LE group and the BPV + LE + K252a group showed increased p75NTR protein expression (P < 0.05), while the BPV + LE group and the BPV + LE + TAT-Pep5 group showed increased p75NTR protein expression (P > 0.05).
The cleaved caspase-3 protein changes in the six groups of hippocampal neurons are shown in Fig. 5, E1–E2. Cleaved caspase-3 protein expression was increased in the BPV group compared with the control group (P < 0.05), while compared with the BPV group, cleaved caspase-3 protein expression in the BPV + LE group was decreased (P < 0.05). Cleaved caspase-3 protein expression was decreased in the BPV + LE + TAT-Pep5 group compared with the BPV + LE group (P < 0.05). Cleaved caspase-3 protein expression was decreased in the BPV + LE + TAT-Pep5 group compared with the BPV + LE + K252a group (P < 0.05) (Fig. 5).
Discussion
These experiments used hippocampal neurons as the research object to study LE treatment’s impact on the toxic effects of BPV on the CNS. The in vitro experiment eliminated body metabolism and adjusted for factors, such as interference, and validated that LE treatment was effective in reducing hippocampal neuron cell toxicity.
Studies have shown that the neurotoxicity of all local anaesthetics is concentration and time-dependent25,26. We tested the influence of BPV on hippocampal neurons and measured its IC50, which was 980.4 μM. In our experiments, 1000 μM or 1 mM BPV inhibited 50% of hippocampal neuron activity after 24 h of treatment. Therefore, this concentration and treatment time were used in the following experiments. However, our study sought to answer the following question: can LE prevent BPV-induced reductions in hippocampal neuronal activity? We chose three LE concentrations of 0.5%, 1%, and 2% and observed their effects on hippocampal neuron cell vitality. We found that the three concentrations of LE had no obvious inhibitory effect on hippocampal neuronal activity. We also found that the 1% and 2% concentrations caused a slight increase in hippocampal neuronal activity. Combined with previous studies on treating BPV toxicity with LE14,15, the effects of 1 mM BPV and 1% LE on BPV-induced neurotoxicity in hippocampal neurons were studied after 24 h of treatment with LE. In this study, the concentrations of the TrkB inhibitor K252a and the p75NTR inhibitor TAT-Pep5 were referenced from the literature27,28.
We observed under the optical microscope that BPV inhibited the growth of hippocampal neurons. Compared with the BPV group, hippocampal neuron projections in the BPV + LE group were increased, with the surrounding hippocampal neurons interwoven into a network, and the connection between hippocampal neurons increased. This also confirmed that local anaesthetic can inhibit the growth of a neuron’s synaptic axon29, and LE treatment can reduce neurotoxic effects and promote both the growth of hippocampal neurons and the connections between them.
Information transmission and functional connections between hippocampal neurons are mainly mediated by synaptic activity. BDNF and its precursor proBDNF are secreted by neuron cell bodies to stimulate TrkB and p75NTR receptors located on axons and dendrites, as well as other receptors involved in neuron development and synaptic plasticity (SP)30,31. However, studies on the role of the BDNF-TrkB pathway have different results. BDNF promotes neuronal survival by activating TrkB in different brain regions and provides a neuroprotective effect32,33. The BDNF-TrkB-dependent ERK pathway is involved in the process of combating glutamate excitatory neurotoxicity and plays a neuroprotective role28,34. It has also been reported that BDNF can stimulate glutamate release by activating TrkB and downstream pathways35. The BDNF-TrkB pathway is involved in the development of epilepsy, depression, Alzheimer’s disease, and other diseases36,37. Blocking this pathway can be used as a therapeutic target for such diseases. In nerve endings, BDNF promotes glutamate release and inhibits the release of γ-aminobutyric acid (GABA) through a variety of mechanisms35,38. Glutamate-induced excitatory toxicity of hippocampal neurons is associated with the downregulation of full-length TrkB receptors and upregulation of truncated TrkB receptors32. Many studies have reported that the proBDNF-p75NTR pathway and the BDNF-TrkB pathway have opposite biological roles in promoting neuron survival and SP37,39,40,41,42.
In this experiment, the protein expression and mRNA levels of TrkB and p75NTR were decreased when bupivacaine induced hippocampal neuronal toxicity, while the expression of BDNF was increased and proBDNF showed no significant change, which was inconsistent with the previous experimental results of neuronal toxicity. This result may be related to the experimental parameters and drugs. Previous studies have confirmed BDNF-TrkB and related pathways downstream of its biological function, most of which adopt methods such as inducing excitatory neurotoxicity by glutamate, creating epileptic convulsions or depression models, or increasing exogenous BDNF. Research reports show that epileptic activity increases the expression of BDNF43,44, and by activating TrkB, BDNF can play a role in neural protection. Therefore, we consider that hippocampus induced neurotoxicity increased BDNF protein expression because hippocampal neurons were noxiously stimulated by bupivacaine and reflexively increased the expression of BDNF, resulting in a self-protective effect. LE increased TrkB protein expression and mRNA levels, activated BDNF, promoted hippocampal neurite growth, and mediated neuroprotective effects. BPV itself exerts a biological effect by preventing the intracellular flow of Na+ required by neurons to generate an action potential and inhibits the transmission of intersynaptic transmitters29,45, as well as the growth of synaptic axons. This may be the reason for the downregulated expression of receptor p75NTR protein and mRNA levels on the dendrites and axons of hippocampal neurons. The expression levels of p75NTR protein and mRNA in the BPV + LE group were slightly increased compared with those in the BPV group, which may be related to the protective effect of LE in hippocampal neuronal processes.
BPV can induce apoptosis in nerve cells46,47. Apoptosis plays an essential role in the removal of unwanted or abnormal cells by multicellular organisms, and is a basic measure to maintain the dynamic balance of cell numbers in vivo. Disorders of apoptosis processes may be directly or indirectly related to the occurrence of many diseases. Caspases (cysteinyl aspartate specific proteinases) are a group of proteases involved in cell growth, differentiation, and apoptosis regulation, and are closely related to eukaryotic cell apoptosis. Caspase is responsible for selectively cleaving certain proteins to cause apoptosis, and Caspase-3 is the ultimate executor of apoptosis48. Cleaved caspase-3, an activated form of caspase-3, is often used as a marker of apoptotic cells49.
For cleaved caspase-3 half quantitative analysis of protein content, the results showed that BPV increased the original generation of cleaved caspase-3 protein content by hippocampal neurons, and LE and BPV decreased the cleaved caspase-3 protein content of hippocampal neurons, showing that BPV induced hippocampal neuron apoptosis, whereas LE could reduce the apoptosis of hippocampal neurons induced by BPV, which is associated with the cell protective effects of BDNF-TrkB. However, after K252a inhibited TrkB activity, hippocampal neuron apoptosis was not statistically significant compared with that in the BPV + LE group, which may be related to decreased TrkB expression. It has been reported in the literature that K252a was not sensitive to BDNF's inhibition of the downstream excitatory cell death pathway32, and thus, the effect of LE was not significantly inhibited. After tat-pep 5 inhibited the activation of the proBDNF-p75NTR pathway, the effect of LE was significantly enhanced, and hippocampal neuron apoptosis was significantly reduced. It was speculated that LE could reduce the hippocampal neuron apoptosis caused by BPV by inhibiting the activity of the proBDNF-p75NTR pathway.
In conclusion, this study confirmed that LE may reduce hippocampal neuron apoptosis by regulating the BDNF-Trkb and probBDNF-p75NTR pathways to promote the survival of hippocampal neurons and reduce the toxicity of rat hippocampal neurons induced by BPV, providing a basis for the treatment of BPV CNS toxicity by LE in the future.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BCA:
-
Bicinchoninic acid
- BDNF:
-
Brain-derived neurotrophic factor
- BPV:
-
Bupivacaine
- BSA:
-
Bovine serum albumin
- CNS:
-
Central nervous system
- EDTA:
-
Ethylene Diamine Tetraacetic Acid
- GABA:
-
γ-Aminobutyric acid
- IC50:
-
Half maximal inhibitory concentration
- LE:
-
Lipid emulsion
- p75NTR :
-
P75 neurotrophic factor receptor
- PVDF:
-
Polyvinylidene fluoride
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TrkB:
-
Tyrosine kinase receptor B
References
Turnbull, Z. A. et al. Anesthesia for the patient undergoing total knee replacement: Current status and future prospects. Local Reg Anesth 10, 1–7. https://doi.org/10.2147/LRA.S101373 (2017).
Abdallah, F. W., Madjdpour, C. & Brull, R. Is sciatic nerve block advantageous when combined with femoral nerve block for postoperative analgesia following total knee arthroplasty? A meta-analysis. Can J Anaesth 63(5), 552–568. https://doi.org/10.1007/s12630-016-0613-2 (2016).
Barrington, M. J. & Kluger, R. Ultrasound guidance reduces the risk of local anesthetic systemic toxicity following peripheral nerve blockade. Reg Anesth Pain Med 38(4), 289–299. https://doi.org/10.1097/AAP.0b013e318292669b (2013).
Neal, J. M. Ultrasound-guided regional anesthesia and patient safety: Update of an evidence-based analysis. Reg Anesth Pain Med 41(2), 195–204. https://doi.org/10.1097/aap.0000000000000295 (2016).
Wolfe, J. W. & Butterworth, J. F. Local anesthetic systemic toxicity: Update on mechanisms and treatment. Curr Opin Anaesthesiol 24(5), 561–566. https://doi.org/10.1097/ACO.0b013e32834a9394 (2011).
Oda, Y. & Ikeda, Y. Effect of lipid emulsion on the central nervous system and cardiac toxicity of bupivacaine and levobupivacaine in awake rats. J Anesth 27(4), 500–504. https://doi.org/10.1007/s00540-013-1581-0 (2013).
Pearn, M. L. et al. Propofol neurotoxicity is mediated by p75 neurotrophin receptor activation. Anesthesiology 116(2), 352–361. https://doi.org/10.1097/ALN.0b013e318242a48c (2012).
Wu, G. et al. Lipid emulsion mitigates local anesthesia-induced central nervous system toxicity in rats. Exp Ther Med 10(3), 1133–1138. https://doi.org/10.3892/etm.2015.2594 (2015).
Weinberg, G. Current evidence supports use of lipid rescue therapy in local anaesthetic systemic toxicity. Acta Anaesthesiol Scand 61(4), 365–368. https://doi.org/10.1111/aas.12870 (2017).
Fettiplace, M. R. & McCabe, D. J. Lipid emulsion improves survival in animal models of local anesthetic toxicity: A meta-analysis. Clin Toxicol (Phila) 55(7), 617–623. https://doi.org/10.1080/15563650.2017.1288911 (2017).
Harvey, M. & Cave, G. Lipid emulsion in local anesthetic toxicity. Curr Opin Anaesthesiol 30(5), 632–638. https://doi.org/10.1097/aco.0000000000000498 (2017).
Yang, J. H. et al. Involvement of TREK-1 channel in cell viability of H9c2 rat cardiomyoblasts affected by bupivacaine and lipid emulsion. Cells https://doi.org/10.3390/cells8050454 (2019).
Kim, H. J. et al. Lipid emulsion restoration of myocardial contractions after bupivacaine-induced asystole in vitro: a benefit of long- and medium-chain triglyceride over long-chain triglyceride. Anesth Analg 131(3), 917–927. https://doi.org/10.1213/ane.0000000000004637 (2020).
Lv, D. et al. Lipid emulsion reverses bupivacaine-induced apoptosis of h9c2 cardiomyocytes: PI3K/Akt/GSK-3β signaling pathway. Environ Toxicol Pharmacol 42, 85–91. https://doi.org/10.1016/j.etap.2016.01.004 (2016).
Yang, L. et al. Rescue effect of lipid emulsion on bupivacaine-induced cardiac toxicity in cardiomyocytes. Mol Med Rep 12(3), 3739–3747. https://doi.org/10.3892/mmr.2015.3852 (2015).
Fettiplace, M. R. et al. Insulin signaling in bupivacaine-induced cardiac toxicity: Sensitization during recovery and potentiation by lipid emulsion. Anesthesiology 124(2), 428–442. https://doi.org/10.1097/aln.0000000000000974 (2016).
Park, W. K. et al. Intralipid restoration of myocardial contractions following bupivacaine-induced asystole: Concentration- and time-dependence in vitro. Anesth Analg 125(1), 91–100. https://doi.org/10.1213/ane.0000000000002124 (2017).
Yang, H. J. et al. Bupivacaine and ropivacaine suppress glycine- and glutamate-induced ion currents in acutely dissociated rat hippocampal neurons. Neurosci Lett 344(1), 33–36. https://doi.org/10.1016/s0304-3940(03)00401-4 (2003).
Dahmani, S. et al. The effects of lidocaine and bupivacaine on protein expression of cleaved caspase 3 and tyrosine phosphorylation in the rat hippocampal slice. Anesth Analg 104(1), 119–123. https://doi.org/10.1213/01.ane.0000249048.56863.08 (2007).
Nie, H. et al. Intravenous lipid emulsion modifies synaptic transmission in hippocampal CA1 pyramidal neurons after bupivacaine-induced central nervous system toxicity. J Neurochem 154(2), 144–157. https://doi.org/10.1111/jnc.14924 (2020).
Courtney, K. R. Structure-activity relations for frequency-dependent sodium channel block in nerve by local anesthetics. J Pharmacol Exp Ther 213(1), 114–119 (1980).
Nair, V. D. & Olanow, C. W. Differential modulation of Akt/glycogen synthase kinase-3beta pathway regulates apoptotic and cytoprotective signaling responses. J Biol Chem 283(22), 15469–15478. https://doi.org/10.1074/jbc.M707238200 (2008).
Ma, R. et al. Dexamethasone attenuated bupivacaine-induced neuron injury in vitro through a threonine-serine protein kinase B-dependent mechanism. Neuroscience 167(2), 329–342. https://doi.org/10.1016/j.neuroscience.2009.12.049 (2010).
Liu, L. et al. Effect of 20% long-chain lipid emulsion on amino acid neurotransmitter imbalance induced by intracerebroventricular injection of local anesthetics. J. Clin. Anesthesiol. 32(03), 273–276 (2016).
Werdehausen, R. et al. Apoptosis induction by different local anaesthetics in a neuroblastoma cell line. Br J Anaesth 103(5), 711–718. https://doi.org/10.1093/bja/aep236 (2009).
Yang, S. et al. Local anesthetic Schwann cell toxicity is time and concentration dependent. Reg Anesth Pain Med 36(5), 444–451. https://doi.org/10.1097/AAP.0b013e318228c835 (2011).
Riffault, B. et al. Pro-brain-derived neurotrophic factor inhibits GABAergic neurotransmission by activating endocytosis and repression of GABAA receptors. J Neurosci 34(40), 13516–13534. https://doi.org/10.1523/jneurosci.2069-14.2014 (2014).
Mao, X. Y. et al. Topiramate protects against glutamate excitotoxicity via activating BDNF/TrkB-dependent ERK pathway in rodent hippocampal neurons. Prog Neuropsychopharmacol Biol Psychiatry 60, 11–17. https://doi.org/10.1016/j.pnpbp.2015.01.015 (2015).
Onizuka, S. et al. Lidocaine treatment during synapse reformation periods permanently inhibits NGF-induced excitation in an identified reconstructed synapse of Lymnaea stagnalis. J Anesth 26(1), 45–53. https://doi.org/10.1007/s00540-011-1257-6 (2012).
Leal, G. et al. Regulation of hippocampal synaptic plasticity by BDNF. Brain Res 1621, 82–101. https://doi.org/10.1016/j.brainres.2014.10.019 (2015).
Leal, G., Bramham, C. R. & Duarte, C. B. BDNF and Hippocampal Synaptic Plasticity. Vitam Horm 104, 153–195. https://doi.org/10.1016/bs.vh.2016.10.004 (2017).
Gomes, J. R. et al. Excitotoxicity downregulates TrkB.FL signaling and upregulates the neuroprotective truncated TrkB receptors in cultured hippocampal and striatal neurons. J Neurosci 32(13), 4610–4622. https://doi.org/10.1523/jneurosci.0374-12.2012 (2012).
Yoo, J. M. et al. Neuroprotective action of N-acetyl serotonin in oxidative stress-induced apoptosis through the activation of both TrkB/CREB/BDNF pathway and Akt/Nrf2/Antioxidant enzyme in neuronal cells. Redox Biol 11, 592–599. https://doi.org/10.1016/j.redox.2016.12.034 (2017).
Xia, Y. et al. Brain-derived neurotrophic factor prevents phencyclidine-induced apoptosis in developing brain by parallel activation of both the ERK and PI-3K/Akt pathways. Neuropharmacology 58(2), 330–336. https://doi.org/10.1016/j.neuropharm.2009.10.009 (2010).
Zhang, Z. et al. The release of glutamate from cortical neurons regulated by BDNF via the TrkB/Src/PLC-γ1 pathway. J Cell Biochem 114(1), 144–151. https://doi.org/10.1002/jcb.24311 (2013).
Hu, M. et al. Hydrogen sulfide protects against chronic unpredictable mild stress-induced oxidative stress in hippocampus by upregulation of BDNF-TrkB pathway. Oxid Med Cell Longev 2016, 2153745. https://doi.org/10.1155/2016/2153745 (2016).
Qiao, H. et al. Role of proBDNF and BDNF in dendritic spine plasticity and depressive-like behaviors induced by an animal model of depression. Brain Res 1663, 29–37. https://doi.org/10.1016/j.brainres.2017.02.020 (2017).
Aroeira, R. I. et al. BDNF modulates glycine uptake in hippocampal synaptosomes by decreasing membrane insertion of glycine transporter 2. Neurochem Int 99, 94–102. https://doi.org/10.1016/j.neuint.2016.06.007 (2016).
Gibon, J., Barker, P. A. & Séguéla, P. Opposing presynaptic roles of BDNF and ProBDNF in the regulation of persistent activity in the entorhinal cortex. Mol Brain 9, 23. https://doi.org/10.1186/s13041-016-0203-9 (2016).
Shirayama, Y. et al. Alterations in brain-derived neurotrophic factor (BDNF) and its precursor proBDNF in the brain regions of a learned helplessness rat model and the antidepressant effects of a TrkB agonist and antagonist. Eur Neuropsychopharmacol 25(12), 2449–2458. https://doi.org/10.1016/j.euroneuro.2015.09.002 (2015).
Guo, J. et al. BDNF pro-peptide regulates dendritic spines via caspase-3. Cell Death Dis 7(6), e2264. https://doi.org/10.1038/cddis.2016.166 (2016).
Riffault, B. et al. Pro-brain-derived neurotrophic factor (proBDNF)-mediated p75NTR activation promotes depolarizing actions of GABA and increases susceptibility to epileptic seizures. Cereb Cortex 28(2), 510–527. https://doi.org/10.1093/cercor/bhw385 (2018).
Wang, Y. et al. BDNF-TrkB signaling pathway mediates the induction of epileptiform activity induced by a convulsant drug cyclothiazide. Neuropharmacology 57(1), 49–59. https://doi.org/10.1016/j.neuropharm.2009.04.007 (2009).
Liu, X. et al. BDNF-TrkB signaling pathway is involved in pentylenetetrazole-evoked progression of epileptiform activity in hippocampal neurons in anesthetized rats. Neurosci Bull 29(5), 565–575. https://doi.org/10.1007/s12264-013-1326-y (2013).
Dickerson, D. M. & Apfelbaum, J. L. Local anesthetic systemic toxicity. Aesthet Surg J 34(7), 1111–1119. https://doi.org/10.1177/1090820x14543102 (2014).
Niu, X. et al. The effects of hispidulin on bupivacaine-induced neurotoxicity: Role of AMPK signaling pathway. Cell Biochem Biophys 70(1), 241–249. https://doi.org/10.1007/s12013-014-9888-5 (2014).
Liang, Y. J. et al. Effect of ganglioglyceride pretreatment on caspase-3 expression after bupivacaine-induced apoptosis of N2a neuronal cells. J. Clin. Anesthesiol. 32(07), 688–691 (2016).
Slee, E. A., Adrain, C. & Martin, S. J. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem 276(10), 7320–7326. https://doi.org/10.1074/jbc.M008363200 (2001).
Logue, S. E. & Martin, S. J. Caspase activation cascades in apoptosis. Biochem Soc Trans 36(Pt 1), 1–9. https://doi.org/10.1042/bst0360001 (2008).
Funding
This study was funded by the Scientific Research Fund of Ningxia Medical University (XZ2018003).
Author information
Authors and Affiliations
Contributions
D.J. and F.W. wrote the main manuscript text, conducted experimented and analyzed data, Z.B. developed the methodology and prepared figures. X.C. administered the project and provide resources. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jia, D., Wang, F., Bai, Z. et al. BDNF-TrkB/proBDNF-p75NTR pathway regulation by lipid emulsion rescues bupivacaine-induced central neurotoxicity in rats. Sci Rep 13, 18364 (2023). https://doi.org/10.1038/s41598-023-45572-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-45572-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.