Abstract
The current meta-analysis aims to investigate the existing articles that evaluated the implications of a positive family history of cancer on the risk of colorectal cancer (CRC) within the EMRO countries. We employed PubMed, Scopus, and Web of Science as search databases for this study. To assess the quality of the selected articles, we utilized the Newcastle–Ottawa (NCO) checklist. In comparing the impact of a family history of cancer between the case and control groups, we computed the odds ratio (OR) along with its corresponding 95% confidence interval (CI). Finally, 27 articles were selected for meta-analysis. The result of the meta-analysis showed a significant association between the presence of a family history of CRC or any cancers and CRC (OR 2.21; 95% CI 1.54–3.17; P < 0.001, OR 1.76; 95% CI 1.27–2.42; P = 0.001, respectively). Our findings underscore the critical importance of timely screening and early identification for individuals with a family history of cancer. By fostering close coordination among healthcare facilities and actively promoting the adoption of screening methods for early detection, we have the potential to significantly reduce both mortality rates and financial burdens of CRC on the general public, ultimately leading to enhanced patient outcomes.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) ranks as the third most frequently diagnosed cancer globally and is the second leading cause of cancer-related deaths worldwide. It constitutes approximately 10% of all cancer cases and is responsible for 9.4% of cancer-related fatalities on a global scale1. The incidence of CRC is higher in highly-developed countries, while the increasing number of CRC cases in middle- and low-income countries may be linked to the process of Westernization2. For example, in a recent analysis conducted by Mehta et al., it was found that adherence to Western dietary patterns was significantly associated with an increased risk of CRC3.
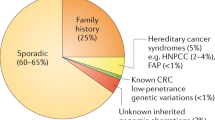
CRC is influenced by a combination of environmental and genetic factors. Hereditary syndromes, including Lynch syndrome (commonly referred as Hereditary Non-Polyposis Colorectal Cancer Syndrome or HNPCC) and familial adenomatous polyposis (also known as Familial Polyposis Coli), are associated with a significantly elevated risk of CRC4,5,6. Hereditary CRCs constitute around 5% of all CRC cases. While the risk of CRC in the general population is approximately 6%, individuals with a positive family history face a significantly higher risk. Those with a family history of CRC have a 2 to 4 times higher chance of CRC compared to individuals without a family history. This increased risk is attributed to the inheritance of mutant genes passed down from one generation to the next, underscoring the role of genetics in cancer development7,8,9. Previous studies reported that the risk for the development of CRC is approximately 1.8–1.9 times higher in patients with positive family history in a first-degree relative reflecting the need for more diagnostic approaches in these patients10.
The Eastern Mediterranean Region (EMRO), one of the six WHO regions, encompasses 22 countries with a total population of 730 million people. In this region, the incidence of CRC stands at approximately 5.9% in both men and women, making it the second most prevalent cause of cancer11. Furthermore, Nikbakht et al. demonstrated that the survival rate of CRC in the USA and European countries is higher compared to EMRO countries. This underscores the need for specific attention and targeted interventions in the EMRO region12.
Since individuals with a positive family history of cancer are classified as a high-risk group for developing CRC, early detection and clinical management are essential to enhance prognosis. Guidelines recommend increased screening interventions for these individuals to ensure timely identification and treatment13,14.
To recognizing the pivotal role of a positive family history in increasing CRC risk, it becomes imperative to determine the precise extent of this impact within the EMRO population. Conducting comprehensive epidemiological studies to evaluate the overall influence of this risk factor can offer invaluable insights into our understanding of the various global risk factors associated with CRC. While a positive family history of cancer has been suggested as a potential risk factor for CRC, its exact strength and significance remain uncertain especially in EMRO countries. In this meta-analysis study, we aimed to assess the impact of a positive family history of CRC or any cancers on the development of CRC in EMRO countries.
Method
Reporting
This systematic review and meta-analysis is conducted according to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)15.
Search strategy
We choose PubMed, Scopus, and Web of Science as our databases for a comprehensive systematic search by utilization of the following keyword: "Colorectal Tumors," "Colorectal Neoplasms," "Colorectal cancer," "Risk Factor," "Iran," "Afghanistan," "Bahrain," "Djibouti," "Egypt," "Iraq," "Jordan," "Kuwait," "Lebanon," "Libya," "Morocco," "Oman," "Pakistan," "State of Palestine," "Palestine," "Qatar," "Saudi Arabia," "Somalia," "Sudan," "Syria," "Tunisia," "United Arab Emirates," and "Yemen". We also examined the reference lists of the included studies. Our search extended up to December 2022.
Study selection
To mitigate the risk of selective reporting bias, a panel of four independent researchers conducted a comprehensive review of all papers. Throughout the screening process, any disagreements among researchers regarding study selection were resolved with the input of the corresponding author.
Data extraction procedure
The data extraction process was carried out by three independent researchers. We extracted all of the essential information from the included studies by designing a checklist with the following sections: First author's name, year of publication, country, sample size, base characteristics of patients (e.g., age, gender), and status of family history (CRC or any cancers). It is noticeable that we considered the positive family history of cancer in first-degree, second-degree, and third-degree families separately. In all stages of reviewing the articles, any conflict between the researchers was resolved with the final decision of the corresponding author.
Inclusion and exclusion criteria
We established a set of criteria to meticulously assess all papers considered for inclusion in our study. Our inclusion criteria consisted of case–control studies conducted within EMRO countries. These studies were required to have at least one relevant data point regarding a family history of cancer in both the case and control groups, with a specific focus on examining its impact on CRC. Additionally, we restricted our selection to English-language literature.
Excluded from consideration were articles where control groups were drawn from sources other than CRC, such as colon polyps or colorectal adenomas. Studies that utilized healthy tissues as control groups were also excluded. Furthermore, we omitted studies that assessed both CRC and colon polyps within the case group.
Quality assessment
To conduct an assessment of the quality of the included articles, we employed the Newcastle–Ottawa (NCO) Quality Assessment Scale Checklist, as depicted in Fig. 1. This checklist comprises three sections: Selection, Comparability, and Exposure. Each part aimed at scrutinizing the overall quality of the studies and identifying potential errors.
Statistical analysis
We employed Comprehensive Meta-Analysis (CMA) software version 3 as our analytical tool. In our analysis, we utilized odds ratio (OR) with 95% confidence intervals to assess the influence of family history of cancer on CRC risk, as reported in the included studies. To assess the degree of heterogeneity, we employed the I2 statistic, considering significance at a level greater than 50%. In instances of significant heterogeneity, we applied a random effects model. To delve deeper into the sources of heterogeneity, we conducted subgroup analyses based on the degree of evaluated relatives. Additionally, we used meta-regression to explore potential causes of heterogeneity, considering factors such as sample size and the Human Development Index (HDI). For an assessment of potential publication bias, we employed a funnel plot. Finally, power analysis was utilized to evaluate whether the mean sample size was sufficient for our study's objectives.
Results
Aim and trends
The primary objective of our present study is to explore the potential influence of a positive family history of cancer in patients with CRC within the EMRO. Through statistical analysis, we have identified a significant correlation between this risk factor and the incidence of CRC.
Description of search
We have meticulously documented our systematic search process in accordance with the PRISMA checklist guidelines, as illustrated in Fig. 2. Our search extended up to December 2022 and covered the databases mentioned above. After eliminating duplicate entries, we identified 1802 articles for initial screening based on their titles and abstracts. Following the exclusion of irrelevant articles, we subjected 75 to a thorough full-text review. Ultimately, we carefully selected 27 articles for inclusion in our meta-analysis.
Characteristics of the included studies
Table 1 provides an overview of the included papers in our analysis. Our meta-analysis comprises 27 studies conducted in EMRO countries, encompassing a total of 13,939 patients. Among these studies, 10 originated from Iran, five from Jordan, and two each from Saudi Arabia, Egypt, and Morocco. Kuwait, Palestine, Oman, Tunisia, Qatar, and Pakistan each contributed one study to our analysis.
All of the selected articles investigated the disparities in the presence of a positive family history of cancer between case and control groups. The publication dates of the included articles span from 1998 to 2021. The mean sample size across these studies was 516, ranging from 72 to 2966.
Main result
Our meta-analysis showed a significant association between the presence of a family history of CRC or any cancers with CRC (OR 2.21; 95% CI 1.54–3.17; P < 0.001 and OR 1.76; 95% CI 1.27–2.42; P = 0.001, respectively) (Fig. 3A,B).
(A) Forest plot for the relationship between positive family history of any cancer and colorectal cancer is performed to see if there is a positive relationship between a positive family history of any cancer and risk of colorectal cancer or not, (B) Forest plot for the relationship between positive family history of CRC cancer and colorectal cancer performed to see if there is a positive relationship between a positive family history of CRC and risk of colorectal cancer or not, (C) Forest plot for the relationship between positive family history of any cancer in first-degree relatives and colorectal cancer is performed to see people having a positive first-degree family history of any cancer can experience a higher risk of colorectal cancer than other types of positive family history of any cancer or not, (D) Forest plot for the relationship between positive family history of CRC in first-degree relatives and colorectal cancer is performed to see people having a positive first-degree family history of CRC can experience a higher risk of colorectal cancer than other types of positive family history of CRC or not.
Subgroup analysis
Obtained results from subgroup analysis revealed that positive family history of any cancers in the first-degree relatives significantly increases the risk of CRC (OR 2.12; 95% CI 1.65–2.73; P < 0.001) (Fig. 3C). A positive family history of CRC in first-degree relatives is also associated with an increased risk of CRC (OR 2.19; 95% CI 1.22–3.91; P = 0.008) (Fig. 3D).
Sensitivity and power analysis
Due to the observed heterogeneity in results, we performed a sensitivity analysis utilizing the 'one study removal' approach to identify potential sources of this heterogeneity. The results of the sensitivity analysis demonstrated that the primary outcome remained consistent, with no significant alterations observed in both CRC and any any cancer group when any single study was excluded (Fig. 4A,B).
(A) Result of sensitivity analysis by one study removing in CRC group to find the potential source of heterogeneity, (B) Result of sensitivity analysis by one study removing in any cancer group to find the potential source of heterogeneity, (C) Result of power analysis to find the source of heterogeneity in CRC relationship, (D) Result of power analysis to find the source of heterogeneity in any cancer relationship.
Additionally, the result of Power analysis showed that the mean number of sample sizes for CRC and any cancer were adequate for the meta-analysis (Fig. 4C,D).
Result of meta-regression
The results of our meta-regression analysis, based on sample size, indicated a significant influence of increased sample size on the impact of a positive family history of CRC on the development of CRC (Coefficient = 0.0005, P = 0.001). Nevertheless, the cumulative effect of a positive family history of any cancer was not found to be influenced by an increase in sample size (Coefficient = − 0.0004, P = 0.538) (Fig. 5A,B).
(A) Result of meta regression based on number of sample size in CRC group. This analysis is performed to see if there is a relationship between increasing number of sample size and risk of CRC among positive family history of CRC, (B) Result of meta regression based on number of sample size in any cancer group. This analysis is performed to see if there is a relationship between increasing number of sample size and risk of CRC among positive family history of any cancer, (C) Result of meta regression based on level of human developmental index (HDI) in CRC group. This analysis is performed to see if there is a relationship between increasing the rate of HDI and risk of CRC among positive family history of CRC, (D) Result of meta regression based on level of HDI in any cancer group. This analysis is performed to see if there is a relationship between increasing the rate of HDI and risk of CRC among positive family history of any cancer, (E) Funnel plot for assessing the source of heterogeneity in CRC group. This analysis is performed to find the source of Heterogeneity, (F) Funnel plot for assessing the source of heterogeneity in any cancer group. This analysis is performed to find the source of Heterogeneity.
We also conducted a meta-regression analysis to evaluate the influence of the HDI level in each study on our final results43. We did not find a significant effect of HDI on the relation between positive family history of CRC and CRC development (Coefficient = − 5.9501, P = 0.057). In addition, our investigations did not show a significant effect of HDI on the positive family history of CRC and any other cancer on CRC development (Coefficient = − 0.3850, P = 0.868) (Fig. 5C,D).
Publication bias
We employed a funnel plot to evaluate potential publication bias. Our primary findings revealed no significant publication bias for both any cancer and CRC (Egger's test P = 0.52; Begg's test P = 0.73, Egger's test P = 0.13; Begg's test P = 0.38, respectively) (Fig. 5E,F).
Discussion
CRC, one of the most prevalent forms of cancer, plays a significant role in global health, leading to numerous fatalities and a substantial burden of morbidity1. Numerous epidemiological studies have assessed the risk of CRC among individuals with a family history of this condition44. CRC is influenced by a combination of environmental and hereditary factors, which are recognized as risk factors for the disease45. Furthermore, individuals with inherited conditions such as HNPCC and Familial adenomatous polyposis, as well as those with a positive family history of CRC among their relatives, face an elevated risk of developing this condition46.
Our study revealed a robust correlation between a positive family history of CRC and an increased risk of CRC within the EMRO region. A study conducted by Ramírez-Díaz further confirmed the crucial role of family history as a significant factor influencing the occurrence of CRC47. These findings may be attributed to shared or similar gene sequences among family members, which could accelerate the mechanisms of CRC. Furthermore, a systematic review conducted by Henrikson et al. confirmed these results, demonstrating that a positive family history of CRC increases the risk of developing this condition48. In addition, the Slattery et al. study identified a positive association between individuals having a positive family history of CRC and an elevated risk of developing CRC49.
Several well-established gene mutations are associated with the development of CRC, and these mutations can be categorized into different classes of genes50. Familial adenomatous polyposis serves as an exemplar of a mutation occurring in a tumor suppressor gene, specifically in the APC gene51. Another similar polyposis syndrome related to CRC development is MUTYH (MYH)-associated polyposis (MAP) which is caused by a DNA repair gene alteration named MYH52. Lynch syndrome also known as HNPCC is a common predisposing condition of CRC accounting for 2–5% of all cases, and is a result of mutation in DNA mismatch repair genes mainly affecting MLH1, MSH2, and MSH6.453.
Our subgroup analysis revealed a notable disparity in CRC incidence within the EMRO region. Specifically, individuals with first-degree relatives affected by CRC were more than twice as likely to develop CRC compared to those without a family history of the disease. This observed risk is significantly higher than the findings reported in Fuchs et al.'s study, which encompassed a prospective study of over 100,000 volunteers in the USA. Remarkably, their investigation concluded that having a first-degree family member with a positive history of CRC could elevate an individual's risk of developing CRC by a substantial 72%. These findings emphasize the importance of familial predisposition as a significant risk factor for CRC within the EMRO region and warrant further exploration and targeted preventive measures8. In a meta-analysis conducted by Adam S., it was found that individuals with at least one positive first-degree family history have a heightened risk of developing CRC54. In a study conducted by Mehraban Far, it was established that a positive family history of CRC in first-degree relatives significantly amplified the risk of developing CRC55. Another noteworthy result from our meta-analysis indicated that having a family history of any type of cancer also increases the risk of developing CRC.
One of the most significant findings from our analysis is that a family history of CRC is more strongly associated with an increased risk of CRC in the EMRO region than having a positive family history of any cancer. These disparities can be attributed to several factors. Certain cancers may be less likely to be inherited or influenced by genetics. Newschaffer et al. demonstrated that a positive history of breast cancer did not elevate the risk of CRC and, in some subgroups, could even be considered a protective factor56. In a recent study conducted by Beebe-Dimmer et al., it was found that a positive family history of prostate cancer did not result in a significant increase in the risk of developing CRC57.
EMRO region people may have a higher susceptibility for CRC than other cancers due to genetical reasons. Within this context, several studies have pinpointed various genes as potential contributors to the risk of CRC development. These genes may vary in prevalence between populations, potentially explaining the differing effect sizes of a positive family history of CRC on increased risk of CRC between our study and others. In a study carried out by Dunlop et al., it was revealed that the median count of risk alleles was higher in cases than in controls58. Furthermore, Yusuf et al. identified several replicated associations as genetic risk factors for CRC59.
Environmental factors and dietary habits in this region may also contribute to an elevated risk of CRC in this region. According to a meta-analysis, smoking emerged as a significant risk factor for CRC in the EMRO region60. Seyyedsalehi et al., through a multicenter case–control study in Iran, identified a significant positive association between CRC and high dietary total fat intake61.
However, these results could also be due to better CRC screening in this region to identify suspicious and possible cases for early treatment. It was shown that due to a recent surge in early-onset diagnoses in the USA, approximately 10% of CRC cases are now detected in individuals under the age of 5062.
Contrary to the good screening of CRC, some cancers may not be diagnosed well until the final stages, and this lack of timely diagnosis can be one of the reasons for the lower percentage of the risk factor of any cancer compared to CRC. For instance, statistics show that in the USA, one-third of cervical cancers are diagnosed in the late stages when treatment is more difficult63.
Lastly, our meta-analysis reveals a compelling discovery: the substantial influence of a first-degree family history of any cancer group on the heightened susceptibility to CRC. This finding sounds a resounding alarm particularly for individuals with this positive familial heritage. It emphasizes the urgency of proactive measures. Timely detection and intervention in cases of CRC can yield remarkable results, primarily in terms of preventing mortality and morbidity.
Limitations
Our study has several limitations. Firstly, some countries in the EMRO region lacked sufficient relevant articles to be included in our study. Secondly, we lacked information regarding family histories of other inheritable cancer types, potentially limiting our comprehensive understanding of inherited cancer risks. Thirdly, we lacked sufficient data to explore whether a positive history of cancer among second- or third-degree relatives could be a potential risk factor. Lastly, the included studies did not provide information on the specific types of cancer that affected the family members, which could have added valuable context to our findings.
Conclusion
In summary, our meta-analysis conclusively establishes a significant positive association between having a family history of CRC or any cancer and the heightened risk of developing CRC. Our study underscores the critical importance of prioritizing screening and early identification for individuals with a family history of malignancies. By fostering collaboration among healthcare facilities and promoting the utilization of screening methods for timely detection, we can substantially mitigate the burdens of morbidity, mortality, and financial costs borne by both governments and the general public. Moreover, individuals with a positive family history should seek consultation with specialists for the screening tests and examinations to facilitate early diagnosis and appropriate treatment.
Data availability
All data analyzed during this study are included in this published article.
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Xi, Y. & Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 14, 101174. https://doi.org/10.1016/j.tranon.2021.101174 (2021).
Mehta, R. S. et al. Dietary patterns and risk of colorectal cancer: Analysis by tumor location and molecular subtypes. Gastroenterology 152, 1944-1953.e1941. https://doi.org/10.1053/j.gastro.2017.02.015 (2017).
Mesher, D. et al. A pooled analysis of the outcome of prospective colonoscopic surveillance for familial colorectal cancer. Int. J. Cancer 134, 939–947. https://doi.org/10.1002/ijc.28397 (2014).
Lynch Syndrome. https://www.ncbi.nlm.nih.gov/books/NBK431096/.
Familial Adenomatous Polyposis. https://www.ncbi.nlm.nih.gov/books/NBK538233/.
Armelao, F. & de Pretis, G. Familial colorectal cancer: A review. World J. Gastroenterol. 20, 9292–9298. https://doi.org/10.3748/wjg.v20.i28.9292 (2014).
Fuchs, C. S. et al. A prospective study of family history and the risk of colorectal cancer. N. Engl. J. Med. 331, 1669–1674. https://doi.org/10.1056/nejm199412223312501 (1994).
Smith, R. A. et al. American Cancer Society guidelines for the early detection of cancer: Update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: Update 2001–testing for early lung cancer detection. CA Cancer J. Clin. 51, 38–75. https://doi.org/10.3322/canjclin.51.1.38 (2001).
Wong, M. C. S. et al. Lower relative contribution of positive family history to colorectal cancer risk with increasing age: A systematic review and meta-analysis of 9.28 million individuals. Am. J. Gastroenterol. 113, 1819–1827. https://doi.org/10.1038/s41395-018-0075-y (2018).
Khazaei, Z., Navabi, M., Darvishi, I. & Goodarzi, E. The incidence and mortality of cancer in Eastern Mediterranean Regional Office (EMRO) and its relationship with Human Development Index (HDI): An ecological study. Asian Pac. J. Environ. Cancer https://doi.org/10.31557/APJEC.2018.1.2.59 (2018).
Nikbakht, H. A. et al. Survival rate of colorectal cancer in eastern Mediterranean region countries: A systematic review and meta-analysis. Cancer Control 27, 1073274820964146. https://doi.org/10.1177/1073274820964146 (2020).
Shaukat, A. et al. ACG clinical guidelines: Colorectal cancer screening 2021. Am. J. Gastroenterol. 116, 458–479. https://doi.org/10.14309/ajg.0000000000001122 (2021).
Leddin, D. et al. Clinical practice guideline on screening for colorectal cancer in individuals with a family history of nonhereditary colorectal cancer or adenoma: The Canadian Association of Gastroenterology Banff Consensus. Gastroenterology 155, 1325-1347.e1323. https://doi.org/10.1053/j.gastro.2018.08.017 (2018).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71. https://doi.org/10.1136/bmj.n71 (2021).
Tayyem, R. F. et al. Macro- and micronutrients consumption and the risk for colorectal cancer among Jordanians. Nutrients 7, 1769–1786. https://doi.org/10.3390/nu7031769 (2015).
Shivappa, N. et al. Dietary inflammatory index and odds of colorectal cancer in a case–control study from Iran. Asian Pac. J. Cancer Prev. 19, 1999–2006. https://doi.org/10.22034/apjcp.2018.19.7.1999 (2018).
Alsheridah, N. & Akhtar, S. Diet, obesity and colorectal carcinoma risk: Results from a national cancer registry-based middle-eastern study. BMC Cancer 18, 1227. https://doi.org/10.1186/s12885-018-5132-9 (2018).
Alqahtani, M. Z. et al. Risk factors of colorectal cancer among Saudi Population: Case-control study. J. Family Med. Prim. Care 9, 5035–5040. https://doi.org/10.4103/jfmpc.jfmpc_895_20 (2020).
Mint Sidi OuldDeoula, M. et al. Behavioral, nutritional, and genetic risk factors of colorectal cancers in Morocco: Protocol for a multicenter case-control study. JMIR Res. Protoc. 9, e13998. https://doi.org/10.2196/13998 (2020).
Khatatbeh, M. M. et al. Factors associated with colorectal cancer among Jordanians: A case–control study. Asian Pac. J. Cancer Prev. 19, 577–581. https://doi.org/10.22034/apjcp.2018.19.2.577 (2018).
Lo, A. C., Soliman, A. S., Khaled, H. M., Aboelyazid, A. & Greenson, J. K. Lifestyle, occupational, and reproductive factors and risk of colorectal cancer. Dis. Colon Rectum 53, 830–837. https://doi.org/10.1007/DCR.0b013e3181d320b1 (2010).
Ghrouz, I. & El Sharif, N. Diet and genetic risk factors of colorectal cancer in Palestine: A case-control study. Nutr. Cancer 74, 2460–2469. https://doi.org/10.1080/01635581.2021.2013507 (2022).
Soliman, A. S. et al. Familial aggregation of colorectal cancer in Egypt. Int. J. Cancer 77, 811–816 (1998).
Azzeh, F. S. et al. Healthy dietary patterns decrease the risk of colorectal cancer in the Mecca Region, Saudi Arabia: A case-control study. BMC Public Health 17, 607. https://doi.org/10.1186/s12889-017-4520-4 (2017).
Farahani, H. et al. Insulin resistance and colorectal cancer risk: The role of elevated plasma resistin levels. J. Gastrointest. Cancer 51, 478–483. https://doi.org/10.1007/s12029-019-00260-7 (2020).
El Kinany, K. et al. Consumption of modern and traditional Moroccan dairy products and colorectal cancer risk: A large case control study. Eur. J. Nutr. 59, 953–963. https://doi.org/10.1007/s00394-019-01954-1 (2020).
Mafiana, R. N. et al. Association between dietary and lifestyle indices and colorectal cancer in Oman: A case-control study. Asian Pac. J. Cancer Prev. 19, 3117–3122. https://doi.org/10.31557/apjcp.2018.19.11.3117 (2018).
Abu Mweis, S. S. et al. Food groups and the risk of colorectal cancer: Results from a Jordanian case-control study. Eur. J. Cancer Prev. 24, 313–320. https://doi.org/10.1097/cej.0000000000000089 (2015).
Gharbi, I. et al. The consumption of red and processed meat and the risk of colorectal cancer: A case-control study among the Tunisian population. Tunis. Med. 98, 726–729 (2020).
Teimoorian, F., Ranaei, M., Hajian Tilaki, K., Shokri Shirvani, J. & Vosough, Z. Association of Helicobacter pylori infection with colon cancer and adenomatous polyps. Iran J. Pathol. 13, 325–332 (2018).
Tayyem, R. F. et al. Consumption of whole grains, refined cereals, and legumes and its association with colorectal cancer among Jordanians. Integr. Cancer Ther. 15, 318–325. https://doi.org/10.1177/1534735415620010 (2016).
Arafa, M. A., Waly, M. I., Jriesat, S., Al Khafajei, A. & Sallam, S. Dietary and lifestyle characteristics of colorectal cancer in Jordan: A case-control study. Asian Pac. J. Cancer Prev. 12, 1931–1936 (2011).
Azizi, H. & Esmaeili, E. D. Stressful life events and risk of colorectal cancer: A case-control study of Iran. Asian Pac. J. Cancer Prev. 16, 2403–2407. https://doi.org/10.7314/apjcp.2015.16.6.2403 (2015).
Rafiee, P. et al. Dietary total antioxidant capacity and colorectal cancer and colorectal adenomatous polyps: A case-control study. Eur. J. Cancer Prev. 30, 40–45. https://doi.org/10.1097/cej.0000000000000577 (2021).
Bener, A., Moore, M. A., Ali, R. & El Ayoubi, H. R. Impacts of family history and lifestyle habits on colorectal cancer risk: A case-control study in Qatar. Asian Pac. J. Cancer Prev. 11, 963–968 (2010).
Safaee, A. et al. Risk of colorectal cancer in relatives: A case control study. Indian J. Cancer 47, 27–30. https://doi.org/10.4103/0019-509x.58855 (2010).
Golshiri, P., Rasooli, S., Emami, M. & Najimi, A. Effects of physical activity on risk of colorectal cancer: A case-control study. Int. J. Prev. Med. 7, 32. https://doi.org/10.4103/2008-7802.175991 (2016).
Moazzen, S. et al. Folic acid supplement intake and risk of colorectal cancer in women; a case control study. Ann. Glob. Health 86, 23. https://doi.org/10.5334/aogh.2664 (2020).
Khan, N. A. et al. Dietary practices, addictive behavior and bowel habits and risk of early onset colorectal cancer: A case control study. Asian Pac. J. Cancer Prev. 16, 7967–7973. https://doi.org/10.7314/apjcp.2015.16.17.7967 (2015).
Abolhassani, M. et al. Organochlorine and organophosphorous pesticides may induce colorectal cancer. A case-control study. Ecotoxicol. Environ. Saf. 178, 168–177. https://doi.org/10.1016/j.ecoenv.2019.04.030 (2019).
Khodaverdi, N., Zeighami, H., Jalilvand, A., Haghi, F. & Hesami, N. High frequency of enterotoxigenic Bacteroides fragilis and Enterococcus faecalis in the paraffin-embedded tissues of Iranian colorectal cancer patients. BMC Cancer 21, 1353. https://doi.org/10.1186/s12885-021-09110-x (2021).
Human Development Index (HDI). https://hdr.undp.org/data-center/human-development-index#/indicies/HDI (2021).
Johns, L. E. & Houlston, R. S. A systematic review and meta-analysis of familial colorectal cancer risk. Am. J. Gastroenterol. 96, 2992–3003. https://doi.org/10.1016/S0002-9270(01)03239-7 (2001).
Lauby-Secretan, B., Vilahur, N., Bianchini, F., Guha, N. & Straif, K. The IARC perspective on colorectal cancer screening. N. Engl. J. Med. 378, 1734–1740 (2018).
Wells, K. & Wise, P. E. Hereditary colorectal cancer syndromes. Surg. Clin. 97, 605–625 (2017).
Ramírez-Díaz, M. D. P., Alvarez-Bañuelos, M. T., Robaina-Castellanos, M. S., Castro-Enríquez, P. P. & Guzmán-García, R. E. Association of family history and life habits in the development of colorectal cancer: A matched case-control study in Mexico. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph18168633 (2021).
Henrikson, N. B. et al. Family history and the natural history of colorectal cancer: Systematic review. Genet. Med. 17, 702–712. https://doi.org/10.1038/gim.2014.188 (2015).
Slattery, M. L. et al. Family history and colorectal cancer: Predictors of risk. Cancer Causes Control 14, 879–887. https://doi.org/10.1023/B:CACO.0000003840.94591.76 (2003).
Hodgson, S. Mechanisms of inherited cancer susceptibility. J. Zhejiang Univ. Sci. B 9, 1–4. https://doi.org/10.1631/jzus.B073001 (2008).
Aghabozorgi, A. S. et al. Role of adenomatous polyposis coli (APC) gene mutations in the pathogenesis of colorectal cancer; current status and perspectives. Biochimie 157, 64–71. https://doi.org/10.1016/j.biochi.2018.11.003 (2019).
Sampson, J. R. et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet 362, 39–41. https://doi.org/10.1016/s0140-6736(03)13805-6 (2003).
Sinicrope, F. A. Lynch syndrome-associated colorectal cancer. N. Engl. J. Med. 379, 764–773. https://doi.org/10.1056/NEJMcp1714533 (2018).
Butterworth, A. S., Higgins, J. P. & Pharoah, P. Relative and absolute risk of colorectal cancer for individuals with a family history: A meta-analysis. Eur. J. Cancer 42, 216–227 (2006).
Mehraban Far, P., Alshahrani, A. & Yaghoobi, M. Quantitative risk of positive family history in developing colorectal cancer: A meta-analysis. World J. Gastroenterol. 25, 4278–4291. https://doi.org/10.3748/wjg.v25.i30.4278 (2019).
Newschaffer, C. J., Topham, A., Herzberg, T., Weiner, S. & Weinberg, D. S. Risk of colorectal cancer after breast cancer. Lancet 357, 837–840. https://doi.org/10.1016/s0140-6736(00)04197-0 (2001).
Beebe-Dimmer, J. L. et al. Family history of prostate and colorectal cancer and risk of colorectal cancer in the Women’s health initiative. BMC Cancer 17, 848. https://doi.org/10.1186/s12885-017-3873-5 (2017).
Dunlop, M. G. et al. Cumulative impact of common genetic variants and other risk factors on colorectal cancer risk in 42 103 individuals. Gut 62, 871–881. https://doi.org/10.1136/gutjnl-2011-300537 (2013).
Yusuf, I. et al. Genetic risk factors for colorectal cancer in multiethnic Indonesians. Sci. Rep. 11, 9988. https://doi.org/10.1038/s41598-021-88805-4 (2021).
Keivanlou, M. H. et al. Association between smoking and colorectal cancer in Eastern Mediterranean Regional Office (EMRO): A systematic review and meta-analysis. Saudi J. Gastroenterol. 29, 204–211. https://doi.org/10.4103/sjg.sjg_163_23 (2023).
Seyyedsalehi, M. S. et al. Association between dietary fat intake and colorectal cancer: A multicenter case-control study in Iran. Front. Nutr. https://doi.org/10.3389/fnut.2022.1017720 (2022).
Thompson, C. A., Begi, T. & Parada, H. Jr. Alarming recent rises in early-onset colorectal cancer. Cancer 128, 230–233. https://doi.org/10.1002/cncr.33919 (2022).
Prevention, C. F. D. A. C. A. Cancers Diagnosed at Late-Stages Despite Available Screening Tests. https://www.cdc.gov/media/pressrel/2010/r101124.html#:~:text=Almost%20half%20of%20the%20colorectal,Disease%20and%20Control%20and%20Prevention. (2010).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Study selection and article screening is performed by N.N., N.L., Z.R., and A.H. Extracting data is performed by E.A.-S., Z.R. and M.S.A. Analyzing the results of our study and is done by E.A.-S. and M.-H.K. The first draft of the manuscript was written by M.-H.K., M.T.A., S.H. and F.M.G. All authors were involved in editing the manuscript for revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Keivanlou, MH., Amini-Salehi, E., Joukar, F. et al. Family history of cancer as a potential risk factor for colorectal cancer in EMRO countries: a systematic review and meta-analysis. Sci Rep 13, 17457 (2023). https://doi.org/10.1038/s41598-023-44487-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-44487-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.