Abstract
Angiotensin II receptor 1(AT1) antagonists are beneficial in focal ischemia/reperfusion (I/R). However, in cases of global I/R, such as cardiac arrest (CA), AT1 blocker's potential benefits are still unknown. Wistar male rats were allocated into four groups: Control group (CG)—animals submitted to CA by ventricular fibrillation induced by direct electrical stimulation for 3 min, and anoxia for 5 min; Group AT1 (GAT1)—animals subjected to CA and treated with 0.2 mg/kg of candesartan diluted in dimethylsulfoxide (DMSO) (0.1%); Vehicle Group (VG): animals subjected to CA and treated with 0.2 ml/kg of DMSO and Sham group (SG)—animals submitted to surgical interventions, without CA. Cardiopulmonary resuscitation consisted of group medications, chest compressions, ventilation, epinephrine (20 mcg/kg) and defibrillation. The animals were observed up to 4 h after spontaneous circulation (ROSC) return, and survival rates, hemodynamic variables, histopathology, and markers of tissue injury were analyzed. GAT1 group had a higher rate of ROSC (62.5% vs. 42.1%, p < 0.0001), survival (100% vs. 62.5%, p = 0.027), lower incidence of arrhythmia after 10 min of ROSC (10% vs. 62.5%, p = 0.000), and lower neuronal and cardiac injury scores on histology evaluation (p = 0.025 and p = 0.0052, respectively) than GC group. The groups did not differ regarding CA duration, number of adrenaline doses, or number of defibrillations. AT1 receptor blockade with candesartan yielded higher rates of ROSC and survival, in addition to neuronal and myocardial protection.
Similar content being viewed by others
Introduction
Cardiac arrest (CA) is the most severe adverse event in the perioperative scenario. Even if promptly assisted, it has a high mortality rate, ranging from 35% immediately after the event to approximately 70% after one year1,2,3,4. Several studies have discussed ways to intervene and reduce the damage caused by global ischemia due to CA5,6,7.
Recent studies have demonstrated the use of renin-angiotensin system (RAS) inhibitors as attenuators of tissue damage in ischemia–reperfusion (I/R)8,9,10,11,12. Angiotensin II, the main active principle of the RAS, exerts its effect primarily by stimulating angiotensin receptors 1 (AT1R), specifically in the brain, AT1R are highly expressed in cerebrovascular endothelial cells and have an essential role in regulating cerebrovascular flow and the function of the blood–brain barrier13, 14. Angiotensin II has also been linked to direct neurotoxic effects, leading to intracellular generation of reactive oxygen species, increased production of inflammatory cytokines and downregulation of the anti-inflammatory peroxisomes13, 15, 16. Thus, angiotensin receptor antagonists (ARA) have been shown to protect cerebral blood flow, maintain blood–brain barrier function, prevent excessive brain inflammation and neuronal injury in animal models of stroke, traumatic brain injury, Alzheimer's and Parkinson's disease. Direct neuroprotection has also been demonstrated, for candesartan and telmisartan, attenuating known mechanisms of neuronal damage by reducing glutamate excitotoxicity, excessive pro-inflammatory interleukin-1beta (IL-1β), and hypoxia induced-lesions17,18,19,20. Blockade of the AT1 receptor also provided cardioprotective effects in the setting of ischemia–reperfusion, and has been shown to reduce infarct size, ischemia-related arrhythmias, and attenuate ventricular dilatation after myocardial infarction21,22,23,24. Specifically, candesartan has been shown to improve the functional recovery of reperfused myocardium, decreasing myocardial stunning25.
Although previous studies have shown the benefits of ARA use under localized I/R, there are no studies with the use of ARA in situations of multiple organ ischemia, such as CRA. Therefore, we aimed to evaluate the role of angiotensin II AT1-receptor blockers on myocardial and brain lesions after a CRA, as well as return of spontaneous circulation rates (ROSC) and survival.
Methods
An experimental study was conducted with male Wistar rats weighing between 380 and 465 g from the Central Animal Facility of the Faculty of Medicine of the University of São Paulo. The Ethics Committee approved the study for the Use of Animals of the University of São Paulo—CEUA-USP, protocol number: 034/16. All experiments were performed in accordance with relevant guidelines and regulations.
After being anesthetized and subjected to surgical procedures, the rats were allocated in a simple random fashion into four different groups.
Sham Group (SH): Anesthetized animals submitted to surgical instrumentation procedures for hemodynamic monitoring but with no ventricular fibrillation (VF) induction.
Control Group (CG): Animals submitted to CRA within 6 min of anoxia, followed by cardiopulmonary resuscitation (CPR). At the start of CPR, 20 mcg/kg of adrenaline and 0.5 ml of 0.9% saline were administered.
Vehicle group (VG): Animals submitted to CRA within 6 min of anoxia, followed by CPR. Adrenaline (20 mcg/kg) and 0.2 ml/kg of 99% dimethylsulfoxide (DMSO) were administered at the beginning of CPR.
Group AT1 (GAT1): Animals submitted to CRA within 6 min of anoxia, followed by CPR. At the beginning of CPR, adrenaline (20 mcg/kg), together with an intravenous dose of the candesartan cilexetil metabolite, Candesartan (> 98%, CAS 139481-59-7), diluted in DMSO (99%) in 0.1%, at a dose of 0.2 mg/kg was injected.
Induction of experimental cardiorespiratory arrest and resuscitation
After induction of general anesthesia with 1,2% isoflurane (1 MAC), electrocardiographic monitoring and orotracheal intubation were performed with a 16 G flexible venous catheter (B. Braun). Afterward, the animals were subjected to mechanical ventilation with a tidal volume of 8 ml/kg and a respiratory rate of 60 breaths per minute (bpm). A rectal thermometer adjusted body temperature to 37 + /− 1 °C. An incision in the cervical region was performed, and the right internal jugular vein was located. The tip of a 3F polyurethane pediatric venous catheter, size 0.6 mm ID and 1.0 mm OD (3F), was cut at a 45° angle for advancement into the right atrium.
The 3F external jugular catheter was marked 4 cm from the tip. This same catheter was used to advance a guidewire into the right ventricle for electrical induction of VF with the subsequent option of using it for drug delivery and blood sampling. Next, the inguinal region was incised, and another PE10 catheter was introduced into the right common femoral artery to collect parameters and invasively measure blood pressure using a data acquisition system (MP-100, BIOPAC, USA). The electrocardiographic (ECG) analysis was performed using a module coupled to the same system.
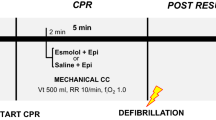
For CPR induction, the catheter was inserted through the jugular and guided by the pressure curve to the right ventricle. Through this, an electrical conductor was introduced, and an electrical discharge of 1 mA (9 V, 60 Hz) was applied and maintained for 3 min. Simultaneously, mechanical ventilation was discontinued. Then, the guide was removed, and the venous catheter was retracted 1 cm and fixed for drug administration. The animals were maintained for another 3 min in CPR, totaling 6 min from the beginning of VF induction. Confirmation of VF induction was confirmed by observing the cessation of aortic pulsations, an exponential drop in aortic pressure below 20 mm Hg and the appearance of disorganized electrical activity on the ECG. After, cardiopulmonary resuscitation maneuvers were started. An automatic compressor performed external chest compressions at a frequency of 200 compressions per minute and chest depth of 1.5 cm26. Together with chest compression, ventilation was reestablished (RR-25ipm), and a mixture of adrenaline (20mcg/kg) and sodium bicarbonate (1 mEq/kg) was administered.
The heart rate was checked every 3 min. If VF was present, a 7 J discharge was performed. After defibrillation, CPR was resumed for another 3 min, together with a new dose of adrenaline. After a heart rate check, arterial pulsations were taken as a ROSC. The maintenance of mean arterial pressure above 25 mmHg for 10 min was considered sustained ROSC as described in the protocol by Lamoureux et al., 201527. If there were no pulsations in the second check and still in VF, a new defibrillation load of 7 J was applied, and chest compressions were resumed, adding a fresh dose of adrenaline. Then, new defibrillations and rhythm checks were performed every 2 min, and adrenaline was administered every 3 min. After 20 min of CPR, efforts were finalized. However, if sustained ROSC and hemodynamic stability were established, the animals were observed for another 4 h (Fig. 1).
The animals surviving the 4-h period had a blood sample collected for troponin measurement. After, they were sacrificed by an overdose of inhalational anesthetic (6% isoflurane). Afterward, the heart and brain were extracted for processing and stored partly in formalin and partially frozen at − 70 °C.
Outcomes assessment
Hemodynamic evaluation
Blood pressure, heart rate, and cardiac rhythm were continuously recorded from the beginning of surgical instrumentation procedures, after anesthetic induction, and until animal sacrifice. The parameters were recorded on data sheets every 10 min, from the ROSC until the end of the experiment.
To analyze the quality of CPR maneuvers, diastolic blood pressure was observed for values above 20 mmHg. Cardiac arrhythmias, when present, were classified as tachyarrhythmia, bradyarrhythmia, ventricular arrhythmia, or atrial arrhythmia, according to the Lambeth Convention guideline28.
Laboratorial determination
Blood samples (1 mL in a heparinized syringe) were collected at pre-CPR induction, after CPR, and after 4 h of observation. Values for pH, pO2, pCO2, lactate, electrolytes (sodium, potassium, chlorine, and calcium), hemoglobin, hematocrit and troponin I were checked. Samples were allocated in specific tubes and analyzed by an ABL800 Flex Radiometer device.
Histology
After the animals were sacrificed, the brain and heart were carefully dissected and immediately fixed in 4% paraformaldehyde in phosphate buffer pH 7.0 for 24 h. After, they were dehydrated in an alcoholic gradient (70º to 100º), cleared in xylol, and embedded in paraffin. 5 µm cuts were obtained for morphological and morphometric evaluation.
The evaluation was performed using tissue morphometry and the percentage of inflammatory cells per unit area of parenchyma. In each histological section, digital images of 10 random fields were obtained, covering the full extension of the tissue, using the Image Pro-Plus program and a light microscope at 400 × magnification. For the analysis, we used a programming tool to calculate the area occupied by the parenchyma.
The pathologist responsible for the analysis was blind to the animal group and the corresponding slide. The histopathological evaluation of both the brain and the myocardium was scored based on the percentage of lesions through manual quantification for each photomicrograph: 1 = (< 30%); score 2 = (31%–60%); score 3 = (> 60%)29.
TUNEL
The Terminal deoxynucleotidyl transferase-mediated (dUTP) nick end Labeling kit, also called the Tunel labeling index, was used to analyze cell injury and apoptosis. Tissue section 4–5 µm thick were placed on silanized slides (Sigma Chemical Co.; St. Louis, Missouri, USA) in a suitable holder.
Using a light microscope (BLUE1600BAL-BAT-Biofocus), the slides were manually counted at 100× magnification. Twenty-five brain fields and 50 heart fields were analyzed. For the heart, 25 were from the right ventricle, and 25 were from the left. Means for each slide and the median for each group were then calculated. The slides were blindly read for each group.
Statistical analysis
The sample calculation was obtained from the troponin value after cardiac arrest in previous studies30, 31. Nine animals per group were needed for a 15% difference between groups, with a type 1 error (α) of 0.05 and a power of 0.80 for a two-tailed test. However, anticipating losses, 10 animals per group were estimated. As the current PCR model presents a mortality of 50% of the animals, the availability of 64 animals for the study was suggested. Initially, descriptive measures were obtained for the minimum and maximum values, mean, and standard deviation variables. Such descriptive measures discriminated the data according to the study period (post and final) and according to the groups.
All continuous variables are presented as Mean ± SD after the Kolmogorov‒Smirnov test confirms the normality. Data were analyzed by one-way analysis of variance (1-way ANOVA) or 2-way ANOVA, where appropriate, followed by post hoc Tukey tests for comparisons between different groups. Non-normal data were analyzed by Kruskal–Wallis. Fisher's exact test (chi-square) was used to analyze CPR rate, survival, and myocardial and brain injury scores. Statistical significance was assumed for p < 0.05. Statistical analyzes were performed in SPSS version 23.0 for Windows (SPSS, Chicago, IL).
Ethical approval
An experimental study was carried out with rats, set in the Laboratory of Medical Investigation of Anesthesiology (LIM-08), at the Faculty of Medicine of the University of São Paulo, approved by the Commission for Ethics in the Use of Animals of the University of São Paulo (CEUA- USP), protocol number: 034/16.
Results
The study was conducted with 93 rats to achieve the sample size of 10 animals surviving 4 h after ROSC in GAT1 and GC. The VG was excluded from the analysis, since among the 26 animals used in the vehicle group, only 3 met ROSC criteria, and none completed the observational period (Fig. 2). Nine animals were discarded (five due to iatrogenic injury and four due to failure to induce CPR).
Baseline and cardiopulmonary resuscitation parameters
There were no significant differences in baseline physiological and hemodynamic parameters among the groups before the CRA (Table 1). In addition, there were no differences in CPR duration, the number of adrenaline doses, or defibrillations (Table 2).
Return of spontaneous circulation
The GAT1 had a ROSC rate of 62.5% (10/16), higher than the control group (42.1% (16/38; X2: 42.9; p = 0.012). Five animals in the control group had a non-shockable rhythm after starting the CPR maneuvers and were not submitted to defibrillation. Three animals in the GAT1 group had a non-shockable rhythm (p = 0.94).
All animals in the GAT1 that met ROSC criteria survived until the end of the study. Ten (62.5%) of the 16 animals in the control group survived until the end of the established period (Fig. 3) (X2: 4 0.87, p = 0.027).
Post-cardiopulmonary resuscitation data
Hemodynamic variables
A significant reduction in blood pressure was observed in the GAT1 group, from 30 min onwards, for the animals that survived during the four hours of the experimental period compared to the GC and Sham groups. The difference was statistically significant up to two hours (p = 0,041; Fig. 4A).
The animals in the CG group had more cardiac arrhythmias in the ten-minute interval after ROSC. Eleven animals had arrhythmias, ten from the CG group. In these, non-sustained ventricular tachycardia (NSVT) was observed in seven animals; three had supraventricular arrhythmia. One animal from the GAT1 group had NSVT. This data was statistically significant between groups (X2: 4.85; p = 0.013). The animals in the Sham group did not have arrhythmias during the observation period. Analyzing the temporal heart rate, it was observed that the GAT1 group had lower median HR than the CG and Sham groups, statistically significant only between 30 and 50 min (CG vs GAT1 p = 0.037; 0.044; Fig. 4B).
Troponin I
The measurement of troponin I was performed 4 h after animal resuscitation. CG group mean was 18,265 ± 8725 pg/mL, while the GAT1 group mean was 18,027 ± 8658 pg/mL, which is not statistically significant. Compared to the Sham group, these values were markedly higher (824 ± 1149 pg/mL).
The animals that met the ROSC criteria showed metabolic acidosis in both groups undergoing CPR. However, the GAT1 group had a higher mean pH than the control group (7.183; 7.101, U: 432, p = 0.032). Similarly, higher values were observed for potassium (3.1; 3.35; U: 456 p = 0.045), base excess (− 9.75; − 12.1; U: 434 p = 0.048), and bicarbonate (17.4; 15.95, U: 509, p = 0.037). Data from arterial blood gas at the end of the study protocol was similar between the variables, and acidosis regulation was observed, moving towards standard values over time. Only the hematocrit of the Sham group was lower than the other groups. Medians for lactate, base excess, and bicarbonate showed no statistical difference between the groups (Table 3).
Cerebral and myocardial injury
On cerebral evaluation, microscopic evaluation showed more red neurons and signs of necrosis throughout the parietal cortex and hippocampus in the CG group compared to the GAT1 group (p = 0.025). The Sham group showed no signs of neuronal injury (Fig. 5A). The distribution of neuronal injury scores between the groups is seen in Fig. 5B.
(A) and (B) Histological analysis of cerebral tissue samples collected from rats subjected to cardiac arrest and treated with adrenaline (GC, n = 10) or candesartan (GAT1, n = 10) and sham-operated rats (Sham, n = 4). (A) Cerebral tissue sections stained with H&E, showing neuronal injury at 4 h after sepsis induction. Magnification 50 μm × 100. The black arrow represents red neurons. (B) Neuronal injury score measured in the brain cortex.
On microscopic evaluation, myocardial cells were orderly arranged with intact nuclei in the Sham group. In the CG group, myocardial cells were irregular, and many immune cells were aggregated (Fig. 6A). Compared to the GAT1 group, fewer myocardial cells were disorganized, and cell infiltrations were lower in the latter group (Fig. 6B). The CG's myocardial damage score increased significantly compared with the Sham group. Furthermore, the myocardial damage score significantly decreased in the GAT1 group compared to the CG group, as seen in Fig. 6B.
(A) and (B). Histological analysis of myocardial tissue samples collected from rats subjected to cardiac arrest and treated with adrenaline (GC, n = 10) or candesartan (GAT1, n = 10) and sham-operated rats (Sham, n = 4). (A) Heart tissue sections stained with H&E, showing myocardial injury at 4 h after cardiac arrest induction. Magnification 50 μm × 100. The black arrow represents lymphocytic infiltrate. (B) Myocardial injury score measured in the subendocardial layer in the left and right ventricle.
When using the TUNEL method, similar medians were observed for the number of apoptotic cells in both brain and heart groups. The Sham group showed no signs of cell injury.
Discussion
To the best of our knowledge, this is the first report that evaluates the effect of AT1 receptor blockade during cardiac arrest as an adjunct to advanced life support. We demonstrated that the antagonism of Angiotensin II AT1 receptor with candesartan leads to a higher rate of ROSC and survival in rats after CA. The results also indicate that candesartan attenuates neuronal and myocardial injury when administered during CPR maneuvers.
Effective cardioprotective strategies after ROSC are scarce in the literature. Therefore, finding interventions that effectively improve post-resuscitation myocardial and neuronal dysfunction is critical for patient prognosis.
Cardiac dysfunction associated with post-CPR syndrome is characterized by severe myocardial impairment and global hypokinesia, affecting the success rate of cardiopulmonary resuscitation. Cardiac function deterioration begins minutes after arrest and peaks within 2 to 5 h after resuscitation, reinforcing early intervention and treatment32. Proposed molecular mechanisms for post-resuscitation myocardial dysfunction involve I/R injury, resulting in large amounts of oxygen free radicals that damage cell membranes and induce myocyte necrosis and apoptosis. Moreover, an intracellular accumulation of Na + occurs through the cytosolic overload of Ca2+ under the action of the Na+/Ca2+ exchanger present in the sarcolemma membrane33.
The presence of endogenous or paracrine RAS in the heart has been recently recognized. RAS components—angiotensinogen and renin messenger RNA, angiotensin I to angiotensin II (Ang II) converting enzyme—have previously been detected in the heart and seem functionally integrated11,12,13,14. Cardiac Ang II may be involved in regulating coronary blood flow, modulation of sympathetic neurotransmission, cardiac contractility, and stimulation of cell hyperplasia and hypertrophy, in addition to repairing the cardiovascular structure. Angiotensin II interacts with two pharmacologically distinct subtypes of cell surface receptors, type 1 and type 2 Ang II receptors. Type 1 receptors mediate the main cardiovascular effects of Ang II25. Such mechanisms may explain the significant differences found in the lower myocardial injury score in the GAT1 group reported here.
Furthermore, limiting Na+ influx into the sarcolemma during ventricular fibrillation resuscitation prevents the accumulation of excess mitochondrial Ca2+ and attenuates myocardial injury34. This mechanism may explain the significant reduction in cardiac arrhythmias observed here since no differences in CPR duration, number of defibrillations, or adrenaline doses between groups were observed. Thus, we suggest that the action of candesartan does not interfere with the reversal of heart rhythm (VF or VT) during CPR. However, as the incidence of cardiac arrhythmias one hour after ROSC was higher in the control group, the drug could potentially affect the reduction of new arrhythmogenic triggers. This mechanism leads to the blockade of Angiotensin II AT1 receptors, explaining the higher survival of the animals in the GAT1 group, as demonstrated in previous studies22,23,24,25.
Since retrospective studies have suggested that high cumulative epinephrine dosage is associated with worse hemodynamic and neurological outcomes and, although it may improve coronary perfusion and increase vascular resistance to promote initial ROSC during CPR, these same effects may lead to increased myocardial dysfunction and occasionally a severe toxic hyperadrenergic state in the post-resuscitation period35,36,37,38,39.
Based on the facts, previous animal studies have shown that using large doses of sodium nitroprusside during CPR significantly improved hemodynamics when used as a simulated ACLS intervention40. Our hypothesis is that ARA could behave similarly, optimizing vital organ perfusion pressures and redirecting blood flow to the brain and thorax, significantly improving myocardial perfusion, and facilitating spontaneous ROSC.
The findings from brain histopathology point to candesartan as a potential neuroprotector in ischemic injury caused by CA. Hyperactivation of the brain AT1 receptor is responsible for the harmful effects associated with the RAS, leading to vasoconstriction, cerebral blood flow decrease, increased oxidative stress, and vulnerability to ischemia, in addition to promoting vascular and tissue inflammation and neurodegeneration exacerbation41. AT2 stimulation counteracts these mechanisms. Therefore, selective blocking of AT1 receptors with Angiotensin Block Receptors (ABRs), especially candesartan, which crosses the blood–brain barrier42, may offer superior protection than simultaneously decreasing AT1 and AT2 receptors, such as angiotensin-converting enzyme inhibitors (ACE inhibitors). Such mechanisms were used by Hajjar et al. (2020)43, demonstrating less neurocognitive impairment in an elderly population that used candesartan instead of Lisinopril, regardless of blood pressure control.
Recently, AT2 receptors have also been linked to neuroprotection, especially after a stroke. These studies have shown that under pathological conditions, such as ischemic insult, AT2 receptor expression may be upregulated and it has been speculated that the increased stimulation of the AT2 receptor may be responsible for some of the therapeutic effects observed during AT1 receptor antagonism44, 45.
It has also been shown that the coadministration of an AT2 receptor antagonist decreases the potential neuroprotection conferred by candesartan or irbesartan on animal models of cerebral artery occlusion, clearly demonstrating that AT2 receptor activation contributed to the effect of AT1 receptor antagonists44, 46. This is also supported by the fact that AT2 receptor-deficient mice have larger infarct volumes and poorer neurological outcomes after stroke45, 47.
Our study lacks a group with AT2 receptor antagonism, making it unclear whether the neuro- and cardioprotective effects were due only to the AT1 receptor antagonism or could be from AT2 stimulation. This is a limitation of our study and further investigations are needed to clear this question.
As for the metabolic analysis of I/R, the GAT1 group had a lower level of acidosis and base intake than the CG group, suggesting that the combination of adrenaline and candesartan can protect myocardial tissue and improve energy metabolism after ROSC. Since candesartan is one of the most potent aldosterone suppressors and higher potassium levels are implicated in lower ROSC rates, we expected higher potassium levels on GAT148,49,50. However, potassium levels were lower 10 min after ROSC. We infer that these findings might be attributed to the higher pH of this group. In addition, aldosterone has been shown to induce myocyte apoptosis in the heart, which candesartan might have blocked, also explaining the better cardioprotection in this group51.
The best survival rate for the GAT1 group corroborates this hypothesis. Furthermore, the lower blood pressure in the GAT1 group compared to the control and Sham groups may be due to the hypotensive effect of angiotensin II antagonism10. This finding may compromise the translation of this experimental study into clinical studies since we do not know the behavior of this drug after CPR in humans.
Limitations
The main limitations of this study include the absence of a group that received candesartan alone during resuscitation maneuvers. However, the use of adrenaline as a significant factor for ROSC increase in rats has already been documented15.
Another limitation of this study is that we have only evaluated the animals for a short period (4 h); thus, long-term effects could not be investigated. In addition, we have not tested other doses of candesartan infusion, which could limit the side effects and still have the potential for organ protection. Since the ROSC rates were lower than the experimental model adopted by our research group27, more animals were required to reach 10 per intervention and control group, as indicated in the study design. Although based on estimated sample calculation, the small sample size may reduce the study's statistical power. Our study is primarily descriptive, and exploratory mechanisms are needed to better clarify our findings.
Our results suggest good perspectives for using angiotensin II AT1 receptor antagonists for cardiopulmonary resuscitation protocols, especially in assisted arrests, where the intervention time is shorter compared to out-of-hospital CAs.
Data availability
Availability of data upon request to the main author Araujo Filho EAF (elsonfilho2@hotmail.com).
References
Yan, S. et al. The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: A systematic review and meta-analysis. Crit. Care. 24(1), 1–13 (2020).
Vane, M. F., Carmona, M. J. C. & Auler, J. O. C. Predictors and their prognostic value for no ROSC and mortality after a non-cardiac surgery intraoperative cardiac arrest: A retrospective cohort study. Rev. Bras. Anestesiol. 67(4), 440–449 (2017).
Girardi, L. N. & Barie, P. S. Improved survival after intraoperative cardiac arrest in noncardiac surgical patients. Arch. Surg. 130, 15–18 (1995).
Schneider, A. P., Nelson, D. J. & Brown, D. D. In-hospital cardiopulmonary resuscitation: A 30-year review. J. Am. Board Family Pract. 6, 91–101 (1993).
Qiu, Y. et al. Rosuvastatin improves myocardial and neurological outcomes after asphyxial cardiac arrest and cardiopulmonary resuscitation in rats. Biomed. Pharmacother. 1(87), 503–508 (2017).
Kato, R. & Foëx, P. Fentanyl reduces infarction but not stunning via δ-opioid receptors and protein kinase C in rats. Br. J. Anaesth. 84(5), 608–614 (2000).
Landoni, G., Bignami, E., Oliviero, F. & Zangrillo, A. Halogenated anaesthetics and cardiac protection in cardiac and non-cardiac anaesthesia. Ann. Card. Anaesth. 12(1), 4–9 (2009).
Ferreira, A. J., Santos, R. A. & Almeida, A. P. Angiotensin-(1–7): Cardioprotective effect in myocardial ischemia/reperfusion. Hypertension 38(3 Pt 2), 665–668 (2001).
Dzau, V. J. Tissue angiotensin and pathobiology of vascular disease a unifying hypothesis. Hypertension 37(4), 1047–1052 (2001).
Hoyer, A., Kempfert, J., Pritzwald-Stegmann, P., Mohr, F. W. & Dhein, S. Acute hemodynamic effects of angiotensin-converting enzyme inhibition after prolonged cardiac arrest with Bretschneider’s solution. Naunyn-Schmiedeberg’s Arch. Pharmacol. 387, 1221–1229 (2014).
Kontogiannis, J. & Burns, K. D. Role of AT1 angiotensin II receptors in renal ischemic injury. Am. J. Physiol. Renal. Physiol. 274(1), F79–F90 (1998).
Blume, A. & Unger, T. The renin-angiotensin system in the brain: Possible therapeutic implications for AT1-receptor blockers. J. Hum. Hypertens. 16, S64-70 (2002).
Min, L. J. et al. Direct stimulation of angiotensin II type 2 receptor initiated after stroke ameliorates ischemic brain damage. Am. J. Hypertens. 27(8), 1036–1044 (2014).
Zhou, J. et al. AT1 receptor blockade regulates the local angiotensin II system in cerebral microvessels from spontaneously hypertensive rats. Stroke 37(5), 1271–1276 (2006).
Kumar, R. & Thomas, C. M. The intracrine renin-angiotensin system. Clin. Sci. (Lond.) 123, 273–328 (2012).
Touyz, R. M. Angiotensin II and endothelin-1 regulate MAP kinases through different redox-dependent mechanisms in human vascular smooth muscle cells. J. Hypertens. 22, 1141–1149 (2004).
Benicky, J. et al. Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology 36(4), 857–870 (2011).
Benicky, J., Sánchez-Lemus, E., Pavel, J. & Saavedra, J. M. Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cell Mol. Neurobiol. 29(6–7), 781–792 (2009).
Danielyan, L. et al. Angiotensin receptor type 1 blockade in astroglia decreases hypoxia-induced cell damage and TNF alpha release. Neurochem. Res. 32(9), 1489–1498 (2007).
Wang, J. et al. Telmisartan ameliorates glutamate-induced neurotoxicity: Roles of AT(1) receptor blockade and PPARγ activation. Neuropharmacology 79, 249–261 (2014).
Jalowy, A., Schulz, R., Dörge, H., Behrends, M. & Heusch, G. Infarct size reduction by AT1-receptor blockade through a signal cascade of AT2-receptor activation, bradykinin and prostaglandins in pigs. J. Am. Coll. Cardiol. 32(6), 1787–1796 (1998).
Shimizu, M., Wang, Q. D., Sjöquist, P. O. & Rydén, L. Angiotensin II type 1 receptor blockade with candesartan protects the porcine myocardium from reperfusion-induced injury. J. Cardiovasc. Pharmacol. 32(2), 231–238 (1998).
Kohya, T. et al. Regression of left ventricular hypertrophy prevents ischemia-induced lethal arrhythmias. Beneficial effect of angiotensin II blockade. Circ. Res. 76(5), 892–899 (1995).
Ozer, M. K., Sahna, E., Birincioglu, M. & Acet, A. Effects of captopril and losartan on myocardial ischemia-reperfusion induced arrhythmias and necrosis in rats. Pharmacol. Res. 45(4), 257–263 (2002).
Dörge, H., Behrends, M., Schulz, R., Jalowy, A. & Heusch, G. Attenuation of myocardial stunning by the AT1 receptor antagonist candesartan. Basic Res. Cardiol. 94(3), 208–214 (1999).
Vane, M. F. et al. Cardiac arrest animal model: A simple device for small animals’ chest compression. Braz. J. Anesthesiol. 67, 440–441 (2017).
Lamoureux, L., Radhakrishnan, J. & Gazmuri, R. J. A rat model of ventricular fibrillation and resuscitation by conventional closed-chest technique. J Vis. Exp. 98, e52413 (2015).
Walker, M. J. A. et al. The Lambeth Conventions: Guidelines for the study of arrhythmias in ischaemia, infarction, and reperfusion. Cardiovasc. Res. 22(7), 441–455 (1988).
Liu, H. et al. Protective mechanisms of the angiotensin II type 1 receptor blocker candesartan against cerebral ischemia: In-vivo and in-vitro studies. J. Hypertens. 26(7), 1435–1445 (2008).
Lin, J. Y. et al. Model of cardiac arrest in rats by transcutaneous electrical epicardium stimulation. Resuscitation 81(9), 1197–1204 (2010).
Kern, K. B., Hilwig, R. W., Rhee, K. H. & Berg, R. A. Myocardial dysfunction after resuscitation from cardiac arrest: An example of global myocardial stunning. J. Am. College Cardiol. 28(1), 232–240 (1996).
Soar, J. et al. Part 4: Advanced life support. 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation 2015(95), e71-120 (2015).
Lee, H.-L., Chen, C.-L., Yeh, S. T., Zweier, J. L. & Chen, Y.-R. Biphasic modulation of the mitochondrial electron transport chain in myocardial ischemia and reperfusion. Am. J. Physiol. 302, H1410–H1422 (2012).
Carden, D. L. & Granger, D. N. Pathophysiology of ischaemia–reperfusion injury. J. Pathol. 190(3), 255–266 (2000).
Rivers, E. et al. The effect of the total cumulative epinephrine dose administered during human CPR on hemodynamic, oxygen transport, and utilization variables in the postresuscitation period. Chest 106, 1499–1507 (1994).
Behringer, W. et al. Cumulative epinephrine dose during cardiopulmonary resuscitation and neurologic outcome. Ann. Intern. Med. 129, 450–456 (1998).
Berg, R. A. et al. A randomized, blinded trial of high-dose epinephrine versus standard-dose epinephrine in a swine model of pediatric asphyxial cardiac arrest. Crit. Care Med. 24, 1695–1700 (1996).
Hornchen, U., Lussi, C. & Schuttler, J. Potential risks of high-dose epinephrine for resuscitation from ventricular fibrillation in a porcine model. J. Cardiothorac. Vasc. Anesth. 7, 184–187 (1993).
Neumar, R. W. et al. Epinephrine and sodium bicarbonate during CPR following asphyxial cardiac arrest in rats. Resuscitation 29, 249–263 (1995).
Schultz, J. C. et al. Sodium nitroprusside-enhanced cardiopulmonary resuscitation improves resuscitation rates after prolonged untreated cardiac arrest in two porcine models. Crit. Care Med. 39(12), 2705–2710 (2011).
Sugawara, T. et al. Candesartan reduces superoxide production after global cerebral ischemia. Neuroreport 16(4), 325–328 (2005).
Stoukides, C. A., McVoy, H. J. & Kaul, A. F. Candesartan cilexetil: An angiotensin II receptor blocker. Ann. Pharmacother. 33(12), 1287–1298 (1999).
Hajjar, .I., Okafor, M., Goldstein, F., & Points, K. Effects of candesartan vs lisinopril on neurocognitive function in older adults with executive mild cognitive impairment a randomized clinical trial.
Li, J. et al. Angiotensin AT2 receptor protects against cerebral ischemia-induced neuronal injury. FASEB J. 19(6), 1–25 (2005).
Mogi, M. et al. Angiotensin II type-2 receptor stimulation prevents neural damage by transcriptional activation of methyl methanesulfonate sensitive 2. Hypertension 48, 141–148 (2006).
Lu, Q., Zhu, Y.-Z. & Wong, P.T.-H. Neuroprotective effects of candesartan against cerebral ischemia in spontaneously hypertensive rats. Neuroreport 16, 1963–1967 (2005).
Iwai, M. et al. Possible inhibition of focal cerebral ischemia by angiotensin II type 2 receptor stimulation. Circulation 110, 843–848 (2004).
Koizumi, G. et al. Analysis of the relationships between multiple endocrine hormones and return of spontaneous circulation (ROSC) in cardiac arrest patients: possible association of the serum free T4 level with ROSC. Int. J. Endocrinol. 30(2020), 4168420 (2020).
Markan, U. et al. The place of ARBs in heart failure therapy: Is aldosterone suppression the key?. Ther. Adv. Cardiovasc. Dis. 13, 1753944719868134 (2019).
Ferraino, K. E. et al. Adrenal angiotensin II type 1 receptor biased signaling: The case for “biased” inverse agonism for effective aldosterone suppression. Cell Signal. 82, 109967 (2021).
Burnistone, J., Saini, A., Tan, L. & Goldspink, D. Aldosterone induces myocyte apoptosis in the heart and skeletal muscles of rats in vivo. J. Mol. Cellular Cardiol. 39(2), 395–399 (2005).
Acknowledgements
We thank the staff and members of the Anesthesiology Department from Faculdade de Medicina da USP for their support.
Funding
There was no funding for the work.
Author information
Authors and Affiliations
Contributions
E.A.F.A.F.: Main author, responsible for the design and execution of the project, data acquisition, data analysis and interpretation; in addition to writing and drafting the article or critically reviewing it for its intellectual content. M.J.C.C.: Contributed to the elaboration of the methodology, revision of the writing and organization of the scientific article D.A.O.: Contributed with the execution of the work, from surgical instrumentation of the animals to data collection. In addition to the literature review and statistical analysis. D.R.R.M.: Participated in data collection, review of statistical analysis and execution of laboratory tests. L.G.C.A.L.: Participated in the histopathological analysis of the brain and myocardial tissues of the animals involved in the study. M.F.V.: Responsible for designing the project, coordinating and guiding the entire execution of the work, as well as reviewing the writing and statistical analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Araújo Filho, E.A.F., Carmona, M.J.C., Otsuki, D.A. et al. Effect of AT1 receptor blockade on cardiovascular outcome after cardiac arrest: an experimental study in rats. Sci Rep 13, 18269 (2023). https://doi.org/10.1038/s41598-023-45568-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-45568-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.